Abstract
The phylogenetic position of tarsiers relative to anthropoids and Paleogene omomyids remains a subject of lively debate that lies at the center of research into anthropoid origins. Omomyids have long been regarded as the nearest relatives of tarsiers, but a sister group relationship between anthropoids and tarsiers has also been proposed. These conflicting phylogenetic reconstructions rely heavily on comparisons of cranial anatomy, but until now, the fossil record of tarsiers has been limited to a single jaw and several isolated teeth. In this article, we describe cranial material of a fossil tarsiid from the middle-Eocene Shanghuang fissure-fillings in southern Jiangsu Province, China. This facial fragment, which is allocated to Tarsius eocaenus, is virtually identical to the corresponding anatomy in living tarsiers and differs substantially from that of early anthropoids such as Bahinia, Phenacopithecus, and Parapithecus. This new specimen indicates that tarsiers already possessed greatly enlarged orbits and a haplorhine oronasal configuration by the time they are first documented in the fossil record during the middle Eocene.
Keywords: China, primate, tarsiid
Lack of consensus regarding the interrelationships among anthropoids, tarsiids, and omomyids is largely due to contradictory similarities among the three groups in cranial anatomy. Tarsiers and anthropoids exclusively share some degree of postorbital closure, an anterior accessory chamber of the middle ear, and loss of the stapedial artery (1–5), whereas omomyids and tarsiers share other features not seen in anthropoids, such as a narrow central stem of the basioccipital, a “peaked” choanal region, and basioccipital flanges that overlap the auditory bulla (6–11). The homology of these features is unclear because few cranial specimens are known for early members of these groups, and none are known for fossil tarsiers (12, 13). The fossil record of tarsiers is currently restricted to three or four species. Tarsius thailandicus, a middle Miocene species from northwestern Thailand, is documented by several isolated teeth and a single lower jaw fragment (14). Tarsius eocaenus, a diminutive species from the middle-Eocene Shanghuang fissure-fillings of Jiangsu Province, China, had been represented by five isolated cheek teeth (15). Xanthorhysis tabrumi from the middle Eocene of the Yuanqu Basin, Shanxi Province, China, is represented by a single lower jaw fragment (12). Afrotarsius chatrathi from the Oligocene Fayum of Egypt is sometimes cited as a fourth potential tarsiid (16, 17), but its tarsiid affinities are disputed (12, 18, 19). The specimen described here illuminates the facial anatomy of fossil tarsiids, and it provides information on the sensory specializations of Eocene tarsiids, which reinforce the antiquity of the distinctive ecological niche of living tarsiers.
Description and Comparisons
Institute of Vertebrate Paleontology and Paleoanthropology (IVPP) V14563 is a left premaxillary-maxillary fragment containing the crown of P3 and complete or partial alveoli for I2, C1, P2, and the mesial roots of P4 (Figs. 1–3). The specimen derives from Shanghuang fissure D, one of several fossiliferous middle-Eocene fissure-fillings located near the village of Shanghuang in southern Jiangsu Province, China. Biostratigraphically, the mammalian faunas (15, –22) suggest that fissure D is significantly older than Shanghuang fissures A and C, from which all described Shanghuang tarsiid dental specimens have been recovered (15). Nevertheless, on the basis of similarities in size and morphology between our new specimen and IVPP V11029, an isolated P3 from fissure C that forms part of the hypodigm of T. eocaenus, we tentatively allocate IVPP V14563 to the latter species here.
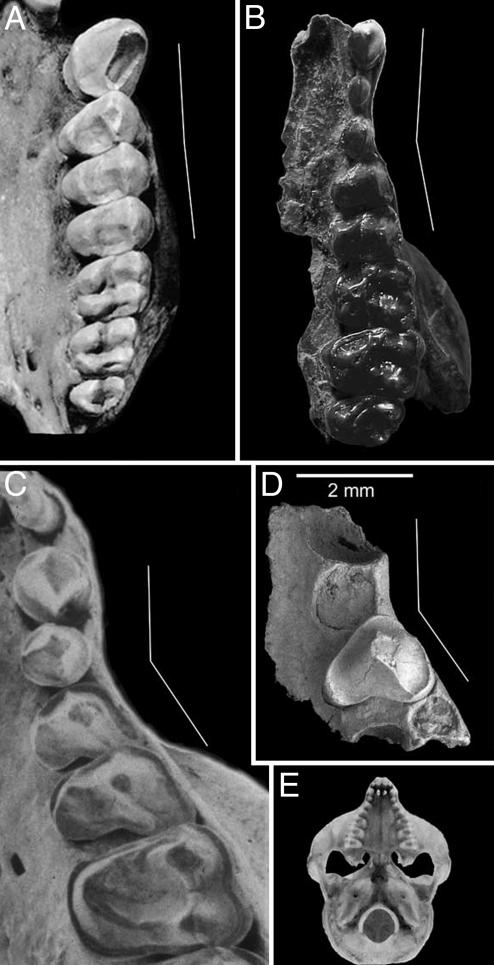
Fig. 1.
