Abstract
Retinoic acid (RA) is commonly used in vitro to differentiate stem cell populations including adult neural stem cells into neurons; however, the in vivo function of RA during adult neurogenesis remains largely unexplored. We found that depletion of RA in adult mice leads to significantly decreased neuronal differentiation within the granular cell layer of the dentate gyrus. RA contribution to neurogenesis occurs early, for RA deficiency also results in a decrease in newborn cells expressing an immature neuronal marker. Furthermore, although proliferation is unaffected during RA absence, cell survival is significantly reduced. Finally, a screen for retinoid-induced genes identifies metabolic targets including the lipid transporters, CD-36 and ABCA-1, the lipogenic master regulator SREBP1c as well as components of the Wnt signaling pathway. Our results reveal RA as a crucial contributor to early stages of adult neurogenesis and survival in vivo.
Keywords: hippocampus, neural stem cells, retinoic acid receptor, vitamin A
Retinoids, which include vitamin A (retinol) and its derivatives, are critical contributors to the proper development of the vertebrate CNS (1) by directing anteroposterior transformation (2) as well as specifying the dorsoventral axis of the spinal cord (3). They also regulate multiple functions in the adult including vision, cellular differentiation, immunity, fertility, and the homeostasis of several tissues. Thus, during both early phases of development and continuing through adulthood, retinoids are vital for normal physiology.
The biological effects of retinoids are mediated by retinoid receptors, members of the nuclear receptor superfamily. Nuclear receptors are ligand-induced transcription factors that respond to a variety of lipophilic steroids, hormones, and fatty acids (4–6). Retinoid receptors consist of the retinoic acid (RA) receptors (RARα, β, and γ), which bind both all-trans RA and 9-cis RA (7–8), and the retinoid X receptors (RXRα, β, and γ), which bind 9-cis RA only (9) but also other non-retinoid lipid ligands such as docosahexaenoic acid (10). RARs and RXRs form heterodimers and bind DNA at specific sequences to promote transcription of their target genes.
RAR, RXR, and RA activity occur in distinct patterns in the mature CNS including the dentate gyrus (DG), a hippocampal region distinguished as being one of the few sites of ongoing proliferation and neuronal development in the adult CNS (11–17). RA is necessary for long-term synaptic plasticity between neurons in the hippocampal Schaffer Collateral pathway (11, 18), but the localized and intense concentration of RA and expression of RAR and RXR in the adult DG suggests that retinoids could also contribute to the region-specific phenomenon of neurogenesis.
Embryonic neurogenesis is closely regulated by RA at various time points and regions; for example, RA signaling controls the number, timing, and subtype produced during motor neuronal differentiation in the spinal cord (19). Although neurogenesis primarily occurs during embryogenesis and early development, it persists in restricted regions in the mature CNS. These areas of proliferation occur in the subventricular zone of the lateral ventricle and the subgranular zone of the hippocampal DG. In the subgranular zone, cells divide, migrate into the granular cell layer (GCL), and differentiate into neuronal or glial cells (20).
To determine whether RA is a natural requirement for adult hippocampal neurogenesis, we studied the effects of retinoid deprivation in mice (see Fig. 1 for experimental design). We found that RA depletion does not influence proliferation within the subgranular zone, but rather inhibits neuronal differentiation. The need for RA occurs early in the differentiation process because the expression of the immature neuronal marker doublecortin (dcx) is dramatically decreased in RAD mice. Although the rate of proliferation is normal, the ability of newborn cells in the DG to survive is lowered in the absence of RA. Using a highly specified population of adult-derived neural stem cells (NSCs), we were able to identify a set of RA responsive genes including lipid modulator proteins (such as SREBP1, CD36, and ABCA1) and components of the Wnt signaling pathway, suggesting that cell signaling and lipid mobilization help direct the earliest steps of neuronal differentiation.
Fig. 1.
Experimental design for the induction of retinoid deficiency in mice. Mothers were placed on RAD diets immediately after giving birth. Pups were maintained on this diet. Once retinoid depletion was attained, BrdUrd injections began for 6 days. On the 7th day, mice were killed, and the brains were used to analyze the effect of retinoid deficiency on proliferation in the DG. Three weeks later, additional mice were killed, and the brains were assessed for neuronal differentiation and survival. In parallel, additional mice were maintained on a RAS diet. For retinoid rescue, some RAD mice were placed on a RAS diet on day 103, at the start of BrdUrd treatment.
