Abstract
Rad is a low molecular weight GTPase that is overexpressed in skeletal muscle of some patients with type 2 diabetes mellitus and/or obesity. Overexpression of Rad in adipocytes and muscle cells in culture results in diminished insulin-stimulated glucose uptake. To further elucidate the potential role of Rad in vivo, we have generated transgenic (tg) mice that overexpress Rad in muscle using the muscle creatine kinase (MCK) promoter–enhancer. Rad tg mice have a 6- to 12-fold increase in Rad expression in muscle as compared to wild-type littermates. Rad tg mice grow normally and have normal glucose tolerance and insulin sensitivity, but have reduced plasma triglyceride levels. On a high-fat diet, Rad tg mice develop more severe glucose intolerance than the wild-type mice; this is due to increased insulin resistance in muscle, as exemplified by a rightward shift in the dose–response curve for insulin stimulated 2-deoxyglucose uptake. There is also a unexpected further reduction of the plasma triglyceride levels that is associated with increased levels of lipoprotein lipase in the Rad tg mice. These results demonstrate a potential synergistic interaction between increased expression of Rad and high-fat diet in creation of insulin resistance and altered lipid metabolism present in type 2 diabetes.
Keywords: diabetes mellitus, glucose transport, RGK GTPase, transgenic mouse
Rad, Gem/Kir, Rem, and Rem2 are members of the Ras-related RGK (Rad, Gem, and Kir) family of small GTP-binding proteins. Rad (Ras Associated with Diabetes) is a 35-kDa GTPase that was identified by subtractive cloning as overexpressed in skeletal muscle of patients with type 2 diabetes mellitus (1, 2). Although some subsequent studies (3, 4) did not confirm this direct association between the Rad mRNA level and diabetes, other studies have shown that Rad is an insulin regulated gene in muscle (5) and that Rad overexpression inhibits glucose transport in muscle cells in culture (6). There is also a correlation between the level of Rad expression and obesity (3), and genetic analysis studies of two different populations found a possible association between trinucleotide-repeat polymorphism of the Rad gene and type 2 diabetes (7, 8).
Rad is most highly expressed in the heart, lung, and skeletal muscle (1) and, in contrast to most other members of the Ras superfamily, is not lipid modified resulting in a primary cytosolic location (9). Rad expression is positively regulated by insulin (5). Previous work in our laboratory has demonstrated that adipocytes and muscle cells in culture in which Rad is overexpressed exhibit a reduction in the rate of insulin-stimulated glucose uptake (6). However, the insulin resistance that was found in the cells overexpressing Rad (6) was not accompanied by any detectable change in GLUT1 and GLUT4 glucose transporter quantity or translocation in response to insulin, suggesting a change in the activity of glucose transporters. To date, neither its exact function nor the ways by which Rad may be related to insulin resistance are known. Rad and other members of the RGK family have been found to be associated with cytoskeletal proteins like β-tropomyosin (10) and calmodulin (CAM) and CAM kinase (9, 11), raising the possibility that vesicle trafficking may be part of the mechanism involved in Rad interfering with glucose uptake. Rad has also been shown to attenuate the migration of vascular smooth muscle cells in generation of atherosclerosis (12).
Because Rad is expressed primarily in muscle and was originally found to be overexpressed in muscle of a Type 2 diabetic patient, and because skeletal muscles are a major site for glucose disposal and considered to be the primary site for insulin resistance in these patients (13), we have created a transgenic (tg) mouse that overexpresses Rad in skeletal muscle to further elucidate the functions of Rad. We find that overexpression of Rad in these animals potentiates the insulin resistance associated with high fat feeding and alters triglyceride metabolism. These data demonstrate how Rad may interact with environmental factors to help create the diabetic state.
Results
Creation and Identification of the Transgenic Mouse.
