Abstract
Marked increase in cell permeability ascribed to open connexin (Cx)43 hemichannels is induced by metabolic inhibition (MI) of cortical astrocytes in culture, but the molecular mechanisms are not established. Dephosphorylation and/or oxidation of Cx43 hemichannels was proposed as a potential mechanism to increase their open probability. We now demonstrate that MI increases the number of hemichannels on the cell surface assayed by biotinylation and Western blot, and that this change is followed by increased dephosphorylation and S-nitrosylation. The increase in rate of dye uptake caused by MI is comparable to the increase in surface expression; thus, open probability and permeation per hemichannel may be unchanged. Reducing agents did not affect dephosphorylation of Cx43 hemichannels but reduced dye uptake and S-nitrosylation. Uptake was also reduced by elevated intracellular but not extracellular levels of reduced glutathione. Moreover, nitric oxide donors induced dye uptake and nitrosylation of surface Cx43 but did not affect its abundance or phosphorylation. Thus, permeability per channel is increased, presumably because of increase in open probability. We propose that increased dye uptake induced by MI is mediated by an increased number of Cx43 hemichannels in the surface and is associated with multiple molecular changes, among which nitrosylation of intracellular Cx43 cysteine residues may be a critical factor.
Keywords: astroglia, ischemia, nitric oxide, permeabilization
Gap junction channels are formed by two hemichannels in series, one provided by each of two contacting cells. Each hemichannel is a hexamer of protein subunits called connexins (Cxs), a family of highly conserved proteins; of these, Cx43 is probably the most commonly expressed. The existence of hemichannels on the cell surface can be demonstrated by using different experimental approaches, including morphological, biochemical, and functional methods. Moreover, recent studies have shown the involvement of functional hemichannels in diverse physiological and pathological conditions (1, 2).
Under physiological conditions, hemichannels composed of Cx43 have a very low open probability (3) but apparently sufficient to release physiologically relevant quantities of signaling molecules (e.g., ATP, glutamate, NAD+, and PGE2) to the extracellular milieu (4–7). Thus, under physiological conditions, hemichannels mediate autocrine and/or paracrine signaling and may be an additional transmembrane pathway for diffusion of cellular nutrients and/or waste products. In addition, excessive opening of hemichannels formed of Cx30, Cx32, or Cx43 may accelerate cell deterioration in pathological conditions (8–11).
Oxygen deprivation during hypoxia and ischemia causes intracellular accumulation of toxic metabolites and ATP depletion, which can lead to cell death. In numerous studies, metabolic inhibition (MI) has been used as model to elucidate the effect of hypoxia with or without substrate deprivation on cells in culture or in ex vivo preparations. In these preparations, hemichannel opening induced by MI or ischemia is thought to accelerate cell death (9, 12). In cardiomyocytes, ischemia activates a large nonselective cationic conductance (13). In cardiomyocytes, cortical astrocytes and renal proximal tubule cells MI or ischemia enhance the plasma membrane permeability to small molecules, such as calcein, ethidium bromide (EtdBr), and Lucifer yellow (9, 12–14). In all these systems, the cellular response to the ischemic insult has been attributed to opening of Cx43 hemichannels. However, the molecular mechanisms remain unknown. Two possible mechanisms have been proposed: (i) dephosphorylation of Cx43 due to ATP depletion and activation of Ca2+-dependent protein phosphatases and (ii) oxidation of Cx43 due to enhanced generation of reactive oxygen-derived species (15), to which we now add, (iii) insertion of additional hemichannels into the surface membrane. In support of the first mechanism, liposomes in which nonphosphorylated (NP) Cx43 hemichannels are reconstituted show much greater permeability than those containing hemichannels phosphorylated by mitogen-activated protein kinase (16). In addition, hemichannels formed of Cx43(S368A), which are missing a demonstrated phosphorylation site, are PKC-unresponsive and remain preferentially open (17). Thus, Cx dephosphorylation may be sufficient to activate opening of Cx43 hemichannels. However, a free-radical scavenger (Trolox) blocks opening of Cx43 hemichannels in metabolically inhibited astrocytes (9, 15, 18), suggesting the involvement of redox potential in the opening. In astrocytes subjected to MI, Trolox does not prevent dephosphorylation of total Cx43. Conversely, in astrocytes subjected to MI and treated with cyclosporin A, dephosphorylation of Cx43 is partially inhibited, but the cells still become permeabilized (15).
In the present work, levels of Cx43 in the surface membrane of astrocytes during MI were assessed by biotinylation. We assume from the work of Musil and Goodenough (19) that the surface Cx43 is in the form of hemichannels assembled before insertion into the membrane. Moreover, their work indicates that Cx43 in gap junctions is biotinylated to only a small degree. We evaluated cell-surface Cx43 levels, phosphorylation, and S-nitrosylation by Western blotting. We found that MI increased the levels of Cx43 on the cell surface and induced dephosphorylation and nitrosylation of the Cx. Both MI-induced cell permeabilization and nitrosylation of surface Cx43 were blocked with reducing agents, i.e., reduced glutathione (GSH) and DTT. Moreover, nitric oxide (NO) donors also induced cell permeabilization and nitrosylation of Cx43 with little change in level or state of phosphorylation of the surface protein. DTT and Trolox, two reducing agents, decreased the MI- or NO-induced dye uptake but did not prevent dephosphorylation of surface Cx43. The MI-induced cell permeabilization was blocked by extracellular application of membrane-permeant but not by -impermeant reducing agents, suggesting that an oxidation reaction on intracellular cysteine residues induces the hemichannel opening. Preliminary findings of this work have been presented (18).