Results
RA Is Not Necessary for Proliferation but Contributes to Cell Survival in the DG
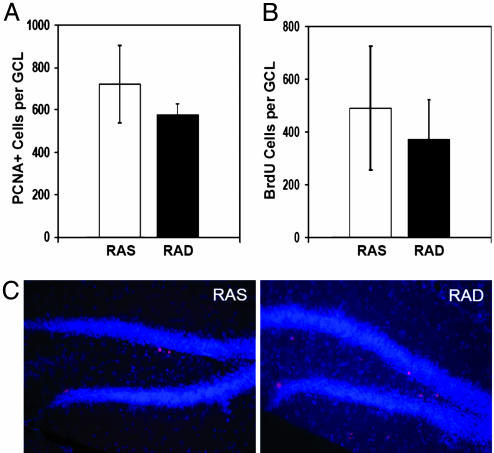
To determine whether RA regulates proliferation in the DG, the number of dividing cells in the GCL of both retinoid-sufficient (RAS) and retinoid-deficient (RAD) mice were compared by using the protocol described in Fig. 1. Proliferating cell nuclear antigen (PCNA), a cofactor for DNA polymerase during S phase and an indicator of proliferating cells, was expressed in an equal number of cells throughout the GCL of both groups (Fig. 2 A and C). Consistent with these results, the number of BrdUrd-labeled cells per GCL in mice killed 24 h after 6 days of BrdUrd injections was also comparable between both groups (Fig. 2B). In contrast, the number of BrdUrd-labeled cells 3 weeks after BrdUrd injections is significantly decreased in retinoid deficient and retinoid replenished mice as compared to retinoid sufficient mice (Fig. 3). This finding suggests that RA is nonessential for normal cell proliferation in the DG but that its absence results in reduced survival of newborn cells.
Fig. 2.
RA status does not affect proliferation in the adult DG. (A and B) Proliferating cells in the subgranular zone of the DG were counted based on expression of PCNA or BrdUrd labeling. (C) No difference in PCNA expression is observed between RAD and RAS mice.
Fig. 3.
Survival of new cells is decreased with retinoid deficiency. Fewer cells labeled with BrdUrd remain in the GCL 3 weeks later, when RA is not present (P = 0.015). Retinoid supplementation also does not rescue this phenotype (P = 0.031).
Neuronal Differentiation Is Disrupted by Retinoid Deficiency
To establish whether RA affects neuronal differentiation in vivo, proliferating cells in the DG were pulsed with BrdUrd and their fates analyzed three weeks later (Fig. 4A). In retinoid sufficient mice, 65.7% of the newborn cells expressed neuronal nuclear antigen (NeuN), a commonly used marker for mature neurons, and 4.2% expressed S100β, an astrocytic marker. These ratios are similar to those found in other strains of mice previously analyzed (21). In contrast, RAD mice displayed a highly significant decrease in number of BrdUrd-labeled cells expressing NeuN in the DG (26.8%, Fig. 4B). These results imply that RA is required for normal neuronal differentiation within the adult DG and disruption of retinoid signaling results in disruption of neurogenesis.
Fig. 4.
Neuronal differentiation is impaired in RAD and retinoid rescued mice. (A) BrdUrd-containing cells were analyzed for signs of differentiation by labeling with antibodies for NeuN (neuronal phenotype) or S100b (astrocytic phenotype). (B) Retinoid deficiency grossly impaired neuronal differentiation, as observed in mice 3 weeks after BrdUrd injection (P = 0.0001). Returning RA to the diet of similarly retinoid-deprived mice did not rescue neurogenesis (P = 0.0001).
Interestingly, replenishing the RAD mice with a RAS diet on the first day of BrdUrd injections did not rescue the loss of neuronal maturation (Fig. 4B), despite our verification that these mice were indeed RA-replenished (data not shown).
Effects of Retinoid Deficiency on Neuronal Differentiation Appear Early and Are Independent of the Effects on Survival
Despite the decrease in neurogenesis, no change in number of new astrocytic cells occurred during retinoid deficiency. Instead, an increase in “unclassified” cells that were neither neuronal nor glial were observed in retinoid deficient and retinoid replenished mice. To assess whether the excess “unclassified” cells in retinoid deficient mice correspond to immature neurons that were not yet expressing NeuN, we compared the expression of dcx, an immature neuronal marker (22), in retinoid sufficient, deficient, and replenished mice (Fig. 5A). The high percentage of BrdUrd-labeled cells expressing dcx in retinoid sufficient mice (78.2%) was decreased significantly in retinoid deficient and retinoid replenished mice (51.2% and 53.7% respectively, Fig. 5B). Importantly, when we differentiate between cells displaying morphology of earlier and later stages of neuronal development (Fig. 5A), the inhibition of dcx expression is already evident in the less mature cells (Fig. 5B). This finding indicates that the disruption of neuronal maturation begins early in the absence of RA, before or at the initiation of dcx expression.
Fig. 5.