Five of 55 FVB offspring were found to carry the Rad transgene using genotyping analysis by Southern blotting. Of these, three transmitted the transgene to their offspring when mated with WT FVB mice. The offspring of the founder with highest level of Rad overexpression (no. 51) were used for all studies (Fig. 1b and c). Western blot analysis of Rad protein in extracts from this line of Tg mice demonstrated a 6- to 20-fold increase of Rad expression as compared to WT mice in all skeletal muscle groups, including quadriceps, gastrocnemius, and gluteus muscles (Fig. 1d). In normal mice, the level of Rad expression in the heart is higher than that in skeletal muscle, and thus there was no demonstrable overexpression of Rad in the heart of the transgenic mice even though the MCK promoter is somewhat activate in that tissue (14) (Fig. 1e). The level of Rad in lungs was also high in both control and Tg mice, whereas no Rad expression was detected in the liver of either Rad-Tg or WT mice (data not shown). The migration of the human Rad product of the transgene on the SDS/PAGE was similar to that of the endogenous protein, indicating that the full-length human Rad protein was expressed from the transgene.
Fig. 1.
Construction of the Rad transgene and Rad expression in the Rad-Tg mice. (a) Schematic structure of the Rad transgene. (b) Southern blot analysis of the two transgenic lines with the highest expression of Rad. Equal amounts of tail DNA digested with EcoRI were blotted with a 1.1-kb MCK probe. (c) Western blotting with anti-Rad serum of quadriceps muscle lysates from the same two lines. Line 51 was used for all studies. (d) Western blot analysis comparing Rad protein level in different muscles from two pairs of WT and Rad-Tg mice. (e) Comparison of Rad protein level in the heart, lung, and skeletal muscle of a WT and a Rad-Tg mouse by Western blotting.
Effects of Rad Overexpression in Muscle on Glucose Homeostasis and Insulin Action.
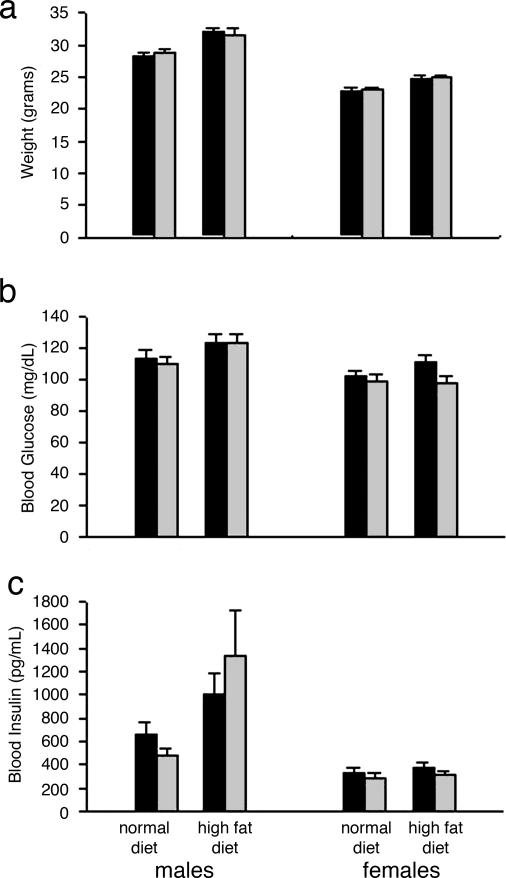
Compared to the WT littermates, the Rad-Tg mice grew normally, were of normal body weight and showed no significant difference in the amount of weight gained on high-fat diet (Fig. 2a). Fasting (Fig. 2b) and fed (not shown) blood glucose levels were similar in WT and Tg mice on the normal chow. After 14–22 weeks on high-fat diet, both the Rad-Tg and WT mice had somewhat higher glucose levels, but there was no difference between the groups (Fig. 2b). Likewise, plasma insulin levels were not significantly different between the Rad-Tg and WT mice on either the normal or high-fat diet in both the fasting (Fig. 2c) and fed (not shown) states.
Fig. 2.
Effects of Rad overexpression of body weight and glucose and insulin levels. (a) Comparison between WT (black bars) and Rad-Tg (gray bars) mice body weight at 4–5 months of age on normal and high-fat diets. High-fat diet was started at 6 weeks of age. (b) Fasting blood glucose levels at 5–6 months of age. (c) Fasting insulin levels. After overnight fasting, mice were anesthetized, and blood samples were taken by retro-orbital bleeding. Each bar represent the mean ± SE of 26–39 mice.