Results
Quantification of Surface Cx43 Hemichannels in Cortical Astrocytes in Culture.
Cx43 is assembled after exit from the endoplasmic reticulum into hexamers or hemichannels in the membrane of vesicles, which are then transported to the cell surface and inserted into the plasma membrane (20). Thus, Cx43 present on the cell surface of isolated cells is in the form of hemichannels, and this Cx43 can be selectively pulled down after biotinylation of cell surface proteins (21). Using this approach, we determined levels of Cx43 in the surface by Western blot analysis (see Fig. 6, which is published as supporting information on the PNAS web site). Under control conditions, close to 15% (14.5 ± 4.3, n = 4) of the total Cx43 expressed by cultured astrocytes was in hemichannels in the plasma membrane; this Cx43 was mostly in P2 and P3 phosphorylated forms (see Figs. 4 and 5) (19).
Fig. 4.
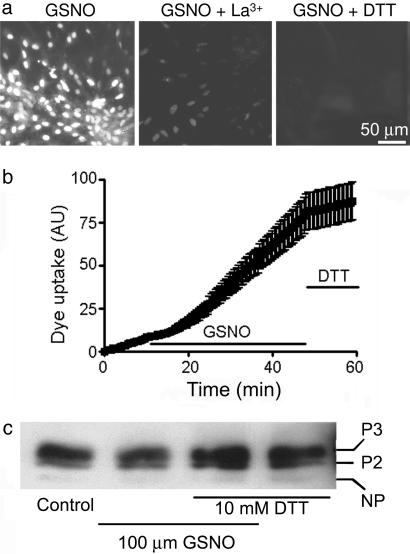
NO induces astrocyte dye uptake. (a) Confluent astrocyte cultures were photographed after incubation with 100 μM GSNO, an NO donor, for 50 min, and then exposed to 100 μM EtdBr for 5 min (Left), incubated with La3+ for the last 5 min of 50 min of GSNO and then exposed to EtdBr for 5 min (Middle), or incubated with DTT for the last 5 min of 50 min of GSNO and then EtdBr for 5 min (Right). La3+ and DTT largely prevented dye uptake, indicating rapid reduction of hemichannel permeability. n = 3. (b) Time-lapse measurement of dye uptake in 10 μM EtdBr. Dye uptake at a low basal rate was increased a few minutes after addition of GSNO. The rate of uptake was markedly reduced by DTT (10 mM) replacing GSNO at ≈48 min. Each point corresponds to mean fluorescence intensity of 21 cells in each of three independent experiments ± SE. (c) Western blot analysis of cell surface Cx43 pulled down with biotin from astrocytes under control conditions (Control), treated for 50 min with 100 μM GSNO, treated for 50 min with 100 μM GSNO, and with 10 mM DTT during the last 10 min or with 10 mM DTT for 10 min. Representative results of three experiments are shown.
Fig. 5.
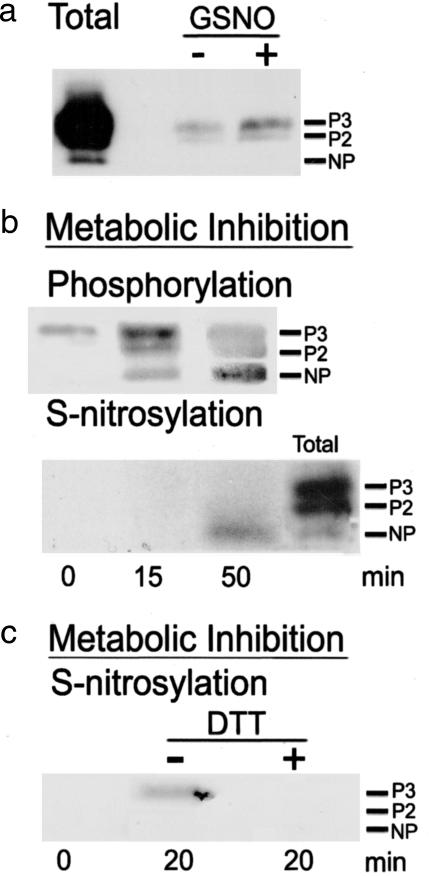
An NO donor and MI increase S-nitrosylation of surface Cx43. S-nitrosylation and phosphorylation states were determined by surface biotinylation and Western blot analysis as described in Supporting Text. (a) After 50 min of treatment with 100 μM GSNO, S-nitrosylation of P2 and P3 bands of surface Cx43 (GSNO/+, right lane) was increased over basal (GSNO/−, middle lane). The left lane (Total) shows Cx43 bands of phosphorylated forms in control homogenate (5 μg of protein). (b, upper gel) Reactive bands of surface Cx43 in astrocytes under control conditions (left lane) and after 15 min (middle lane) and 50 min (right lane) of MI. (b, lower gel) Bands of S-nitrosylated surface Cx43 in astrocytes under conditions identical to those in b (upper gel). Nitrosylation was detected at 50 min of MI but not at 0 or 15 min (left and middle lanes). The lane labeled Total is as in a. (c) MI for 20 min caused nitrosylation of surface Cx43 (−DTT, middle lane), whereas DTT (10 mM) for the last 5 min of 20 min of MI prevented or reversed nitrosylation (+DTT, right lane). No basal nitrosylation was detected (left lane). The electrophoretic mobilities of the NP and two phosphorylated (P2 and P3) forms of Cx43 are indicated.