RA is essential during early phases of neuronal differentiation. (A) Early dcx+ cells exhibit no processes or processes horizontal to the dentate blades. Late dcx+ cells exhibit long processes perpendicular to the blades. Dcx expression in newborn cells is reduced in the absence of RA, during early stages as well as during later stages (total P = 0.005, early P = 0.05, late P = 0.008). Retinoid supplementation does not rescue dcx expression in RAD mice (total P = 0.005, late P = 0.05). (B) Retinoid deficiency results in a significant decrease in dcx expression in RAD mice examined immediately after BrdUrd injections when neither proliferation nor survival were affected (P < 0.005). (C) Contribution by RA to neuronal differentiation is independent of survival requirements. Retinoid deficiency results in a significant decrease in dcx expression in retinoid deficient mice (P < 0.005) immediately after BrdUrd injections when neither proliferation nor survival was affected by retinoid depletion. (D) Rate of neurogenesis is affected by both rates of survival and neuronal differentiation. Total number of BrdUrd-labeled cells expressing dcx in RAD and retinoid-replenished mice is reduced to 29.1% and 28.5%, respectively, compared to RAS mice. Total number of BrdUrd and NeuN double-labeled cells are reduced to 18.2% and 16.2%, respectively, compared to RAS mice.
To differentiate between the effects of RA on neuronal differentiation and survival, we examined the brains from mice killed immediately after the completion of BrdUrd injections. At this time point, the number of PCNA-expressing and BrdUrd-labeled cells were equivalent between RAD and RAS groups, indicating that neither proliferation nor survival had been significantly affected by RA absence (see above). In contrast, the expression of dcx by newborn cells in RAD mice was already significantly decreased at this time point (Fig. 5C), indicating that RA is essential during early stages of neuronal differentiation and its function is independent of its role in cell survival.
The above observations reveal that the reduction in neuronal differentiation during retinoid deficiency is independent of the survival deficit. However, the total rate of neurogenesis during RA deficiency was significantly reduced as a result of combined decreased neuronal differentiation and decreased survival, resulting in an overall neurogenesis rate of 18.2% in retinoid deficient and 16.2% in retinoid replenished mice when compared to control levels (Fig. 5D). Because survival impacts the rate of neurogenesis, it is likely that the cells that should be destined for a neuronal fate are selectively lost.
Analysis of Genes Induced by RA Using an in Vitro Neural Progenitor System
RA control of neurogenesis appears to occur through the retinoid receptors, because RAR-specific agonists and VP16-RAR fusion proteins are also highly potent inducers of neuronal differentiation (23). To identify retinoid target genes in adult NSCs, we used isolated Tlx-expressing cells from adult mouse forebrain, which are able to proliferate, self-renew, and differentiate into all neural cell types in vitro (24). We used these isolated Tlx-expressing cells collected from the forebrains of adult mice because they provide homogeneous populations that function as neural progenitor cells in the adult DG. Comparing the RNA content of these cells in the presence and absence of RA on an Affymetrix GeneChip revealed a small number of genes induced during an 8-h exposure to all-trans RA, and even fewer genes that were depressed. Several of these induced genes are known targets of RAR and RXR in other cell types, such as cellular retinol binding protein I (CRBPI; ref. 25), protein kinase C alpha (PKCα; ref. 26), cd36 (27), and ATP-binding cassette transporter A1 (ABCa1; ref. 28).
To determine whether the observed RA-dependent gene inductions were biologically significant retinoid-dependent inductions, alternate populations of adult NSCs from the forebrains of wild-type C57BL/6 mice were analyzed. In addition to incubations with and without RA, we added the RAR antagonist AGN193109 (29) to cells incubated with RA. All incubations were also repeated with the addition of cycloheximide, a protein synthesis inhibitor. Seventy-one percent of the genes highlighted by the Affymetrix analysis showed consistent retinoid regulation in wild-type NSCs incubated with both RA and cycloheximide for 4 or 8 h (Table 1). Of these 15 validated genes, the inductions of 9 were prevented when AGN109 was added along with RA. These genes comprise a group of retinoid targets induced by RA through the retinoid receptors in NSCs and include both cell signaling and metabolic regulators.
Table 1.