During intraperitoneal glucose tolerance tests (GTTs), the pattern of blood glucose levels of mice on normal chow was similar for the Rad-Tg and WT mice (Fig. 3a, filled circles). After the high-fat diet, glucose levels rose in both groups, but the glucose intolerance was more prominent in the Rad-Tg than for the WT mice in both males and females (Fig. 3a, open circles). When the results were expressed as the area under the curves (Fig. 3b), the Rad-Tg mice on high-fat diet are significantly different (P < 0.05) from the other groups both for male and female mice. Insulin tolerance testing (ITT), on the other hand, revealed no difference in the blood glucose response between the Rad-Tg and the WT mice on either the normal or high-fat diet (Fig. 4); however, both the control and transgenic strains did exhibit a decreased response to exogenous insulin on the high-fat diet, reflecting increased insulin resistance at these pharmacological levels of hormone.
Fig. 3.
Glucose tolerance tests. (a) Glucose tolerance test of WT (circles) and Rad-Tg (triangles) mice on normal chow (filled symbols) and high-fat (open symbols) diet. The GTTs were performed at the age of 5–6 months after overnight fasting. (b) Area under the curves for GTTs. Each bar represents the mean ± SE of 18–28 mice. *, P < 0.05 for Rad-Tg on high-fat diet vs. the other groups.
Fig. 4.
Insulin tolerance tests. Insulin tolerance tests for WT (circles) and Rad-Tg (triangles) mice on either normal chow (filled symbols) or high fat (open symbols) diets at 5 months of age. Results are expressed as mean percent of basal blood glucose concentration ± SEM from at least seven mice of each group.
Isolated Muscle Studies of Glucose and Amino Acid Transport.
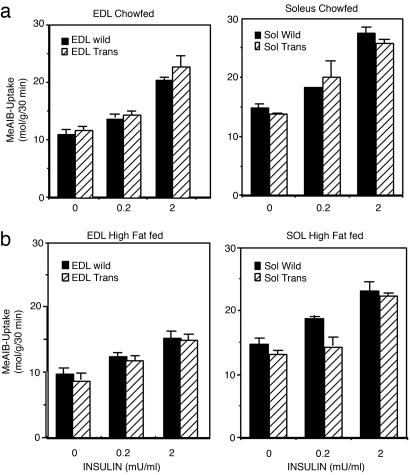
To more precisely evaluate skeletal muscle insulin resistance, isolated skeletal muscles was taken from WT mice and Rad-Tg mice and studied in vitro. On normal diet, insulin increased 2-deoxy-glucose (2-DOG) uptake in a dose-dependent manner, reaching 2- to 3-fold stimulation with the maximal insulin concentration (2 milliunits/ml) both in the soleus and extensor digitorum longis (EDL) muscles of WT mice (Fig. 5a). After high-fat diet, 2-DOG uptake into the EDL, but not the soleus, was reduced both in the basal state and at all insulin concentrations. The 2-DOG uptake dose–response curves in the Rad-Tg mice were similar to those of WT mice when fed with normal diet (Fig. 5a). However, the effect of high-fat diet to induce insulin resistance was greater in the Rad-Tg mice, as demonstrated by a more prominent reduction of insulin-stimulated glucose uptake and a rightward shift of the dose-response curve both in soleus and EDL (Fig. 5b). In the EDL, this insulin resistance was overcome at higher levels of insulin, but with the soleus muscle, there was reduced glucose transport in muscle of the Rad-Tg mice even at the highest insulin concentration tested (Fig. 5b). Rad overexpression did not change basal glucose transport into the muscles. The difference between Rad-Tg and WT mice was also more prominent in muscle of male mice, as compared to females (data not shown).
Fig. 5.
Glucose transport in isolated muscles. (a) Glucose transport in isolated muscles from WT (open circle) and Rad-Tg (filled circle) fed a normal diet. Results are expressed as mean ± SEM of muscles from at least five mice. (b) Glucose transport on high-fat diet. Results are from at least five mice.