Ischemia Increases the Amount of Surface Cx43 and Induces Cx43 Dephosphorylation and Dye Uptake.
It has been shown that MI enhances surface permeability to various gap junction-permeable molecules, an effect attributed to hemichannel opening (3, 9, 13, 14). However, it was not determined whether the permeabilization involved increase in the number of Cx43 hemichannels in the plasma membrane or a modification that increased their open probability or permeability. To approach these questions, we evaluated both the level and phosphorylation state of Cx43 in the surface membrane by biotinylation and Western blot analysis.
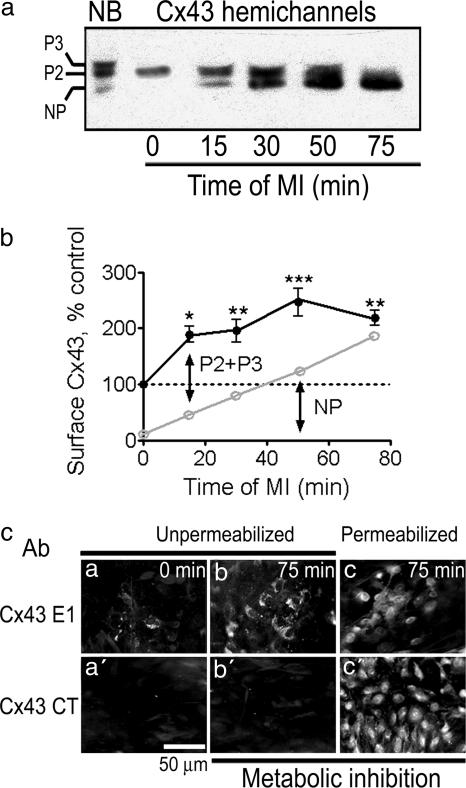
During the first 15 min of MI, induced by antimycin A (5 ng/ml) and iodoacetic acid (270 μM), there was a large increase in levels of surface Cx43, to 189 ± 28% (Fig. 1, n = 4; ∗, P < 0.05) of that detected in control astrocytes (summing densities for all bands) (Fig. 1 a and b). The maximal increase in surface Cx43 was observed at 50 min of MI (to 246 ± 55% of control; ∗∗∗, P < 0.001). The increase in surface Cx43 was also assessed by indirect immunofluorescence applied to nonpermeabilized cells by using an antibody directed to the extracellular loop 1 (Fig. 1c Upper). Although in control cultures of astrocytes, <20% showed fluorescent label (Fig. 1ca), MI for 75 min increased the fraction of immunoreactive cells to ≈50% (Fig. 1cb). Labeling with a C-terminal antibody did not label nonpermeabilized cells before or after MI (Fig. 1 ca′ and cb′). After 75 min of MI and then permeabilization, both antibodies labeled all cells present in the field (Fig. 1 cc and cc′, there were fewer cells in Fig. 1cc).
Fig. 1.
MI induces dephosphorylation of Cx43 hemichannels and increases surface expression. Cultured astrocytes (≈80% confluent) were subjected to MI for different periods of time (0, 15, 30, 50, and 75 min), and relative levels of surface Cx43 were measured by Western blot analysis of biotinylated proteins from intact cells. (a) Sample blot. On the left, the phosphorylated (P3 and P2) and NP forms of total Cx43 in homogenates of control astrocytes (not biotinylated, NB) are indicated. MI induced progressive loss of phosphorylated forms as well as a progressive increase in surface expression. (b) Graph showing the densities of immunoreactive bands (P3 + P2 and NP) of surface Cx43 for astrocytes after different durations of MI (n = 4). The upper line shows the total amount of Cx43 relative to that present in the cell surface of control astrocytes (denoted by dotted line). The lower line shows the NP density. The difference between upper and lower lines is the amount of P2 + P3 (∗, P < 0.05; ∗∗, P < 0.01; and ∗∗∗, P < 0.001 as compared to the value of control astrocytes). (c) Immunofluorescence detection of Cx43 on the surface of control and metabolically inhibited astrocytes. In nonpermeabilized astrocyte monolayers, an anti-Cx43 E1 antibody (directed to the first extracellular loop of Cx43) labeled a few cells in control cultures (ca) but numerous cells after 75 min of MI (cb). In sister cultures, an anti-Cx43 CT antibody (directed to the C terminus, which is located intracellularly) did not react with cells either under control conditions (ca′) or after 75 min of MI (cb′). Both antibodies showed extensive Cx43 immunoreactivity in astrocytes permeabilized after 75 min of MI (cc and cc′). (n = 2.)