A small group of genes are up-regulated by RA according to Affymetrix GeneChip analysis
| Affy (8 h) | QPCR |
Gene name | GenBank accession no. | |
|---|---|---|---|---|
| 4 h | 8 h | |||
| 8.72 | 26 | 56 | CRBPI | NM_011254 |
| 4.6 | 4.8 | 4.1 | ABCa1* | NM_013454 |
| 4.0 | 2.9 | 3.0 | Calcng5 | NM_080644 |
| 3.36 | 4.7 | 3.7 | Ril-pending | NM_019417 |
| 2.42 | 1.5 | 1.4 | SREBP1* | AI326423 |
| 2.04 | 2.2 | 1.6 | CD36 Ag* | AK004192 |
| 2.06 | 2.5 | 2.6 | Wnt7b* | NM_009528 |
| 1.93 | 2.5 | 2.0 | PKCα* | NM_011101 |
| 1.87 | 1.3 | 1.8 | Ptp4a3 | NM_008975 |
| 1.71 | NC | 2.4 | PHR1 (Evectins1) | NM_013746 |
| 1.65 | 1.9 | 3.1 | AGT* | NM_007428 |
| 1.6 | 2.4 | 1.9 | PLCε1* | NM_019588 |
| 1.57 | 1.6 | 1.7 | Kit ligand* | NM_013598 |
| 1.54 | 2.4 | 5.2 | ApoE* | NM_009696 |
| ND | 4.3 | 3.1 | fTG | M55154 |
| −1.62 | −1.5 | −1.3 | Sox9 | BC024958 |
These genes were confirmed to be induced by RA in multiple NSC populations. Some of these genes are known nuclear receptor targets. Affy, Affymetrix GeneChip result; QPCR, quantitative PCR; ND, not determined; NC, no change.
*Genes whose inductions are completely prevented with the use of RAR antagonist.
Discussion
RA application has become a common tool for directing proliferating cultured cells including adult NSCs into a differentiated neuronal fate. The in vitro studies suggest an in vivo function of RA, an implication supported by the specific pattern of retinoid signaling at sites of adult neurogenesis in the mature CNS (12). Furthermore, a function in adult neurogenesis mimics the well established role of retinoids during embryonic neurogenesis. Little is known at present about the requirements that make possible neurogenesis in the adult brain, but our results reveal that RA is an important contributor to early stages of neuronal differentiation and the survival of proliferating or newborn cells in the adult DG. In contrast, RA is unnecessary for cellular proliferation in this region.
Previously, we have shown that both RA and its receptors (RARs) are critical components of adult synaptic plasticity, a correlate of learning and memory, in the hippocampus (11, 18). In contrast, the role of adult neurogenesis in the hippocampus in learning and memory is speculated but not firmly established (30–32). Our discovery of a retinoid requirement during adult neurogenesis suggests that RA may modify memory performance from multiple angles via the promotion of both adult neurogenesis and adult synaptic plasticity. Previously observed changes in memory performance that result from retinoid deficiency (33–34) may therefore result from disruption of multiple aspects of adult hippocampal physiology including both synaptic plasticity and neurogenesis.
Our results reveal that RA contributes significantly to neuronal differentiation, functioning very early during the differentiation process. The immature neuronal marker, dcx, can be identified in BrdUrd-labeled cells 2 days after BrdUrd incorporation (35). In fact, within 2 h of BrdUrd injections, some cells in the DG containing BrdUrd already express dcx (22). Because dcx expression was decreased in the absence of RA, RA must act early during the differentiation process. Importantly, it appears that the decrease of immature and mature neurons during retinoid deficiency is not a secondary effect of the lowered survival rate, because fewer dcx+ cells are present in RAD hippocampi at a time point before survival has taken a significant toll. On the other hand, the loss of survival resulting from retinoid deficiency may result from the inability for these cells to progress normally through neurogenesis. This could explain why RA replenishment, unable to rescue neuronal differentiation, also does not rescue survival.
It is interesting to note that none of the aspects of neurogenesis that we examined were rescued by RA replenishment during the time frame studied. Although the hippocampi were not directly studied to verify retinoid replenishment, mice supplemented with RA overcame signs of RAD, including squamous metaplasia of the tracheal epithelium and deteriorated physical appearances. Others have shown that the hippocampi of vitamin A-deficient rats injected with radiolabeled RA contain significant quantities of RA within 10 h (36). In fact, 2 days of dietary RA supplementation are adequate to recover retinoid-dependent hippocampal functions in RAD mice (11). There are multiple possibilities that could explain the lack of rescued neuronal differentiation with RA supplementation. It may be that the fast dividing cells proliferating in the DG are not the true stem cells but instead are lineage-committed, as has been proposed (37). In this case, the majority of cells labeled by BrdUrd would be lineage-committed cells, and their more restrictive identity may not be reversed after being altered by the absence of RA. Alternatively, the dividing cells may be normally multipotent but the absence of RA may have altered them such that, at least within the time range studied, the multipotency could not be rescued. It is likely that RA regulates neuronal differentiation at multiple timepoints, each requiring reversibility for rescue to occur. It would be interesting to determine whether neurogenesis can be rescued with a longer replenishment period that would affect the slower proliferating cells as well.