Similar to its effect on glucose transport, high-fat diet causes a reduction of insulin stimulated amino acid transport in muscle (Fig. 6). This effect was also more prominent in the EDL than in soleus muscle. However, in contrast to the glucose transport, no significant difference could be detected between Rad-Tg and WT mice with regards to amino acid transport both in the basal and insulin-stimulated states and both on normal and high-fat diets (Fig. 6).
Fig. 6.
Amino acid transport into isolated muscles. (a) Amino acid transport in isolated muscles from WT (black bars) and Rad-Tg (hatched bars) fed a normal diet. Results are expressed as mean ± SEM of muscle from at least five male mice. (b) Amino acid transport on high-fat diet. Results are from at least five replicate studies.
Effects of Rad Overexpression on Lipid Metabolism.
In the fed and fasting state, Rad-Tg mice have lower triglycerides level than controls by 7–12% (Fig. 7a). This effect was statistically significant (P < 0.05) for both males and females. As previously observed in rodents, after 14–22 weeks on a high-fat diet, triglyceride levels were decreased by ≈12% (P ≤ 0.01). This effect was further exaggerated by Rad overexpression and was additive with the effect of high-fat diet. Free fatty acid (FFA) levels were not significantly different between the Rad-Tg and the WT mice on the normal diet, on the high-fat diet FFA levels in both decreased in parallel (Fig. 7b). Cholesterol levels were similar in all of the groups (data not shown).
Fig. 7.
Triglyceride and free fatty acid levels. (a) Fasting triglyceride levels of WT (black bars) and Rad-Tg (gray bars) mice on normal chow and high-fat diets at 5–6 months of age. Each bar represents the mean from 8–40 mice ± SE. *, P < 0.05 for the combined effect of diet and Rad overexpression. (b) FFA levels after overnight fasting at the same age. Each bar represents the mean of 9–18 mice ± SE.
To elucidate the mechanism by which Rad overexpression might cause lower triglyceride levels, thigh muscles were taken from fasted male mice and were immunoblotted with anti-lipoprotein-lipase antibody. The level of lipoprotein lipase (LPL) protein in these muscles was found to be increased by both Rad overexpression and high-fat diet, and this effect was additive with much higher levels when these two factors were combined (Fig. 8). The level of Rad expression by Western blotting was also slightly increased by high-fat diet (data not shown), but this effect was hard to quantitate due to the low level of Rad in muscle of the nontransgenic mice.
Fig. 8.
LPL level in muscle. (a) Western blot analysis of LPL level in muscle. Thigh muscles were taken from mice after overnight fasting, homogenized, and blotted with anti-LPL antibody. (b) Quantitative analysis of LPL level in muscle by using imagequant. The data are expressed as mean ± SEM of Western blots from at least three mice.
Discussion
Rad is a prototypic member of the RGK family of Ras-related GTPases, which also includes Gem/Kir, Rem, and Rem2 (1, 15, 16). Members of this family share conserved GTP binding domains regions with Ras, but are extended at both the N and C termini and lack the C-terminal CAAX motif involved in prenylation of other Ras family members (9). Rad was originally found to be overexpressed in skeletal muscle of a patient with type 2 diabetes mellitus using subtraction cloning (1). Although two larger human studies (3, 4) could not confirm a direct association between the Rad level and diabetes, Garvey et al. (3) did find a positive correlation between the level of Rad in skeletal muscle and body–mass index or percentage of body fat. In addition, genetic studies in two of three different Caucasian populations found a possible association between trinucleotide-repeat polymorphism of the region of the Rad gene and type 2 diabetes (7, 8, 17), and in a Chinese population, a novel Rad gene polymorphism was found to be associated with an increased risk for type 2 diabetes when combined with obesity (18). At the cellular level, previous work by our laboratory has demonstrated a reduction of insulin stimulated glucose uptake in both cultured adipocytes and muscle cells overexpressing Rad, consistent with a role in insulin resistance (6).
Whether the change in Rad expression seen in some populations with diabetes is primary or secondary is unclear; however, several studies have shown that Rad expression in muscle and other tissues can be acutely regulated by insulin and other factors. In vivo insulin infusion in the context of a euglycemic clamp produces a 2- to 3-fold increase in Rad mRNA level within 3 h (5). Rad expression in vascular smooth muscle cells is highly induced by platelet-derived growth factor, TNF-α, and balloon injury of the endothelium (12). Rad is also up-regulated in regenerating limb muscle of the newt (19), in some human breast cancers (20), and in human peripheral blood mononuclear cells in response to acute heat shock (21). Thus, there are a number of normal and pathological situations in which Rad expression is increased.