In astrocytes under control conditions, 87 ± 2% (n = 4) of surface Cx43 was in the phosphorylated forms, P2 and P3. During MI, the amount in the P2 and P3 forms increased and then decreased, whereas that in the NP form progressively increased (Fig. 1 a and b). At 75 min of MI, 84.2 ± 2.6% of surface Cx43 was in the NP form. The decline of P2 + P3 forms after the initial increase suggests that Cx43 hemichannels undergo dephosphorylation while in the surface membrane. Alternatively, if less likely, phosphorylated Cx43 may be retrieved from the cell surface and replaced by NP Cx43.
To evaluate the rate of dye uptake, we used time-lapse imaging before and during application of metabolic inhibitors (Fig. 2a). A low basal rate of uptake of EtdBr (10 μM) was increased within minutes of application. The mean increase in rate of uptake was to 2.3 ± 0.1 times the basal value (n = 62 cells in three experiments, P < 0.001, basal compared with uptake after 30–40 min). Thus most, if not all, of the increase in uptake is ascribable to increased number of hemichannels in the cell surface.
Fig. 2.
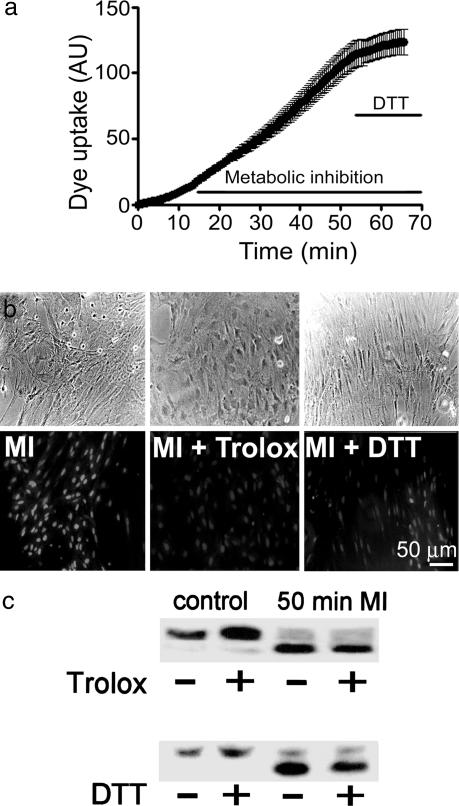
Dye uptake but not Cx43 dephosphorylation induced by MI is reduced by antioxidants. (a) Time-lapse measurements of EtdBr (10 μM) uptake during MI. Later application of 10 mM DTT reduced the rate of uptake. Mean and standard error of >20 cells in an experiment representative of seven. (b) Phase micrographs (Upper), fluorescence (Lower), from a different experiment. Astrocytes after 50 min of MI showed prominent EtdBr (100 μM) uptake during a 5-min application of the dye (MI). In sister cultures with 100 μM Trolox (MI + Trolox) added 20 min or 10 mM DTT (MI + DTT) added 10 min before the end of 50 min of MI, the dye uptake was greatly reduced (n = 3). (c) Western blot analysis of cell surface Cx43 pulled down with biotin from control astrocytes incubated for 20 min with or without Trolox (Upper Left) or 10 min with or without DTT (Lower Left) or from astrocytes subjected to 50 min of MI without or with application of 100 μM Trolox 20 min (Upper) or 10 mM DTT 10 min before the end of the period of inhibition (Lower). Representative results of three experiments are shown. The reducing agents did not prevent dephosphorylation of surface Cx43 induced by MI.
Reducing Agents Prevent Induction of Cell Permeabilization by MI but Do Not Prevent Dephosphorylation of Surface Cx43.
Our group previously reported that dye uptake induced by MI is almost abolished by Trolox or melatonin, two potent free-radical scavengers, but that these agents do not affect the dephosphorylation of total Cx43 (9, 15). In agreement, at 50 min of MI, astrocytes treated with 100 μM Trolox during the last 20 min showed much less EtdBr uptake after a 5-min dye application (100 μM) than cells subjected only to MI (Fig. 2b, MI + Trolox, n = 3). Moreover, Trolox applied at this time did not prevent dephosphorylation of cell surface Cx43 (Fig. 2c, n = 4). Independence of the dephosphorylation and permeabilization was suggested by the earlier finding that dye uptake induced by MI is not affected by cyclosporin A, which partially inhibits the dephosphorylation of total Cx43 (9, 15).
To elucidate the redox reaction responsible for the dye uptake elicited by MI, we tested the effect of DTT, a reducer of oxidized sulfhydryl groups that has more limited antioxidant activity than Trolox but is known to reduce oxidized cysteine residues. Application of 10 mM DTT during the last 10 min of a 50-min period of MI reduced dye uptake (Fig. 2b, MI + DTT); moreover, DTT, like Trolox, did not significantly affect the phosphorylation of surface Cx43 in astrocytes either under control conditions or undergoing dephosphorylation during MI (Fig. 2c, n = 4). We have not carefully examined the interaction of reducing agents and MI on surface expression of Cx43.
Intracellular but Not Extracellular GSH Blocks the Dye Uptake Induced by MI.