In vitro studies of adult neurogenesis indicate that RA promotes the early events of neuronal differentiation, perhaps by priming them with neurotrophin responsiveness, whereas neurotrophins act later to promote neuronal maturation (38). In fact, some studies reveal that RA induces the neurotrophin receptors TrkA in immature chick sympathetic neurons (39) and TrkB during the differentiation of human neuroblastoma cells (40). Although a number of studies have identified genes induced by RA in cultured cell lines including the neurotrophin receptors (15, 38, 41), we chose a comprehensive approach to view retinoid-dependent gene inductions in the adult NSC environment. Our results suggest several attractive pathways for retinoid regulation of neurogenesis, and of these genes, a promising candidate is Wnt7b. The family of Wnt genes has been attributed to controlling early cell fate decisions, cell proliferation and migration, apoptosis, embryonic patterning including the development of the CNS, and the formation of neuronal connections (42–47). Wnt7b specifically functions in early neural patterning (46). RA treatment results in the up- and down-regulation of various Wnts and Wnt receptors, including the up-regulation of Wnt7b in NT2 cells, during the early phases of neuronal differentiation (48). Thus, Wnt7b may be downstream of retinoid signaling in the promotion of adult neurogenesis in the DG.
Other interesting genes include PLCε1, whose expression appears to correlate with the commitment of neural precursors to the neuronal fate (49), and PKCα, which functions in RA-dependent neuronal differentiation in vitro (50). Finally, the prevalence of proteins involved in cholesterol and fatty acid homeostasis, such as ABCa1, SREBP1a, ApoE, and cd36, offers the possibility that RA prepares the cell for changes in energy requirements and cellular morphology associated with neurogenesis by adjusting lipid content.
There is little known at present about what confers stem cell status, but it is likely that the microenvironment plays a major role. Thus, it is important to identify and classify the molecular components associated with stem cell renewal and neurogenesis. Our results, in this and earlier studies, help to decode the requirements of RA for in vivo adult neurogenesis, and imply that it may be possible to manage neuronal differentiation and survival pharmacologically in adults.
Materials and Methods
Induction of Retinoid Deficiency in Mice
RAD and RAS mice were produced as described (50). Briefly, pregnant inbred SENCAR dams at 2 weeks postcoitum were obtained from the National Cancer Institute (Frederick Research Development Center and Animal Production, Frederick, MD) and placed on rodent diet (TD 8604; Harlan Teklad, Madison, WI). At parturition, one group (dam and offspring) was fed a RAD rodent diet (TD 69523; Harlan Teklad) containing no sources or precursors of vitamin A. The other group was fed a RAS rodent diet containing 3 μg of all-trans RA per gram of diet (TD 87373; Harlan Teklad). At 3 weeks of age, pups were weaned onto either diet for the specified time. Daily side observations were performed.
BrdUrd Injections and Perfusions
Animals received i.p. injections of BrdUrd (50 mg/kg; Sigma) once for 6 consecutive days beginning at 103 days of age. RA-rescued mice were returned to a RAS diet at the time of initial BrdUrd injections.
Mice were anesthetized with ketamine or avertin the day after BrdUrd injections ceased or 3 weeks after. Mice were perfused transcardially with 4% paraformaldehyde in PBS, pH 7.4. Brains were removed, fixed overnight in the same solution, and transferred to 30% sucrose/PBS. Brains were cut coronally into 40-μm slices and stored at −20°C in a cryoprotectant solution.
Verification of Retinoid Deficiency in Mice
Mice were anesthetized as described and perfused with 4% paraformaldehyde at ages of 15.5 and 18.5 weeks. Trachea were transferred to 10% formalin at 4°C. Tissues were sent to American HistoLab (Gaithersburg, MD) for paraffin embedding and horizontal sectioning (5 μm). Sections were examined for signs of squamous metaplasia.
Diamidobenzidine/Hoescht Staining
To determine the number of BrdUrd-labeled cells, we stained for BrdUrd by using the peroxidase method (ABC system, with biotinylated donkey anti-mouse IgG antibodies and diaminobenzidine as chromogen; Vector Laboratories).
Antibody Staining
Free-floating 40-μm coronal sections were rinsed with Tris-buffered saline (TBS) and blocked for 30 min at room temperature in TBS containing 0.3% Triton X-100 and 5% preimmune donkey serum (TBS++). Samples were incubated in TBS++ containing primary antibodies for 24–72 h at 4°C. Samples were washed three times with TBS for 10 min at room temperature and blocked in TBS++ for 1 h. Samples were then incubated for 2 h with secondary antibodies. Samples were washed three times with TBS, treated with 10 mg/ml DAPI (Sigma) for 10 min, and coverslipped in 20% poly(vinyl alcohol) (20,000–30,000 molecular weight; Air Products and Chemicals, Allentown, PA) in 50% glycerol (wt/vol) containing 2.5% wt/vol 1,4-diazobicyclo-[2.2.2]-octane (Sigma).