Because insulin resistance in muscle is a major component of the pathophysiology of type 2 diabetes (13) and Rad is overexpressed in skeletal muscle of some patients with type 2 diabetes, we decided to determine the effect of Rad overexpression in vivo by creating of a transgenic mouse overexpressing Rad specifically in muscle; this was achieved by using the MCK promoter to drive the full-length human Rad gene in the transgenic mice. This promoter is known to be highly expressed in skeletal muscle, weakly expressed in heart and not expressed in other nonmuscle tissues (22). In the mouse, MCK expression begins at embryonic day 17, reaches maximal level at postnatal day 10, and remains high throughout the rest of life. Not surprisingly, the level of Rad protein in skeletal muscles of the Tg mice driven by the MCK promoter was 6–12 times higher than the level in WT mice, but in a range observed in some diabetic patients.
Rad-Tg mice exhibit normal growth and development and normal glucose homeostasis and insulin sensitivity, as measured by fasted and fed glucose and insulin levels, glucose tolerance testing and insulin tolerance testing. However, when fed a high-fat diet, Rad-Tg mice become more insulin resistant and glucose intolerant than normal mice on the same diet. This was clearly shown by the area under the curve of the GTT and further supported by the finding of insulin resistance in isolated muscle glucose transport studies of male mice. The combination of high-fat diet and Rad overexpression causes more severely diminished insulin-stimulated glucose uptake than high-fat diet alone, even though Rad overexpression alone did not change glucose transport. Thus, Rad overexpression interacts with high-fat diet to worsen insulin resistance in muscle. This finding is consistent with clinical studies suggesting that Rad may interact with obesity in increasing diabetes risk (3, 18) and is an example of how a genetic factor (Rad overexpression) can act together with an environmental factor (high-fat diet and obesity) to alter glucose homeostasis. Although this was not accompanied by a change in the ITT, this is not surprising because, in mice, the ITT is rather insensitive to muscle insulin resistance and even mice with a muscle specific insulin receptor knockout (MIRKO) mice exhibit normal ITTs, despite having severe insulin resistance as measured by 2-DOG uptake in isolated muscles (22).
The insulin resistance in isolated muscle of the Rad-Tg mice on high-fat diet was specific to glucose transport. Thus, insulin-stimulated amino acid transport remains normal in these mice, indicating that the effect of Rad to cause this resistance lies somewhere in the insulin signaling pathway after the divergence between signals to glucose transport and amino acid transport. This finding is similar to previous in vitro cell culture studies (6), which demonstrated that overexpression of Rad in muscle or fat could cause a block in insulin stimulated glucose transport with no change in early insulin signaling events, such as insulin receptor or substrate (IRS) phosphorylation or phosphatidylinositol 3-kinase activity.
In addition to its effects on muscle glucose uptake, Rad overexpression in muscle had effects on whole body lipid metabolism. Both male and female Rad-Tg mice had lower plasma triglyceride level as compared to WT mice both on normal and high-fat diets. For the mice fed with high-fat diet, Rad has an additional effect to that of the high-fat diet to lower triglyceride levels. The level of triglyceride at fasting reflects the balance between triglyceride production, mostly by the liver, and triglyceride breakdown by the enzyme lipoprotein lipase. This enzyme is produced by parenchymal cells of many tissues and is then secreted and transported to the luminal surface of vascular endothelium, where it functions to breakdown lipoproteins (23). In agreement with the plasma triglyceride level, the LPL protein level was found to be increased by high-fat diet and by Rad overexpression, and to be even further increased when combining both effects. This may imply two different pathways (high-fat diet and Rad) that increase LPL expression.