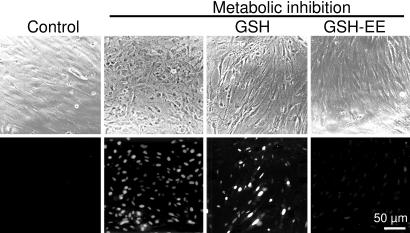
Cx43 has four transmembrane domains, and both the N and C termini are located on the cytoplasmic side. The first and second extracellular loops and the C-terminal tail of Cx43 each contain three cysteine residues (22) that may be susceptible to oxidation. To localize cysteine residues that may be involved in the EtdBr uptake induced by MI, we studied the effect on dye uptake of GSH (10 mM), which is membrane-impermeant, and GSH ethyl ester (GSH-EE, 10 mM), which is membrane-permeant, and from which GSH is generated intracellularly by the action of cytoplasmic esterases (23). Extracellular GSH had no effect on EtdBr uptake induced by MI, whereas GSH-EE reduced it to levels similar to those in cells under control conditions (Fig. 3).
Fig. 3.
Intra- but not extracellular GSH blocks the dye uptake induced by MI. Astrocyte cultures near to confluency were subjected to a 30-min period of MI followed by a 5-min application of EtdBr (100 μM). (Upper) Phase contrast; (Lower) fluorescence. Control astrocytes did not show dye uptake (control, first column). MI induced dye uptake and changes in appearance (second column), which were prevented by cell-permeant GSH ethyl ester (10 mM, fourth column) but not by cell-impermeant GSH (10 mM, third column) applied for the last 10 min of MI. (n = 3.)
NO Induces Dye Uptake by Astrocytes.
Because the generation of NO, a free radical, is increased in astrocytes during MI (25, 26), and NO can oxidize cysteine residues (24, 27, 28), we tested whether NO donors induce dye uptake by astrocytes. Application of 100 μM nitrosoglutathione (GSNO) (Fig. 4a Left) or 100 μM NOR-3 (data not shown), two NO donors, increased dye uptake, which at 50 min of treatment was similar to that seen in metabolically inhibited astrocytes. In cells treated with an NO donor, this permeabilization was markedly reduced by the hemichannel blocker La3+ (200 μM) during last 5 min of a 50-min application of NO donor (just before dye application at 50 min; Fig. 4a Center). Gd+3 (50 μM) gave similar results (data not shown). The NO-induced dye uptake was greatly reduced by 10 mM DTT applied during the last 5 min of a 50-min NO donor treatment (Fig. 4a Right). In time-lapse studies, application of GSNO within minutes increased the rate of EtdBr uptake (10 μM) to 2.3 ± 0.3 (P < 0.01) times the basal rate, and application of 10 mM DTT rapidly reduced the rate of dye uptake to 0.5 ± 0.1 times the basal rate (n = 3 experiments, 21 cells) (Fig. 4b). The permeabilization induced by the NO donor, NOR-3, was not affected by the addition of 150 μM oATP (data not shown), indicating that ionotropic purinergic receptors are not involved in NO-induced permeabilization. Level and phosphorylation of surface Cx43 in astrocytes treated for 50 min with 100 μM GSNO with or without 10 mM DTT during the last 10 min were similar to those measured in astrocytes under control conditions or treated for 10 min with 10 mM DTT (Fig. 4c). Surface expression was increased by 20.4 ± 2.1% of control (n = 2 with GSNO and 2 with NOR-3 treatment, P > 0.05).
NO Donors and MI Induce Reversible S-Nitrosylation of Surface Cx43.
S-nitrosylation is a protein oxidation mediated by NO and other oxides of nitrogen that is reversed by DTT (29–32). Therefore, we determined whether NO donors and MI induced this covalent modification in surface Cx43 of astrocytes. In three of six experiments, a weak signal indicating basal S-nitrosylation was detected under control conditions (Fig. 5a), whereas in the other three experiments, a band was detected only after prolonged exposure of the x-ray film yielding a high background (data not shown). In cultures treated with 100 μM GSNO for 50 min, Cx43 S-nitrosylation was detected in all six experiments and was always greater than in control cells (Fig. 5a). S-nitrosylation was detected in different forms of Cx43. In astrocytes under control conditions or treated with NO for 50 min, surface Cx43 was predominantly phosphorylated (Fig. 4c), and both forms were S-nitrosylated, the P3 form being the most evident (Fig. 5a). The NP form also appeared to be S-nitrosylated but required longer periods of exposure of the film for detection (data not shown). Similar results were obtained with the NO donor NOR-3 (data not shown).
S-nitrosylation of Cx43 was also detected after MI and was greater at 50 min of treatment than at 15 min of treatment (Fig. 5b, n= 3). At 50 min, little of the P2 and P3 forms remained, and S-nitrosylation occurred mainly in the NP form. Because DTT reduced EtdBr uptake, we also tested whether DTT reduced Cx43 S-nitrosylation. In agreement with the effect on dye uptake, S-nitrosylation was undetectable in cells treated with 10 mM DTT during the last 5 min of a 20-min period of MI (Fig. 5c, +DTT). In contrast, S-nitrosylation was evident in Cx43 from astrocytes subjected to 20 min of MI but not treated with DTT (Fig. 5c, −DTT, n = 2).