Upper left dentitions of selected living and fossil primates illustrating the morphological diversity in palatal shape that occurs within this group. Taxa depicted are as follows: the anthropoid Cebus (A), the strepsirrhine Notharctus (B), a modern tarsier Tarsius sp. (C), and T. eocaenus (D). Occlusal view of modern Tarsius sp. (E) shows a bell-shaped dental arcade characteristic of this taxon. The white lines in A–D illustrate the angle of divergence between the anterior and posterior parts of the dental arcade, with the root of P2 arbitrarily defining these segments. Specimens are not depicted at the same magnifications; the scale bar refers only to the IVPP V14563 maxillary fragment of T. eocaenus. Images C and E are modified from ref. 23.
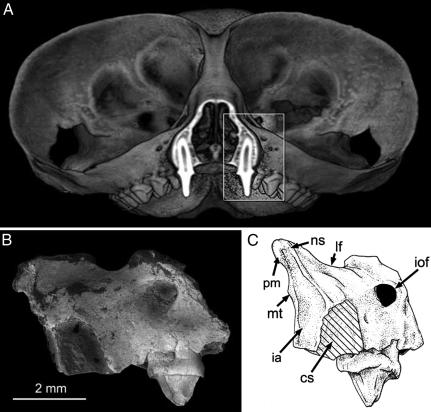
Fig. 2.
Modern Tarsius sp. (A) compared with T. eocaenus in a lateral (B) and anterior (C) view. The 3D computed tomography reconstruction of modern tarsier in A is cut in the coronal plane of the canine to facilitate comparison with the fossil in anterior view (C). cs, canine alveolus; ia, incisor alveolus; iof, infraorbital foramen; lf, lacrimal foramen; mt, maxilloturbinal base; ns, nasomaxillary suture; pm, premaxillary-maxillary suture.
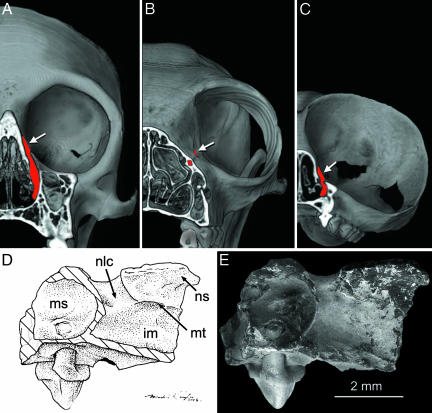
Fig. 3.
Internal anatomy of T. eocaenus. 3D computed tomography reconstructions of Cebus (A), Eulemur (B), and modern Tarsius sp. (C) compared with line art (D) and an SEM image (E) of T. eocaenus in medial view. The computed tomography reconstructions of modern skulls are cut in a coronal plane to show the course of the nasolacrimal ducts (filled in red). White arrows indicate their orbital opening. Note that in Eulemur the orbital opening is anterior to the orbit, and the duct travels horizontally, instead of vertically, so that the nasal opening is located much farther anterior than the plane of dissection. im, inferior meatus; ms, maxillary sinus; n/c, nasolacrimal duct.
