Abstract
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) participates in a cell death cascade wherein a variety of stimuli activate nitric oxide (NO) synthases with NO nitrosylating GAPDH, conferring on it the ability to bind to Siah, an E3-ubiquitin-ligase, whose nuclear localization signal enables the GAPDH/Siah protein complex to translocate to the nucleus where degradation of Siah targets elicits cell death. R-(−)-Deprenyl (deprenyl) ameliorates the progression of disability in early Parkinson’s disease and also has neuroprotective actions. We show that deprenyl and a related agent, TCH346, in subnanomolar concentrations, prevent S-nitrosylation of GAPDH, the binding of GAPDH to Siah, and nuclear translocation of GAPDH. In mice treated with the dopamine neuronal toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), low doses of deprenyl prevent binding of GAPDH and Siah1 in the dopamine-enriched corpus striatum.
Keywords: nitric oxide, apoptosis, Parkinson’s disease, S-nitrosylation, Siah
Efforts to prevent neurodegeneration in disorders such as Alzheimer’s disease, Parkinson’s disease (PD), Huntington’s disease, and stroke have proceeded along two pathways. One involves identifying the fundamental molecular causes of these diseases, although even for Huntington’s disease whose molecular causation is established, curative therapy has not followed directly. Alternatively, elucidating mechanisms of cell death may lead to agents that prevent neurodegeneration without knowing the specific disease etiology.
The monoamine oxidase-B (MAO-B) inhibitor, R-(−)-Deprenyl (selegiline, hereafter designated deprenyl) (Fig. 1a) has been used in the therapy of PD with the initial goal of elevating dopamine levels (1–4). Deprenyl can delay the progression of disability in early Parkinson’s disease, and recent studies also indicated that deprenyl reduces neuronal death in a variety of in vivo and in vitro experimental models. These include death of neuronal cultures induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), nitric oxide (NO), or peroxynitrite, as well as in vivo hypoxia models and peripheral or optic nerve crush (5–13). Neuroprotection by deprenyl has been suggested to be independent of MAO-B (14–16). Such a concept has been reinforced by the fact that the deprenyl derivative TCH346 (Fig. 1a) with no inhibitory action for MAO-B, also displays neuroprotective effects in culture models at concentrations as low as 0.1 pM and in intact animals at oral doses as low as 0.3 μg/kg (17–20).
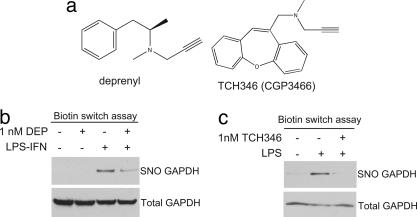
Fig. 1.
Deprenyl and TCH346 inhibit S-nitrosylation of GAPDH. (a) Chemical structure of deprenyl and TCH346 (i.e., CGP3466). (b) Deprenyl (DEP) inhibits S-nitrosylation of GAPDH (SNO GAPDH). RAW264.7 cells were untreated, treated with deprenyl (1 nM), treated with LPS and IFN-γ (LPS-IFN), or treated with LPS-IFN and deprenyl for 24 h. (c) TCH346 inhibits S-nitrosylation of GAPDH. RAW264.7 cells were untreated, treated with LPS, or treated with LPS and TCH346 for 24 h.
Recently we described a cell death signaling system whereby cell stressors activate inducible or neuronal nitric oxide synthase (NOS) with a specific S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by NO (21). S-nitrosylation of GAPDH abolishes catalytic activity and confers upon GAPDH the ability to bind to Siah1 (hereafter designated Siah), an E3-ubiquitin-ligase whose nuclear localization signal mediates nuclear translocation of the GAPDH/Siah complex. In the nucleus, GAPDH extends the rapid turnover of Siah, leading to the degradation of selected nuclear targets of Siah and apoptosis. In efforts to identify the target for the neuroprotective actions of TCH346, Waldmeier and colleagues (22) used affinity binding, affinity labeling, and BIAcore technology, and identified GAPDH as its specific target.
We now demonstrate that deprenyl and TCH346 at subnanomolar concentrations prevent the S-nitrosylation of GAPDH, inhibit GAPDH/Siah binding and prevent the nuclear translocation of GAPDH. These actions also occur in intact animals at drug doses as low as 0.01 mg/kg. Thus, the neuroprotective actions of these drugs appear to reflect inhibition of the GAPDH/Siah cell death cascade.