Discussion
In the present study, we investigated the mechanisms of the pronounced increase in dye uptake mediated by Cx43 hemichannels in cultures of cortical astrocytes subjected to MI. We found that MI increases the levels of surface Cx43, presumably already incorporated into hemichannels (20), and most if, not all, of the increase in dye uptake can be ascribed to the increased insertion. The surface protein becomes NP and S-nitrosylated over a low basal level, and it appears likely that these covalent changes occurred at least in part after insertion into the surface. In contrast, increased dye uptake induced by NO donors could not be accounted for by the insertion of new hemichannels. We propose that NO donors increase open probability by oxidative reactions of surface Cx43 rather than by dephosphorylation, because nitrosylation of surface Cx43 and dye uptake were inhibited by reducing agents with no obvious effect on dephosphorylation evaluated by Western blot analysis. We demonstrated S-nitrosylation and permeabilization by NO donors that do not increase the level of surface Cx43 (Fig. 4c) but cannot exclude a contribution from other oxidizing reactions (33). The difference between MI and NO donors will require further investigation.
Because surface Cx43 isolated by biotinylation was only ≈15% of the total in control cultures, assaying changes required separation of this material from the Cx43 in gap junctions and intracellular membranes. In control cultures, surface Cx43 was mainly phosphorylated, and the cells showed little dye uptake, probably due to infrequent hemichannel opening. Furthermore, open probability is likely very low, even under conditions of increased dye uptake (3), and we cannot exclude that the open hemichannels are among the small NP fraction on the surface. Cx43 hemichannels phosphorylated by mitogen-activated protein kinase or PKC and reconstituted in liposomes show decreased activity and/or permeability (16, 17); thus, dephosphorylation may contribute to the increase in dye uptake induced by MI, but increased insertion appears adequate to account for our observations, and we found no obvious effect of hemichannel dephosphorylation in dye uptake induced by MI. The rapid reduction in dye uptake by application of reducing agents (Fig. 2a) seems unlikely to be due to internalization of surface hemichannels and instead to be due to reduction in open probability. The underlying mechanism might involve changes in sensitivity to intracellular regulators, such as Ca2+. It has recently been proposed that Cx32 hemichannels open over a narrow range of cytoplasmic free Ca2+ (34). Future experiments will clarify these issues.
We used biotin labeling and immunofluorescence with an antibody to a region of the (extracellular) E1 domain and observed with biotinylation that levels of surface Cx43 increased within minutes of MI. The increase in surface Cx43 could result from enhanced insertion into the plasma membrane from an intracellular pool, reduced endocytosis, or reduced recruitment to gap junctions. Recently, it was demonstrated that oxidant stress reduces the degradation of endocytosed Cx43 by interfering with its targeting and/or transport to the lysosome, possibly by increasing the level of unfolded protein in the cytosol (35).
Our group has previously shown that cyclosporin A, an inhibitor of calcineurin, reduces the dephosphorylation of total Cx43 but not the dye uptake induced by MI (15), suggesting that dephosphorylation is not the main mechanism of opening. Moreover, the dye uptake but not the dephosphorylation is almost completely prevented by reducing agents such as melatonin and Trolox, suggesting oxidation of −SH group(s) as an activating mechanism (9, 15). Here, we confirmed those findings for surface Cx43; Trolox and DTT did not prevent the dephosphorylation induced by MI but rapidly blocked the dye uptake. We did not determine whether reducing agents caused internalization of surface Cx43; the rapidity of the effects on dye uptake (Figs. 2 and 4) suggests that the primary action was reduction in open probability or permeability of hemichannels.
In support of a role of cysteine residue oxidation in Cx43 hemichannel opening, we found that a brief application of DTT to metabolically inhibited astrocytes decreased dye uptake and S-nitrosylation of surface Cx43 without apparent effect on the degree of dephosphorylation of this protein. Moreover, we demonstrated that dye uptake induced by NO donors was greatly reduced by DTT and by hemichannel blockers. Notably, NO donors had little effect on the amount of surface Cx43 or its phosphorylation state.
Hemichannels formed of Cx43 lacking the extracellular cysteine residues are permeable to carboxyfluorescein, as are hemichannels formed of wild-type Cx43, and this permeability is decreased by PKC-mediated phosphorylation (36), suggesting that those amino acid residues are not relevant for the normal activity of Cx43 hemichannels. Here we showed that extracellular application of the cell permeant GSH–ethyl ester markedly reduced the MI -induced activation of hemichannels, whereas the membrane-impermeant GSH had no effect. These data suggest that the affected cysteine residues are located intracellularly. Cx43 has only three such cysteines, all of which are in the cytoplasmic C-terminal domain (37). S-nitrosylation of one or more of these cysteines may be sufficient to induce opening of surface Cx43 hemichannels in astrocytes treated with metabolic inhibitors or NO donors. Mutation studies should help determine which residue(s) is involved.