For BrdUrd staining, samples were pretreated with 50% formamide in 2× SSC for 2 h at 65°C, followed by 15 min in 2× SSC, 30 min in 2 M HCl at 37°C, 10 min in 0.1 M borate buffer, and six 15-min rinses in TBS, pH 7.5.
Primary antibodies were used at the following concentrations in TBS++: mouse anti-NeuN (1:20; hybridoma supernatant kindly provided by R. Mullen, University of Utah, Salt Lake City), rabbit anti-S100β (1:2,500; Swant, Bellinzona, Switzerland), rat anti-BrdUrd (1:250; Accurate Chemicals, Westbury, NY), goat anti-doublecortin (1:200, Santa Cruz Biotechnology), rabbit anti-calbindin (1:500; Swant), mouse anti-PCNA (1:500; Santa Cruz Biotechnology), and rabbit anti-cleaved Caspase-3 (1:500; Cell Signaling Technology, Beverly, MA). Fluorescent samples were evaluated by using a Bio-Rad MRC1024UV confocal imaging system. Secondary antibodies (donkey; Jackson ImmunoResearch), conjugated to fluorescein isothiocyanate (FITC), cyanin 3 (Cy3), or cyanin 5 (Cy5), were used at a final dilution of 1:250 in TBS++.
Confocal Analysis
Fifty randomly selected, BrdUrd-positive cells per animal were evaluated for colabeling with each phenotypic marker. The complete cell nucleus was analyzed throughout the z axis, and only cells with a well circumscribed, immunopositive cell body or nucleus were considered positive for colabeling.
The labeling index was calculated by dividing the number of cells that were double labeled for BrdUrd and a phenotypic marker by the number of total evaluated BrdUrd-positive cells per animal. The Student’s t test was applied to evaluate significance.
Stereology
BrdUrd-positive cells were counted in a one-in-six series of sections (240 μm apart) through a ×40 objective (Leitz) throughout the rostrocaudal extent of the GCL to measure GCL volume. A one-in-six series of the same or adjacent sections stained with 0.5 mg/ml Hoechst dye 33342 in TBS (Molecular Probes) or DAPI for 15 min was analyzed with a semiautomatic stereology system (StereoInvestigator; MicroBrightfield) and a ×10 objective.
Culture of Neural Stem Cells
Neural stem cells were cultured as described (24). Tlx+/− cells were collected by β-gal-based sorting using fluorescein digalactoside as substrate (Molecular Probes). Cells were grown in N2 supplement (GIBCO/BRL, Gaithersburg, MD), EGF (20 ng/ml), FGF2 (20 ng/ml), and heparin (5 μg/ml). For the Affymetrix analysis, proliferating Tlx cells were cultured for 8 h in fresh N2 media with or without 1 μM all-trans RA. For quantitative PCR validation, wild-type proliferating NSCs were incubated in N2 media alone for 24 h. The N2 media was then supplemented with 1 μM RA, 1 μM RA and 1 μM AGN193109 (Allergan, Irvine, CA), or nothing at all. Cycloheximide was used at 10 μg/ml.
RNA Extraction for PCR and Affymetrix
Cells were placed in TRIzol solution (Invitrogen) and left at room temperature for a few minutes before chloroform was added. The mixture was then vigorously shaken briefly and spun to separate aqueous from organic layer. The aqueous layer was then removed and added to isopropanol to precipitate the RNA which was later washed with 75% ethanol, air-dried, and resuspended in DEPC water. RNA quality was evaluated on a Mops/agarose gel.
Affymetrix Analysis
Five micrograms of RNA was applied to mouse expression GeneChip 430A (Affymetrix) as prescribed. Data analysis was performed by using bullfrog software (51). Criteria used to detect differences in gene expression were 3/4 comparisons with a fold change of 1.5 or greater; a difference call of increase, marginal increase, decrease, or marginal decrease; and a signal change >50.
Real-Time PCR Validation
RNA was converted to cDNA by using SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen). Standard curve DNA was produced by combining cDNA from different samples to be studied as well as adding some mouse whole-brain cDNA. Serial dilutions for the standard curve ranged from 0.08 to 5 ng/μl. cDNA was used at ≈2 ng/μl. Primers were stocked at 1 μM. Final volumes of PCRs were 10 μl. Sybr Green PCRs were performed at 40 cycles by the ABI Prism 7700 Sequence Detection System using 96- or 384-well plates, and results were analyzed by using sds 2.0 software and Microsoft excel.
Acknowledgments
We thank Elaine Stevens and members of R.M.E.’s laboratory for helping to read and prepare the manuscript. R.M.E. is an Investigator of the Howard Hughes Medical Institute at the Salk Institute for Biological Studies and March of Dimes Chair in Molecular and Developmental Biology. This work was supported by National Institutes of Health Grant HD27183.