Results and Discussion
In the macrophage cell line RAW264.7, stimulation by LPS and IFN-γ (LPS–IFN), components of endotoxin, provokes a massive activation of inducible NOS with NO formed mediating the cytotoxic actions of macrophages and also eliciting apoptotic cell death of the macrophages themselves (23). In this system, NO S-nitrosylates GAPDH eliciting GAPDH/Siah binding and nuclear translocation of the protein complex, a process blocked by NOS inhibitors (21). We have replicated these findings revealing S-nitrosylation of GAPDH after induction of inducible NOS. At a 1 nM concentration, deprenyl and TCH346 both prevent GAPDH nitrosylation (Fig. 1 b and c).
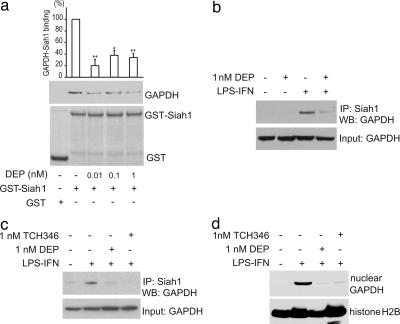
We directly demonstrate that the binding of GST-tagged Siah to GAPDH in vitro is prevented by deprenyl with as little as 0.01 nM deprenyl eliciting detectable diminution of binding (Fig. 2a).
Fig. 2.
Deprenyl and TCH346 inhibit the binding of GAPDH and Siah1. (a) Deprenyl (DEP) inhibits the binding of GAPDH and Siah1 in vitro. GAPDH was preincubated with deprenyl at 0 nM (n = 5), 0.01 nM (n = 2), 0.1 nM (n = 3), or 1 nM (n = 4). GST-Siah1 was added, and binding was assessed by GSH-agarose pull down followed by Western blotting. The graph represents densitometry analysis of Western blotting (error bars indicate SEM; ∗, P < 0.0001; ∗∗, P < 0.00005). (b) Deprenyl inhibits the binding of GAPDH and Siah1. RAW264.7 cells were untreated, treated with deprenyl (1 nM), treated with LPS and IFN-γ (LPS-IFN), or treated with LPS-IFN and deprenyl for 24 h. Cell lysates were immunoprecipitated with α -Siah1 antibody. (c and d) RAW264.7 cells were treated the same as in b. (c) TCH346 inhibits the binding of GAPDH and Siah1. (d) Deprenyl and TCH346 inhibit the nuclear translocation of GAPDH. Nuclear fractions were analyzed by Western blotting.
We also monitored drug effects upon GAPDH/Siah binding in intact cells. In RAW264.7 cells treatment with LPS-IFN elicits robust binding of GAPDH to Siah (Fig. 2 b and c). At 1 nM, both deprenyl and TCH346 abolish GAPDH/Siah binding.
We wondered whether the prevention of GAPDH S-nitrosylation and binding to Siah by drugs would influence GAPDH nuclear translocation. In RAW264.7 cells 1 nM deprenyl or TCH346 abolish the nuclear translocation of GAPDH after LPS–IFN treatment (Fig. 2d).
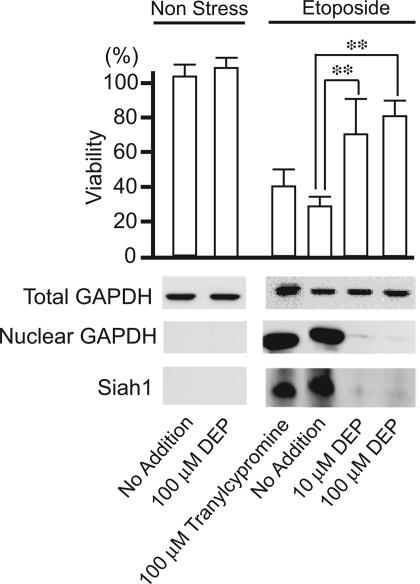
To ascertain the influence of deprenyl upon cell death, we used cerebellar granule cells (Fig. 3). Etoposide reduces cell viability by 70% associated with profound augmentation of Siah levels and with nuclear translocation of GAPDH. Treatment with deprenyl abolishes the Siah augmentation and the nuclear translocation of GAPDH and markedly reduces cell death.
Fig. 3.