S-nitrosylation is a common protein modification that can occur under oxidative stress and may be a common mediator of NO effects (22, 38). Other possible protein modifications include S-glutathionylation ( S
S SG; reaction with oxidized GSH (GSSG) (16), formation of disulfide bonds (
SG; reaction with oxidized GSH (GSSG) (16), formation of disulfide bonds ( S
S S
S ) with another cysteine residues (38) and S-hydroxylation (
) with another cysteine residues (38) and S-hydroxylation ( S
S OH; oxidation by H2O2) (16, 39). Further studies are required to elucidate any functional differences conferred by oxidative changes in Cx43 hemichannels, as has been done for other proteins (16). Moreover, quantitation of S-nitrosylation of cysteine residues per protein subunit and hemichannel might suggest the function of basal Cx43 nitrosylation and illuminate the structure–activity relations in opening of hemichannels by this or other oxidative covalent modification.
OH; oxidation by H2O2) (16, 39). Further studies are required to elucidate any functional differences conferred by oxidative changes in Cx43 hemichannels, as has been done for other proteins (16). Moreover, quantitation of S-nitrosylation of cysteine residues per protein subunit and hemichannel might suggest the function of basal Cx43 nitrosylation and illuminate the structure–activity relations in opening of hemichannels by this or other oxidative covalent modification.
Our findings and interpretations are consistent with restricted opening of hemichannels in normal cells adequate to allow release of signaling molecules and with greater opening that may accelerate death under pathological conditions such as ischemia, in which there is enhanced NO generation (25, 26). Mutations of the cysteine residues should give a clearer view of hemichannels as a sensor of redox potential and possible target for therapeutic intervention. The findings are likely to have wide application, because Cx43 is expressed in numerous organs subject to ischemia, including brain and heart.
Materials and Methods
Details are described in Supporting Text, which is published as supporting information on the PNAS web site. Methods for culture and surface labeling of astrocytes, Western blotting, dye uptake, and light microscopy were as routinely applied. MI was induced by application of antimycin A (5 ng/ml) and iodoacetic acid (270 μM). Detection of S-nitrosylated Cx43 used the NitroGlo kit (PerkinElmer). After surface biotinylation and pull down, unmodified  SH groups were blocked, nitrosyl groups were removed, and the unmasked
SH groups were blocked, nitrosyl groups were removed, and the unmasked  SH groups were reacted with NitroGlo HPDP-Biotin to allow for protein isolation with NeutrAvidin and resolution by Western blotting. Results are presented as means ± SE.
SH groups were reacted with NitroGlo HPDP-Biotin to allow for protein isolation with NeutrAvidin and resolution by Western blotting. Results are presented as means ± SE.
Supplementary Material
Acknowledgments
We are thankful for the outstanding technical assistance of Ms. Teresa Vergara, who prepared all astrocyte cultures. This work was funded in part by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) Grant 1030945 (to J.C.S.), National Institutes of Health Grants GM068586 (to L.R.) and NS045287 (to M.V.L.B.), and the F. M. Kirby Program in Neural Repair and Protection at the Albert Einstein College of Medicine. M.V.L.B. is the Sylvia and Robert S. Olnick and Distinguished Professor of Neuroscience.
Abbreviations
- Cx
connexin
- GSH
glutathione
- NP
nonphosphorylated
- EtdBr
ethidium bromide
- MI
metabolic inhibition
- GSNO
nitrosoglutathione
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Saéz J. C., Contreras J. E., Bukauskas F. F., Retamal M. A., Bennett M. V. L. Acta Physiol. Scand. 2003;179:9–22. doi: 10.1046/j.1365-201X.2003.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett M. V. L., Contreras J. E., Bukauskas F. F., Saéz J. C. Trends Neurosci. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Contreras J. E., Saéz J. C., Bukauskas F. F., Bennett M. V. L. Proc. Natl. Acad. Sci. USA. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stout C. E., Costantin J. L., Naus C. C., Charles A. C. J. Biol. Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 5.Ye Z. C., Wyeth M. S., Baltan-Tekkok S., Ransom B. R. J. Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruzzone S., Guida L., Zocchi E., Franco L., De Flora A. FASEB J. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- 7.Cherian P. P., Siller-Jackson A. J., Gu S., Wang X., Bonewald L. F., Sprague E., Jiang J. X. Mol. Biol. Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrams C. K., Bennett M. V. L., Verselis V. K., Bargiello T. A. Proc. Natl. Acad. Sci. USA. 2002;99:3980–3984. doi: 10.1073/pnas.261713499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contreras J. E., Sánchez H. A., Eugenín E. A., Speidel D., Theis M., Willecke K., Bukauskas F. F., Bennett M. V. L., Saéz J. C. Proc. Natl. Acad. Sci. USA. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Essenfelder G. M., Bruzzone R., Lamartine J., Charollais A., Blanchet-Bardon C., Barbe M. T., Meda P., Waksman G. Hum. Mol. Genet. 2004;13:1703–1714. doi: 10.1093/hmg/ddh191. [DOI] [PubMed] [Google Scholar]