Interestingly, there was no significant difference in free fatty acids, a product of triglyceride breakdown, between WT and Rad-Tg on either normal or high-fat diet. High-fat diet itself tended to lower FFA acid levels, a phenomenon also observed by others (24). This finding is somewhat surprising because high-fat diet causes more triglyceride breakdown. The explanation may be that when more triglycerides are being broken down by the muscle LPL, more FFA are being driven into the muscles for energy use and storage. At the same time the hyperinsulinemia that accompanies this condition inhibits lipolysis in the adipocytes and causes less FFA to be released from the adipocytes into the circulation. The effect of insulin on the liver to inhibit triglyceride production and secretion (25) may also play a role in lowering the triglyceride level. The level of the high-fat diet-induced insulin resistance in these tissues may be different (26), causing more muscle insulin resistance, whereas inhibition of lipolysis can still be significant. Rad-Tg mice have normal or even slightly lower insulin levels than WT mice, and this may also contribute to the fact that FFA levels are not lower than WT mice on the same diet.
It is interesting to compare the Rad-Tg mice with mice overexpressing LPL in the muscles described by Jensen et al. (24). These investigators found that high-fat diet causes a reduction of triglyceride and FFA level, and that similar effects are observed in mice overexpressing LPL in the muscles. In their transgenic mice, however, adding high-fat diet did not cause an additional effect, suggesting that the effect of high-fat diet alone to cause these changes was likely mediated through LPL itself. Unlike our mice, overexpression of LPL did reduce the FFA levels, but this was accompanied by some increase of insulin level (a condition similar to high-fat diet feeding). This hyperinsulinemia is probably secondary to other effects of LPL overexpression. Interestingly, mice that overexpress LPL in muscle were found to be protected from high-fat diet-induced weight gain. We observed this phenomenon in two of our three study groups, and it was more prominent in males (data not shown). However, in one study group, this was not the case, so that the final results did not show a significant difference in weight gain between the transgenic and WT mice. It is possible that another yet unknown factor may influence these effects of Rad or LPL overexpression. In any case, activating triglyceride breakdown by muscle LPL will produce a higher level of FFA in the muscle, and this may further contribute to the insulin resistance (27–30, ‖ Exactly how Rad induce these changes in glucose and lipid metabolism is still unclear. At the molecular level, Rad and other members of the RGK family have been shown to associate with cytoskeletal proteins, 14–3-3 proteins and calmodulin-related proteins (10, 11, 31). Rad has also been shown to interact with the NDP kinase and putative tumor metastasis suppressor nm23, and this interaction promotes conversion of Rad-GDP to Rad-GTP, providing a unique mechanism of GTPase regulation (32). In neural cells, RGK proteins have been shown to regulate voltage-dependent Ca2+ channel activity and cell-shape remodeling (16). In these cells, both Gem and Rad have also been shown to interface with the Rho pathway through association with the Rho kinase (ROK) alpha and beta (33), and recently, Fu et al. (12) demonstrated that Rad inhibits the attachment/migration of vascular smooth muscle cells and reduces the formation of focal contacts and stress fibers by blocking the Rho/ROK signaling pathway. This finding is interesting in light of recent data suggesting that Rho kinase may play a role in insulin stimulation of glucose transport in skeletal muscle, via interaction with IRS-1 (34). Insulin-stimulated glucose transport in muscle has also been shown to involve cortical actin remodeling in a pathway including the Rho related GTPase TC10 (35).
In summary, transgenic mice overexpressing Rad specifically in muscle have lower plasma triglyceride levels than control mice on both normal and high-fat diets, probably secondary to an increase in muscle LPL expression. These mice also exhibit insulin resistance in muscle and mild glucose intolerance, but are not “clinically” insulin resistant as measured by ITT. Type 2 diabetes mellitus is a polygenic disease, but many environmental factors including obesity, can affect its course. The finding that Rad together with high-fat diet can affect glucose homeostasis further supports the possibility that Rad may be one of the many genes contributing to the development of diabetes.
Methods
Construction of the Rad Transgene and Rad Transgenic Mice.