Deprenyl prevents apoptotic cell death in cerebellar granule neurons. Etoposide (100 μM) was added to primary cerebellar granule neuron cultures 4–5 days after plating. Cytotoxicity was monitored 20 h after the addition of etoposide. Deprenyl (DEP) exerts cytoprotective effects (P < 0.01), whereas tranylcypromine, a monoamine oxidase inhibitor that lacks GAPDH binding properties, is ineffective. The extent of cell death parallels increased levels of Siah1 and nuclear GAPDH.
To determine whether deprenyl actions in intact animals involve the GAPDH/Siah system, we used an animal model of PD in which the dopamine neuronal toxin MPTP destroys dopamine neurons (24). MPTP treatment markedly augments levels of S-nitrosylated GAPDH in the brain (Fig. 4a). MPTP also leads to GAPDH/Siah binding complexes in the corpus striatum, the locus of the highest dopamine terminal density (Fig. 4b). Treatment with 0.01 mg/kg deprenyl markedly reduces GAPDH/Siah binding in the striatum.
Fig. 4.
Deprenyl inhibits the binding of GAPDH and Siah1 in MPTP-treated mice. (a) S-nitrosylation of GAPDH (SNO GAPDH) in MPTP-treated mouse brain. Mice were treated as described in Materials and Methods, and the brain lysate was subjected to the biotin switch assay. (b) Deprenyl (DEP) inhibits the binding of GAPDH and Siah1 in MPTP treated mouse striatum. Mice were treated as described in Materials and Methods. Striatum region was dissected, and its lysate was used for coimmunoprecipitation assay.
In summary, our findings provide compelling evidence that the neuroprotective actions of deprenyl and TCH346 reflect their preventing GAPDH/Siah binding and the nuclear translocation of GAPDH. It appears that the initial action of the drugs is to bind to GAPDH, as Waldmeier and colleagues (22) directly demonstrated binding of TCH346 to GAPDH. Such binding would inhibit S-nitrosylation of GAPDH and its binding to Siah. Recently, Youdim and colleagues (25, 26) observed that rasagiline, a monoamine oxidase inhibitor used in the therapy of PD, is also neuroprotective in multiple animal models and prevents the nuclear translocation of GAPDH.
Although the principal focus for the therapeutic actions of deprenyl has been PD, deprenyl and related drugs might be useful in other neuronal conditions as well as non-nervous system conditions, because the GAPDH/Siah cascade appears fairly universal (21, 27–29). Thus, a wide range of stressors in diverse cell lines elicits nuclear translocation of GAPDH, with antisense to GAPDH preventing nuclear translocation and cell death. The most investigated GAPDH systems involve apoptotic death. Whether GAPDH plays a role in necrosis or in nonapoptotic programmed cell death is unclear.
In addition to the therapeutic relevance of our findings, evidence that cytoprotective actions of these drugs involve blockade of the GAPDH/Siah system provides support for the concept that the GAPDH/Siah signaling cascade is an important component of cell death.
Materials and Methods
Biochemistry
GST-tagged proteins were prepared according to the manufacturer’s recommendations (Amersham Pharmacia) and purified through glutathione-Sepharose (Amersham Pharmacia). Five hundred nanograms of GAPDH from human erythrocytes (Sigma) were preincubated with deprenyl or TCH346 in 1 ml of binding buffer (50 mM Tris, pH 7.4/150 mM NaCl/0.2 mg/ml BSA) at 4°C overnight. Five micrograms of GST or GST-Siah1 was added and incubated for 2 h at 4°C. Twenty microliters of 50% glutathione-Sepharose was added to the mixture and incubated for 1 h at 4°C. The beads were washed four times with 1 ml of washing buffer (50 mM Tris, pH 7.4/150 mM NaCl/0.1 mg/ml BSA/0.1% Chaps), separated by SDS/PAGE, and analyzed by Western blotting. Coimmunoprecipitation with an anti-Siah1 antibody was performed as described (21). Subcellular fractionation was performed as described (27). The S-nitrosylation biotin switch assay was performed as described (30).
Cell Culture and Cytotoxicity
RAW264.7 macrophages were maintained in DMEM with 10% FBS and 2 mM l-glutamine at 37°C with a 5% CO2 atmosphere in a humidified incubator. To stimulate macrophages, LPS (1 μg/ml) with or without IFN-γ (10 ng/ml) was added to medium. Primary cerebellar granule cells were prepared from 6- to 7-day-old rats and were cultured in DMEM plus 10% FBS. Four to five days after plating, 100 μM etoposide was added to induce cell death. Deprenyl (10 or 100 μM) or tranylcypromine (100 μM) were added twice directly to cultured media, 7 h before and immediately after the etoposide addition. The viability of primary neurons was monitored by MAP2 staining as described (27).