- 11.Liang G. S., de Miguel M., Gomez-Hernandez J. M., Glass J. D., Scherer S. S., Mintz M., Barrio L. C., Fischbeck K. H. Ann. Neurol. 2005;57:749–754. doi: 10.1002/ana.20459. [DOI] [PubMed] [Google Scholar]
- 12.Vergara L., Bao X., Cooper M., Bello-Reuss E., Reuss L. J. Membr. Biol. 2003;196:173–184. doi: 10.1007/s00232-003-0636-9. [DOI] [PubMed] [Google Scholar]
- 13.John S. A., Kondo R., Wang S. Y., Goldhaber J. I., Weiss J. N. J. Biol. Chem. 1999;274:236–240. doi: 10.1074/jbc.274.1.236. [DOI] [PubMed] [Google Scholar]
- 14.Kondo R. P., Wang S. Y., John S. A., Weiss J. N., Goldhaber J. I. J. Mol. Cell Cardiol. 2000;32:1859–1872. doi: 10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- 15.Contreras J. E., Sánchez H. A., Véliz L. P., Bukauskas F. F., Bennett M. V. L., Saéz J. C. Brain Res. Brain Res. Rev. 2004;47:290–303. doi: 10.1016/j.brainresrev.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D. Y., Kam Y., Koo S. K., Joe C. O. J. Biol. Chem. 1999;274:5581–5587. doi: 10.1074/jbc.274.9.5581. [DOI] [PubMed] [Google Scholar]
- 17.Bao X., Reuss L., Altenberg G. A. J. Biol. Chem. 2004;279:20058–20066. doi: 10.1074/jbc.M311137200. [DOI] [PubMed] [Google Scholar]
- 18.Retamal M. A., Córtes C. J., Bukauskas F. F., Bennett M. V. L., Saéz J. C. J. Physiol. (London) 2005. 565P, PC161. [Google Scholar]
- 19.Musil L. S., Goodenough D. A. J. Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musil L. S., Goodenough D. A. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- 21.Lampe P. D. J. Cell Biol. 1994;127:1895–1905. doi: 10.1083/jcb.127.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laird D. W., Revel J. P. J. Cell Sci. 1990;97:109–117. doi: 10.1242/jcs.97.1.109. [DOI] [PubMed] [Google Scholar]
- 23.Minhas H. S., Thornalley P. J. Biochem. Pharmacol. 1995;49:1475–1482. doi: 10.1016/0006-2952(94)00518-q. [DOI] [PubMed] [Google Scholar]
- 24.Martínez-Rúiz A., Lamas S. Cardiovasc. Res. 2004;62:43–52. doi: 10.1016/j.cardiores.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Gibson C. L., Coughlan T. C., Murphy S. P. Glia. 2005;50:417–426. doi: 10.1002/glia.20143. [DOI] [PubMed] [Google Scholar]
- 26.Scorziello A., Pellegrini C., Secondo A., Sirabella R., Formisano L., Sibaud L., Amoroso S., Canzoniero L. M., Annunziato L., Di Renzo G. F. J. Neurosci. Res. 2004;76:812–821. doi: 10.1002/jnr.20096. [DOI] [PubMed] [Google Scholar]
- 27.Hara M. R., Agrawal N., Kim S. F., Cascio M. B., Fujimuro M., Ozeki Y., Takahashi M., Cheah J. H., Tankou S. K., Hester L. D., et al. Nat. Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 28.Sun J., Xin C., Eu J. P., Stamler J. S., Meissner G. Proc. Natl. Acad. Sci. USA. 2001;98:11158–11162. doi: 10.1073/pnas.201289098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eu J. P., Sun J., Xu L., Stamler J. S., Meissner G. Cell. 2000;102:499–509. doi: 10.1016/s0092-8674(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 30.Garban H. J., Marquez-Garban D. C., Pietras R. J., Ignarro L. J. Proc. Natl. Acad. Sci. USA. 2005;102:2632–2636. doi: 10.1073/pnas.0409854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gow A. J., Buerk D. G., Ischiropoulos H. J. Biol. Chem. 1997;272:2841–2845. doi: 10.1074/jbc.272.5.2841. [DOI] [PubMed] [Google Scholar]
- 32.Park H. S., Huh S. H., Kim M. S., Lee S. H., Choi E. J. Proc. Natl. Acad. Sci. USA. 2000;97:14382–14387. doi: 10.1073/pnas.97.26.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper C. D., Lampe P. D. J. Biol. Chem. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- 34.DeVuyst E., Decrock E., Cabooter L., Dubyak G. R., Naus C. C., Evans W. H., Leybaert L. EMBO J. Epub. 2005 doi: 10.1038/sj.emboj.7600908. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanslyke J. K., Musil L. S. Mol. Biol. Cell. 2005;16:5247–5257. doi: 10.1091/mbc.E05-05-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bao X., Altenberg G. A., Reuss L. Am. J. Physiol. 2004;286:C647–C654. doi: 10.1152/ajpcell.00295.2003. [DOI] [PubMed] [Google Scholar]
- 37.Beyer E. C., Paul D. L., Goodenough D. A. J. Cell Biol. 1987;105:2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hess D. T., Matsumoto A., Kim S. O., Marshall H. E., Stamler J. S. Nat. Rev. Mol. Cell. Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 39.Poole L. B., Karplus P. A., Claiborne A. Annu. Rev. Pharmacol. Toxicol. 2004;44:325–347. doi: 10.1146/annurev.pharmtox.44.101802.121735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.