Abbreviations
- RA
retinoic acid
- RAR
RA receptor
- RXR
retinoid X receptor
- DG
dentate gyrus
- GCL
granular cell layer
- dcx
doublecortin
- NSC
neural stem cell
- RAS
retinoid sufficient
- RAD
retinoid deficient
- PCNA
proliferating cell nuclear antigen
- TBS
Tris-buffered saline
- NeuN
neuronal nuclear antigen
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Maden M. Nat. Rev. Neurosci. 2002;3:843–853. doi: 10.1038/nrn963. [DOI] [PubMed] [Google Scholar]
- 2.Durston A. J., Timmermans J. P., Hage W. J., Hendriks H. F., de Vries N. J., Heideveld M., Nieuwkoop P. D. Nature. 1989;340:140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- 3.Pierani A., Brenner-Morton S., Chiang C., Jessell T. M. Cell. 1999;97:903–915. doi: 10.1016/s0092-8674(00)80802-8. [DOI] [PubMed] [Google Scholar]
- 4.Mangelsdorf D. J., Thummel C., Beato M., Herrlich P., Shutz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., Evans R. M. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beato M., Herrlich P., Schutz G. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 6.Chambon P. Recent Prog. Horm. Res. 1995;50:317–332. doi: 10.1016/b978-0-12-571150-0.50019-6. [DOI] [PubMed] [Google Scholar]
- 7.Giguere V., Ong E. S., Segui P., Evans R. M. Nature. 1987;330:624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- 8.Petkovich M., Brand N. J., Krust A., Chambon P. Nature. 1987;330:444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- 9.Mangelsdorf D. J., Borgmeyer U., Heyman R. A., Zhou J. Y., Ong E. S., Oro A. E., Kaakizuka A., Evans R. M. Genes Dev. 1992;6:329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- 10.de Urquiza A. M., Liu S., Sjöberg M., Zetterström R. H., Griffiths W., Sjövall J., Perlmann T. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 11.Misner D. L., Jacobs S., Shimizu Y., de Urquiza A. M., Solomin L., Perlmann T., De Luca L. M., Stevens C. F., Evans R. M. Proc. Natl. Acad. Sci. USA. 2001;98:11714–11719. doi: 10.1073/pnas.191369798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corcoran J., Maden M. Nat. Neurosci. 1999;2:307–308. doi: 10.1038/7214. [DOI] [PubMed] [Google Scholar]
- 13.Wagner E., Luo T., Drager U. C. Cereb. Cortex. 2002;12:1244–1253. doi: 10.1093/cercor/12.12.1244. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan M. S., Hinds J. W. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan M. S., Bell D. H. Exp. Brain Res. 1983;52:1–5. doi: 10.1007/BF00237141. [DOI] [PubMed] [Google Scholar]
- 16.Cameron H. A., Woolley C. S., McEwen B. S., Gould E. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn H. G., Dickinson-Anson H., Gage F. H. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang M. Y., Misner D., Kempermann G., Schikorski T., Giguere V., Sucov H. M., Gage F. H., Stevens C. F., Evans R. M. Neuron. 1998;21:1353–1361. doi: 10.1016/s0896-6273(00)80654-6. [DOI] [PubMed] [Google Scholar]
- 19.Sockanathan S., Jessell T. M. Cell. 1998;94:503–514. doi: 10.1016/s0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- 20.Gage F. H. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 21.Kempermann G., Gage F. H. Brain Res. Dev. Brain Res. 2002;134:1–12. doi: 10.1016/s0165-3806(01)00224-3. [DOI] [PubMed] [Google Scholar]
- 22.Kempermann G., Gast D., Kronenberg G., Yamaguchi M., Gage F. H. Development (Cambridge, U.K.) 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 23.Lipkin S. M., Grider T. L., Heyman R. A., Glass C. K., Gage F. H. J. Virol. 1996;70:7182–7189. doi: 10.1128/jvi.70.10.7182-7189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y., Lie D. C., Taupin P., Nakashima K., Ray J., Yu R. T., Gage F. H., Evans R. M. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 25.Smith W. C., Nakshatri H., Leroy P., Rees J., Chambon P. EMBO J. 1991;10:2223–2230. doi: 10.1002/j.1460-2075.1991.tb07758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai D. S., Hirai S., Karnes W. E., Niles R. M., Ohno S. Biochem. Biophys. Res. Commun. 1999;263:28–34. doi: 10.1006/bbrc.1999.1307. [DOI] [PubMed] [Google Scholar]