Animals and Treatment
All experiments were approved and conformed to the guidelines set by the Institutional Animal Care Committee. Eight-week-old male CD1 mice (The Jackson Laboratory) were used. Two groups of treatment schedules were followed in our studies. In one group, mice received four i.p. injections of MPTP-HCl (20 mg/kg of free base; Sigma) in saline at 2-h intervals and were killed after 48 h (n = 5). The second group received four i.p. injections of MPTP-HCl (20 mg/kg of free base) at 2 h intervals on the first day, and 24 h after the first dose of MPTP, they received four i.p. injections of deprenyl (0.01 mg/kg; Sigma) in saline at 2 h intervals and were killed at 24 h after the first dose of deprenyl (n = 5). Control mice received saline only.
Acknowledgments
We thank B. Ziegler and Y. Lema for organizing the manuscript; A. S. Huang, S. K. Tankou, and M. D. Kornberg for discussion and technical assistance; and Dr. P. C. Waldmeier (Novartis, Basel, Switzerland) for providing TCH346. This work was supported by U.S. Public Health Service Grants DA-00266, Research Scientist Award DA-00074 (to S.H.S.), MH-069853 (to A.S.), and grants from National Alliance for Research on Schizophrenia and Depression, The Stanley Foundation, Hereditary Disease Foundation, and S-R Foundation (to A.S.), as well as National Institutes of Health Grant NS-38377 (to T.M.D. and V.L.D.).
Abbreviations
- PD
Parkinson’s disease
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NOS
nitric oxide synthase.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Kofman O. S. N. Engl. J. Med. 1993;328:1715. doi: 10.1056/nejm199306103282316. [DOI] [PubMed] [Google Scholar]
- 2.The Parkinson Study Group. N. Engl. J. Med. 1989;321:1364–1371. doi: 10.1056/NEJM198911163212004. [DOI] [PubMed] [Google Scholar]
- 3.Correspondences to The Parkinson Study Group. N. Engl. J. Med. 1990;322:1526–1528. [Google Scholar]
- 4.The Parkinson Study Group. N. Engl. J. Med. 1993;328:176–183. doi: 10.1056/NEJM199301213280305. [DOI] [PubMed] [Google Scholar]
- 5.Paterson I. A., Barber A. J., Gelowitz D. L., Voll C. Neurosci. Biobehav. Rev. 1997;21:181–186. doi: 10.1016/s0149-7634(96)00008-5. [DOI] [PubMed] [Google Scholar]
- 6.Buys Y. M., Trope G. E., Tatton W. G. Curr. Eye Res. 1995;14:119–126. doi: 10.3109/02713689508999923. [DOI] [PubMed] [Google Scholar]
- 7.Knollema S., Aukema W., Hom H., Korf J., ter Horst G. J. Stroke. 1995;26:1883–1887. doi: 10.1161/01.str.26.10.1883. [DOI] [PubMed] [Google Scholar]
- 8.Lahtinen H., Koistinaho J., Kauppinen R., Haapalinna A., Keinanen R., Sivenius J. Brain Res. 1997;757:260–267. doi: 10.1016/s0006-8993(97)00227-8. [DOI] [PubMed] [Google Scholar]
- 9.Ravikumar R., Lakshmana M. K., Rao B. S., Meti B. L., Bindu P. N., Raju T. R. Exp. Neurol. 1998;149:123–129. doi: 10.1006/exnr.1997.6682. [DOI] [PubMed] [Google Scholar]
- 10.Salo P. T., Tatton W. G. J. Neurosci. Res. 1992;31:394–400. doi: 10.1002/jnr.490310223. [DOI] [PubMed] [Google Scholar]
- 11.Maruyama W., Takahashi T., Naoi M. J. Neurochem. 1998;70:2510–2515. doi: 10.1046/j.1471-4159.1998.70062510.x. [DOI] [PubMed] [Google Scholar]
- 12.Tatton W. G., Greenwood C. E. J. Neurosci. Res. 1991;30:666–672. doi: 10.1002/jnr.490300410. [DOI] [PubMed] [Google Scholar]