The number and relative sizes of the alveoli preserved in IVPP V14563 correspond precisely with those found in modern species of Tarsius. The most mesial alveolus preserved in IVPP V14563, which can only be viewed from an anterior perspective (Fig. 2C), is interpreted as the distal part of the alveolus for I2. Its close proximity to the much larger canine alveolus suggests that there would not have been any appreciable diastema between I2 and C1 in this specimen. In this respect, IVPP V14563 resembles extant Tarsius bancanus more closely than it does other living species of Tarsius (see figure 11 in ref. 23). The canine alveolus, although only partially preserved, is clearly larger than that for P2 (Table 1). Dorsally, the large size of the upper canine root is reflected on the external topography of the maxilla, where a distinct canine jugum occurs rostral and inferior to the lacrimal foramen. The relatively small, single alveolus for P2 lies directly distal to the canine alveolus in IVPP V14563, as it does in living species of Tarsius. In contrast, the roots and alveoli for P3–4 are displaced distolaterally (Fig. 1), as in primates with a “bell-shaped” dental arcade (e.g., Tarsius and Pseudoloris). Such morphology, which is very rare in primates, is probably related to orbital hypertrophy because the lateral displacement of the distal part of the tooth row places the alveolar process of the maxilla beneath the laterally expanded orbits (24). In contrast, the postcanine tooth row is relatively straight in living and fossil anthropoids, including the most primitive genera for which the condition is observable [Bahinia and Parapithecus (25, 26)].
Table 1.
Measurements of IVPP V14563
| Mesiodistal length, mm | Buccolingual breadth, mm | |
|---|---|---|
| C1 alveolus | >1.08 | >1.15 |
| P2 alveolus | 0.88 | 0.88 |
| P3 crown | 1.39 | 1.63 |
| IOF height = 0.54 mm | ||
| IOF breadth = 0.58 mm | ||
| IOF area = 0.31 mm2 |
The crown of P3 is roughly triangular in occlusal outline, being dominated by a single buccal cusp, which can be identified as the paracone. A moderately developed preparacrista descends the mesial face of the paracone, becoming confluent with the cingulum near the mesiobuccal margin of the tooth. The postparacrista is more trenchant, connecting the apex of the paracone with the cingulum that lines the distobuccal corner of the tooth. The mesiolingual and distolingual faces of the paracone are relatively flat, and they meet at an acute angle to form a rounded, but generally crest-like, hypoparacrista that runs down the lingual side of the paracone. This structure is more trenchant and crestiform in modern species of Tarsius and in the previously described specimen of T. eocaenus (IVPP V11029). The reduced lingual lobe of the tooth, like the rest of the crown, is completely encircled by a cingulum. The combination of the concave distal margin, the absence of a protocone, and the extreme reduction of the lingual lobe are found only in Tarsius and allow us to refer this specimen to this genus on the basis of dental anatomy alone.
A tiny portion of the inferior orbital rim is preserved above the level of P2, but the rim is broken away dorsal and lateral to the infraorbital foramen (Fig. 2). This foramen is relatively small, like those of extant haplorhines and unlike those of other primates including omomyids (27, 28). It is not yet clear exactly how the size of the infraorbital foramen (IOF) varies systematically among primates of such diminutive body size, but the omomyid Teilhardina asiatica has an inferred body mass (28 g) very similar to that of T. eocaenus (29 g; see ref. 29), whereas its IOF area (0.83 mm2) is nearly three times as large as that of the present specimen (0.31 mm2). The IOF transmits vasculature and nerves to the rhinarium and vibrissae, and its uniquely reduced size in living haplorhines apparently reflects the absence of a naked rhinarium and mystacial vibrissae in these taxa (27). If so, the relatively large IOF in T. asiatica (and probably Necrolemur; see ref. 27) supports the view that most, if not all, Eocene omomyids lacked this derived nasal morphology (27, 30, 31), and the relatively small IOF in T. eocaenus may indicate that this early tarsier was already anatomically haplorhine.
Immediately rostral to the orbit is the inferior margin of a large lacrimal foramen. The lacrimal foramen lies outside the orbit in many nonanthropoid primates, but the relatively large size of this foramen and its close proximity to the orbital rim in IVPP V14563 is most similar to that of tarsiers among living primates (23). Among fossil haplorhines, the lacrimal foramen is small and farther from the orbital rim in Necrolemur and Rooneyia (8, 32), but it appears to be larger in Pseudoloris (33). In Shoshonius, it lies outside the orbit, but details of its morphology are obscured by crushing. Rostral to the lacrimal foramen in IVPP V14563 the maxilla forms a short peaked rostrum that bulges slightly under the influence of the canine root, as in Tarsius (Fig. 2). The dorsal half of the rostrum slopes medially toward the midline to the point of the nasomaxillary suture, producing a very restricted snout like that pinched between the orbits in Tarsius. Overall, the maxilla is very shallow dorsoventrally, particularly beneath the orbit. This condition contrasts markedly with the deeper maxillae of early anthropoids such as Phenacopithecus (34), Bahinia (17), and Parapithecus (26).