- 27.Wuttge D. M., Romert A., Eriksson U., Torma H., Hansson G. K., Sirsjo A. FASEB J. 2001;15:1221–1223. doi: 10.1096/fj.00-0488fje. [DOI] [PubMed] [Google Scholar]
- 28.Costet P., Lalanne F., Gerbod-Giannone M. C., Molina J. R., Fu X., Lund E. G., Gudas L. J., Tall A. R. Mol. Cell. Biol. 2003;23:7756–7766. doi: 10.1128/MCB.23.21.7756-7766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal C., Chandraratna R. A., Johnson A. T., Rorke E. A., Eckert R. L. J. Biol. Chem. 1996;271:12209–12212. doi: 10.1074/jbc.271.21.12209. [DOI] [PubMed] [Google Scholar]
- 30.Shors T. J., Miesegaes G., Beylin A., Zhao M., Rydel T., Gould E. Nature. 2001;410:372–375. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 31.Kempermann G. J. Neurosci. 2002;22:635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prickaerts J., Koopmans G., Blokland A., Scheepens A. Neurobiol. Learn. Mem. 2004;81:1–11. doi: 10.1016/j.nlm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Cocco S., Diaz G., Stancampiano R., Diana A., Carta M., Curreli R., Sarais L., Fadda F. Neuroscience. 2002;115:475–482. doi: 10.1016/s0306-4522(02)00423-2. [DOI] [PubMed] [Google Scholar]
- 34.Etchamendy N., Enderlin V., Marighetto A., Pallet V., Higueret P., Jaffard R. Behav. Brain Res. 2003;145:37–49. doi: 10.1016/s0166-4328(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 35.Cooper-Kuhn C. M., Kuhn H. G. Dev. Brain Res. 2002;134:13–21. doi: 10.1016/s0165-3806(01)00243-7. [DOI] [PubMed] [Google Scholar]
- 36.Werner E. A., DeLuca H. F. Am. J. Physiol. 2002;282:E672–E678. doi: 10.1152/ajpendo.00280.2001. [DOI] [PubMed] [Google Scholar]
- 37.Gage F. H., Kempermann G., Palmer T. D., Peterson D. A., Ray J. J. Neurobiol. 1998;362:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi J., Palmer T. D., Gage F. H. J. Neurobiol. 1999;38:65–81. [PubMed] [Google Scholar]
- 39.von Holst A., Rodriguez-Tebar A., Michaille J. J., Dhouailly P., Backstrom A., Ebendal T., Rohrer H. Mol. Cell. Neurosci. 1995;6:185–198. doi: 10.1006/mcne.1995.1016. [DOI] [PubMed] [Google Scholar]
- 40.Lucarelli E., Kaplan D., Matsumoto K., Sickafuse S., Thiele C. J. Prog. Clin. Biol. Res. 1994;385:185–198. [PubMed] [Google Scholar]
- 41.Kobayashi M., Kurihara K., Matsuoka I. FEBS Lett. 1994;356:60–65. doi: 10.1016/0014-5793(94)01238-5. [DOI] [PubMed] [Google Scholar]
- 42.Siegfried E., Chou T. B., Perrimon N. Cell. 1992;71:1167–1179. doi: 10.1016/s0092-8674(05)80065-0. [DOI] [PubMed] [Google Scholar]
- 43.Lucas F. R., Salinas P. C. Dev. Biol. 1997;192:31–44. doi: 10.1006/dbio.1997.8734. [DOI] [PubMed] [Google Scholar]
- 44.Thomas K. R., Capecchi M. R. Nature. 1990;346:847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- 45.McMahon A. P., Bradley A. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- 46.Chang C., Hemmati-Brivanlou A. Dev. Biol. 1998;194:129–134. doi: 10.1006/dbio.1997.8820. [DOI] [PubMed] [Google Scholar]
- 47.Lie D. C., Colamarino S. A., Song H. J., Desire L., Mira H., Consiglio A., Lein E. S., Jessberger S., Lansford H., Dearie A. R., Gage F. H. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 48.Katoh M. Int. J. Mol. Med. 2002;10:683–687. [PubMed] [Google Scholar]
- 49.Wu D., Tadano M., Edamatsu H., Masago-Toda M., Yamawaki-Kataoka Y., Terashima T., Mizoguchi A., Minami Y., Satoh T., Kataoka T. Eur. J. Neurosci. 2003;17:1571–1580. doi: 10.1046/j.1460-9568.2003.02591.x. [DOI] [PubMed] [Google Scholar]
- 50.Ponnamperuma R. M., Kirchhof S. M., Trifiletti L., De Luca L. M. Am. J. Clin. Nutr. 1999;70:502–508. doi: 10.1093/ajcn/70.4.502. [DOI] [PubMed] [Google Scholar]
- 51.Zapala M. A., Lockhart D. J., Pankratz D. G., Garcia A. J., Barlow C., Lockhart D. J. Genome Biol. 2002;3:1–9. doi: 10.1186/gb-2002-3-6-software0001. [DOI] [PMC free article] [PubMed] [Google Scholar]