- 13.Tatton W. G., Chalmers-Redman R. M., Elstner M., Leesch W., Jagodzinski F. B., Stupak D. P., Sugrue M. M., Tatton N. A. J. Neural Transm. Suppl. 2000;60:77–100. doi: 10.1007/978-3-7091-6301-6_5. [DOI] [PubMed] [Google Scholar]
- 14.Ansari K. S., Yu P. H., Kruck T. P., Tatton W. G. J. Neurosci. 1993;13:4042–4053. doi: 10.1523/JNEUROSCI.13-09-04042.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finnegan K. T., Skratt J. J., Irwin I., DeLanney L. E., Langston J. W. Eur. J. Pharmacol. 1990;184:119–126. doi: 10.1016/0014-2999(90)90672-s. [DOI] [PubMed] [Google Scholar]
- 16.Tatton W. G., Chalmers-Redman R. M. Neurology. 1996;47:S171–S183. doi: 10.1212/wnl.47.6_suppl_3.171s. [DOI] [PubMed] [Google Scholar]
- 17.Waldmeier P. C., Boulton A. A., Cools A. R., Kato A. C., Tatton W. G. J. Neural Transm. Suppl. 2000;60:197–214. doi: 10.1007/978-3-7091-6301-6_13. [DOI] [PubMed] [Google Scholar]
- 18.Sagot Y., Toni N., Perrelet D., Lurot S., King B., Rixner H., Mattenberger L., Waldmeier P. C., Kato A. C. Br. J. Pharmacol. 2000;131:721–728. doi: 10.1038/sj.bjp.0703633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andringa G., van Oosten R. V., Unger W., Hafmans T. G., Veening J., Stoof J. C., Cools A. R. Eur. J. Neurosci. 2000;12:3033–3043. doi: 10.1046/j.1460-9568.2000.00181.x. [DOI] [PubMed] [Google Scholar]
- 20.Carlile G. W., Chalmers-Redman R. M., Tatton N. A., Pong A., Borden K. E., Tatton W. G. Mol. Pharmacol. 2000;57:2–12. [PubMed] [Google Scholar]
- 21.Hara M. R., Agrawal N., Kim S. F., Cascio M. B., Fujimuro M., Ozeki Y., Takahashi M., Cheah J. H., Tankou S. K., Hester L. D., et al. Nat. Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 22.Kragten E., Lalande I., Zimmermann K., Roggo S., Schindler P., Muller D., van Oostrum J., Waldmeier P., Furst P. J. Biol. Chem. 1998;273:5821–5828. doi: 10.1074/jbc.273.10.5821. [DOI] [PubMed] [Google Scholar]
- 23.Eu J. P., Liu L., Zeng M., Stamler J. S. Biochemistry. 2000;39:1040–1047. doi: 10.1021/bi992046e. [DOI] [PubMed] [Google Scholar]
- 24.Mandir A. S., Simbulan-Rosenthal C. M., Poitras M. F., Lumpkin J. R., Dawson V. L., Smulson M. E., Dawson T. M. J. Neurochem. 2002;83:186–192. doi: 10.1046/j.1471-4159.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- 25.Mandel S., Weinreb O., Amit T., Youdim M. B. Brain Res. Brain Res. Rev. 2005;48:379–387. doi: 10.1016/j.brainresrev.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 26.Maruyama W., Akao Y., Youdim M. B., Davis B. A., Naoi M. J. Neurochem. 2001;78:727–735. doi: 10.1046/j.1471-4159.2001.00448.x. [DOI] [PubMed] [Google Scholar]
- 27.Sawa A., Khan A. A., Hester L. D., Snyder S. H. Proc. Natl. Acad. Sci. USA. 1997;94:11669–11674. doi: 10.1073/pnas.94.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuang D. M., Hough C., Senatorov V. V. Annu. Rev. Pharmacol. Toxicol. 2005;45:269–290. doi: 10.1146/annurev.pharmtox.45.120403.095902. [DOI] [PubMed] [Google Scholar]
- 29.Sirover M. A. Biochim. Biophys. Acta. 1999;1432:159–184. doi: 10.1016/s0167-4838(99)00119-3. [DOI] [PubMed] [Google Scholar]
- 30.Jaffrey S. R., Erdjument-Bromage H., Ferris C. D., Tempst P., Snyder S. H. Nat. Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]