Inside the nasal cavity, the nasolacrimal foramen opens immediately into the nasal cavity (Fig. 3). As in living haplorhines, the course of the lacrimal duct is vertical, opening beneath the maxilloturbinal, the basal lamella of which runs horizontally rostral to the canal. As in living tarsiers, the canal is short (Fig. 3B), and its posterior wall is shared with a small maxillary sinus that lies above P3 and P4. The course of the duct in anthropoids is also vertical (Fig. 3A) but longer because of their deeper facial skeletons. As a result, this structure in anthropoids often has an independent posterior wall that extends ventral to the anterior wall of the maxillary sinus. Furthermore, in most anthropoids, the maxillary sinus extends lateral to the canal (Fig. 3A), whereas in tarsiers and IVPP V14563, it is restricted to the area posterior to the canal. In contrast, in living strepsirrhines, the nasolacrimal duct travels a considerable distance rostroventrally through a bony canal (Fig. 3D), usually within the base of the maxilloturbinal, to open below the rostral end of the maxilloturbinal or atrioturbinal into the nasal vestibule (35). The morphology of the nasolacrimal duct and surrounding structures in IVPP V14563 is found only in tarsiers among living primates. The nasolacrimal region of omomyids remains largely unknown, although Shoshonius cooperi (Carnegie Museum of Natural History no. 31366) exhibits the tarsier-like pattern of having an extra-orbital lacrimal foramen with a vertical drop into the nasal cavity below the maxilloturbinal.
Conclusion
Cranial characters have been cited as evidence for either a close relationship between tarsiers and omomyids or a sister-group relationship between tarsiers and anthropoids. Both of these hypotheses have suffered from the absence of fossils documenting the cranial anatomy of early Cenozoic tarsiers. IVPP V14563 is the first such evidence to come to light. Unfortunately, the Shanghuang facial fragment does not bear directly on the most controversial crossing synapomorphies among tarsiers, anthropoids, and omomyids because these characters are found in the basicranial, auditory, and circumorbital regions. However, the very small infraorbital foramen in T. eocaenus may represent a derived feature shared with living haplorhines to the exclusion of omomyids. This possibility is intriguing, but the relative size of the foramen has only been documented for two omomyids (Necrolemur and Teilhardina) (27, 28) because Rooneyia is no longer regarded as an omomyid (9, 17). Regardless, the small infraorbital foramen probably indicates the presence of an anatomically haplorhine oronasal condition in this fossil tarsiid. Beyond this, the bell-shaped dental arcade, short and peaked rostrum, short and vertical lacrimal canal, and shallow infraorbital region indicate that much of the distinctive suite of facial morphology found in Tarsius was already present in this 45-million-year-old species.
The unique craniofacial morphology of living tarsiers is generally considered to be related to their hypertrophied eyeballs, each of which exceeds the tarsier’s brain in volume (4, 24, 36). It has been argued that the enormous eyes of tarsiers evolved as part of a shift to nocturnality from a diurnal ancestor that lacked the reflective tapetum lucidum of the retina (4, 37). If this were the case, the last common ancestor (LCA) of tarsiers and anthropoids would have been more monkey-like than tarsier-like in its cranial anatomy (4). In the absence of cranial material for fossil tarsiids, it has not been possible to determine when the transition from a diurnal to a nocturnal activity pattern occurred along the tarsiid stem lineage. The new material of T. eocaenus indicates that a virtually modern tarsier-like facial morphology (and presumably the associated nocturnal habits) was present at least 45 million years ago.
The preceding scenario assumes that the LCA of tarsiers and anthropoids, as well as the earliest anthropoids, were diurnal. Most workers agree that the most basal anthropoids currently known are members of the middle-Eocene Eosimiidae (15, 17, 25, 29, 34, 38). All eosimiids for which the relevant anatomy is known (Phenacopithecus and Bahinia) appear to have retained relatively small orbits, suggesting a diurnal activity pattern (17, 34, 39). In contrast, the recently described stem anthropoid Biretia megalopsis from Egypt apparently possessed orbits nearly as enlarged as those of Tarsius (38). Not only does this provide evidence of nocturnality in a stem anthropoid, but also the degree of orbital hypertrophy strongly suggests, as in the case of Tarsius, that Biretia had lost its reflecting tapetum lucidum. Biretia is argued to be an early member of the phylogenetically more advanced parapithecoid clade (38), a group that also includes seemingly diurnal taxa such as Parapithecus (40). Accordingly, rather than suggesting that anthropoids and tarsiers shared a nocturnal LCA, these findings support the previous inference that the two groups shared a diurnal ancestor that lacked a tapetum lucidum (4, 37). The disparity in facial morphology between T. eocaenus and basal anthropoids like eosimiids implies that their phylogenetic divergence occurred well before the middle Eocene.
Alternatively, if tarsiids are more closely related to omomyids than they are to anthropoids (6–11), then their hypertrophied eyes and associated facial morphology are simply an exaggerated state of a trend toward enlarged orbits that is already evident in omomyids such as Shoshonius (6, 9). The new material of T. eocaenus described in this article indicates that middle-Eocene tarsiers were at least as derived in this respect as the slightly older Shoshonius and far more derived than basal omomyids such as T. asiatica (28). One plausible phylogenetic interpretation of this pattern is that tarsiids are nested within Omomyidae rather than being a sister group of that clade (7, 10). If the relatively small orbits of T. asiatica indicate diurnality in this presumably basal haplorhine (28), then the inference of a diurnal LCA for living tarsiers and anthropoids is once again supported. Additional specimens of early Cenozoic tarsiids will be required to clarify their phylogenetic relationships with other living and extinct primate clades. Regardless of these lingering phylogenetic uncertainties, the specimen described in this article, along with recently reported material of B. megalopsis, suggests that different groups of crown haplorhines independently adopted nocturnal lifestyles during the Eocene.
Acknowledgments
We thank Erik Seiffert and two anonymous reviewers for constructive comments; Mark Klingler for illustrations; and Qi Tao, Wang Banyue, Li Chuankui, Wang Yuanqing, and Guo Jianwei for their assistance and advice. Funding has been provided by National Science Foundation Grants BCS-0100825 (to J.B.R.) and BCS-0309800 (to K.C.B.) and by the National Science Foundation of China (X.N.).
Abbreviations
- IVPP
Institute of Vertebrate Paleontology and Paleoanthropology
- IOF
infraorbital foramen
- LCA
last common ancestor.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Cartmill M., Kay R. F. In: Recent Advances in Primatology. Chivers D. J., Joysey K. A., editors. Vol. 3. London: Academic; 1978. pp. 205–214. [Google Scholar]
- 2.MacPhee R. D. E., Cartmill M. In: Comparative Primate Biology: Systematics, Evolution and Anatomy. Swindler D. R., Erwin J., editors. Vol. 1. New York: Alan R. Liss; 1986. pp. 219–275. [Google Scholar]
- 3.Ross C. F. In: Anthropoid Origins. Fleagle J. G., Kay R. F., editors. New York: Plenum; 1994. pp. 469–547. [Google Scholar]
- 4.Cartmill M. In: Evolutionary Biology of the New World Monkeys and Continental Drift. Ciochon R. L., Chiarelli A. B., editors. New York: Plenum; 1980. pp. 243–274. [Google Scholar]
- 5.Cartmill M., MacPhee R. D. E., Simons E. L. Am. J. Phys. Anthrop. 1981;56:3–21. [Google Scholar]
- 6.Beard K. C., Krishtalka L., Stucky R. K. Nature. 1991;349:64–67. doi: 10.1038/349064a0. [DOI] [PubMed] [Google Scholar]
- 7.Gingerich P. D. J. Hum. Evol. 1981;10:345–374. [Google Scholar]
- 8.Simons E. L., Russell D. E. Breviora. 1960;127:1–14. [Google Scholar]
- 9.Beard K. C., MacPhee R. D. E. In: Anthropoid Origins. Fleagle J. G., Kay R. F., editors. New York: Plenum; 1994. pp. 55–97. [Google Scholar]
- 10.Rosenberger A. Folia Primatol. 1985;45:179–194. [Google Scholar]
- 11.Szalay F. S. Bull. Am. Mus. Nat. Hist. 1976;156:157–450. [Google Scholar]
- 12.Beard K. C. Bull. Carnegie Mus. Nat. Hist. 1998;34:260–277. [Google Scholar]
- 13.Fleagle J. G. Primate Adaptation and Evolution. San Diego: Academic; 1999. [Google Scholar]
- 14.Mein P., Ginsburg L. Geodiversitas. 1997;19:783–844. [Google Scholar]
- 15.Beard K. C., Qi T., Dawson M. R., Wang B., Li C. Nature. 1994;368:604–609. doi: 10.1038/368604a0. [DOI] [PubMed] [Google Scholar]
- 16.Simons E. L., Bown T. M. Nature. 1985;313:475–477. [Google Scholar]
- 17.Kay R. F., Williams B. A., Ross C. F., Takai M., Shigehera N. In: Anthropoid Origins: New Visions. Ross C. F., Kay R. F., editors. New York: Kluwer/Plenum; 2004. pp. 91–135. [Google Scholar]
- 18.Fleagle J. G., Kay R. F. J. Hum. Evol. 1987;16:483–532. [Google Scholar]
- 19.White J., Gebo D. L. Am. J. Primatol. 2004;64:293–308. doi: 10.1002/ajp.20079. [DOI] [PubMed] [Google Scholar]
- 20.Wang B., Dawson M. R. Ann. Carnegie Mus. 1994;63:239–256. [Google Scholar]
- 21.Dawson M. R., Huang X., Li C., Wang B. Vert. PalAsiatica. 2003;41:249–270. [Google Scholar]
- 22.Qi T., Zong G., Wang Y. Vert. PalAsiatica. 1991;29:59–63. [Google Scholar]
- 23.Musser G. G., Dagosto M. Am. Mus. Novitates. 1987;2867:1–53. [Google Scholar]
- 24.Cartmill M. Doctoral thesis. Chicago: Univ. of Chicago; 1970. [Google Scholar]
- 25.Jaeger J. J., Thein T., Benammi M., Chaimanee Y., Soe A. N., Lwin T., Tun T., Wai S., Ducrocq S. Science. 1999;286:528–530. doi: 10.1126/science.286.5439.528. [DOI] [PubMed] [Google Scholar]
- 26.Simons E. L. Proc. Natl. Acad. Sci. USA. 2001;98:7892–7897. doi: 10.1073/pnas.051003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kay R. F., Cartmill M. J. Hum. Evol. 1977;6:19–53. [Google Scholar]
- 28.Ni X., Wang Y., Hu Y., Li C. Nature. 2004;427:65–68. doi: 10.1038/nature02126. [DOI] [PubMed] [Google Scholar]
- 29.Gebo D. L., Dagosto M., Beard K. C., Qi T. J. Hum. Evol. 2000;38:585–594. doi: 10.1006/jhev.2000.0395. [DOI] [PubMed] [Google Scholar]
- 30.Beard K. C. Int. J. Primatol. 1988;9:83–96. [Google Scholar]
- 31.Martin R. D. Primate Origins and Evolution: A Phylogenetic Reconstruction. Princeton: Princeton Univ. Press; 1990. [Google Scholar]
- 32.Wilson J. A. Folia Primatol. 1966;4:227–248. doi: 10.1159/000155056. [DOI] [PubMed] [Google Scholar]
- 33.Szalay F. S., Delson E. Evolutionary History of the Primates. New York: Academic; 1979. [Google Scholar]
- 34.Beard K. C., Wang J. J. Hum. Evol. 2004;46:401–432. doi: 10.1016/j.jhevol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Maier W. In: Evolutionary Biology of the New World Monkeys and Continental Drift. Ciochon R., Chiarelli A. B., editors. New York: Plenum; 1980. pp. 219–241. [Google Scholar]
- 36.Starck D. In: Biology of Tarsiers. Niemitz C., editor. Stuttgart: Fischer; 1984. pp. 275–290. [Google Scholar]
- 37.Ross C. F. Am. J. Primatol. 1996;40:205–230. doi: 10.1002/(SICI)1098-2345(1996)40:3<205::AID-AJP1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Seiffert E. R., Simons E. L., Clyde W. C., Rossie J. B., Attia Y., Bown T. M., Chatrath P., Mathison M. E. Science. 2005;310:300–304. doi: 10.1126/science.1116569. [DOI] [PubMed] [Google Scholar]
- 39.Heesy C. P., Ross C. F. In: Anthropoid Origins: New Visions. Ross C. F., Kay R. F., editors. New York: Kluwer/Plenum; 2004. pp. 665–698. [Google Scholar]
- 40.Simons E. L. In: Anthropoid Origins: New Visions. Ross C. F., Kay R. F., editors. New York: Kluwer/Plenum; 2004. pp. 183–204. [Google Scholar]