A vector containing the muscle creatine kinase (MCK) promoter (22) and the human Rad gene was used to create the transgene construct as illustrated in Fig. 1a and introduced into pronuclei of fertilized FVB mouse embryos. Mouse-tail DNA was isolated by using phenol/chloroform extraction and subjected to restriction digest with EcoRI. A 1.1-kb BamHI–EcoRI fragment from the 3′ end of the plasmid MCK promoter was used as a probe for Southern blotting. This resulted in a single 3.3-kb band in transgenic mice versus vs. no band in controls. In later studies, genotyping was conducted by PCR using primers specific for the human Rad gene (5′-ACTGGCCCGAGGACTCCGAG and 3′-AATTCCACAGTATTGGCCAGGTGC).
Animal Studies.
Mice were housed in pathogen-free animal facilities at The Joslin Diabetes Center (Boston, MA) and Brandies University (Waltham, MA). All animal protocols were approved by the Animal Care Committee of the Joslin Diabetes Center and were in accordance with National Institutes of Health guidelines. The physiologic studies were conducted on three cohorts of mice, each containing between 65 and 120 mice. Each cohort was divided into eight subgroups by gender, presence, or absence of the Rad-Tg and normal versus high-fat diet. The normal diet contained 9% fat by weight (16.5% of calories) (Mouse chow 9F, Purina, St. Louis), whereas the high-fat diet had 29% fat by weight (≈55% of calories) [Modified 9F Purina chow]. The study groups were created by mating Rad-Tg with WT FVB mice, so that the Rad-Tg were all heterozygote for the transgene. At 3 weeks of age, mice were weaned and genotyped. High-fat diet was started at the 6 weeks of age for the high-fat diet groups and maintained 14–22 weeks.
Physiologic Studies and Analytical Procedures.
Fasted blood samples were taken after overnight fasting (12–16 h); fed samples were drawn between 9–11 a.m. Blood glucose values were determined from whole venous blood by using a glucometer (One Touch II, Lifescan). Insulin levels were measured by RIA (Linco) using rat insulin as a standard or ELISA (Crystal Chem) using mouse standards. Cholesterol and triglyceride levels in serum were measured by colorimetric enzyme assay in a Beckman CX7 spectrophotometer, except for the third study group in which triglycerides were measured by using the Triglyceride 10 kit (Sigma). FFA levels were analyzed by using NEFA-C FFA kits (Wako). Insulin and glucose tolerance tests (ITT and GTT, respectively) were performed on animals that had been fasted overnight. Animals were injected with either 0.75 unit/kg body weight of human regular insulin (Lilly) or 2 g/kg body weight of glucose into the peritoneal cavity.
Glucose and amino acid transport was measured in isolated muscles as described by using 2-[3H]-2-deoxy-d-glucose (36) and the nonmetabolizable amino acid analog, 2-(methylamino)isobutyric acid (MeAIB) (37).
Immunoprecipitation and Western Blot Analysis.
Tissues were removed and homogenized in homogenization buffer (50 mM Hepes, pH 7.4/1% Triton X-100/50 mM sodium pyrophosphate/0.1 M NaF/10 mM EDTA/10 mM sodium orthovanadate/10 μg/ml leupeptin/2 mM benzamidine/2 mM phenylmethylsulfonyl fluoride) with a Polytron homogenizer. Particulate matter was removed by centrifugation at 47,000 rpm (140,000 × g) for 1 h at 4°C in a Beckman Ti-70 rotor. The lysates were used either for immunoprecipitation with Rad antiserum (500 μg of protein) or for direct (200 μg of protein) immunoblotting with Rad antiserum as described (3). For LPL, 100 μg of protein was subjected to SDS/PAGE and immunoblotted with 5D2 monoclonal anti-LPL antibody (39) kindly provided by J. Brunzell (University of Washington, Seattle). The results were quantified by using imagequant software.
Statistical Analysis.
All values are expressed as mean ± SEM. A two-tailed Student’s unpaired t test was used to analyze the results, except for the triglyceride levels, which were analyzed by using the general linear models procedure (SAS Software) to assess the interaction between the effects of Rad and high-fat diet.
Acknowledgments
We thank M. Petruzelli, M. Ginsberg, J. N. Winnay, and J. O’Keefe for technical assistance and T. L. Azar, K. Frayjo, and J. Konigsberg for secretarial assistance. This work was supported by National Institutes of Health Grants DK45935 (to C.R.K.) and Joslin’s Diabetes and Endocrinology Research Center Grant DK36836.
Abbreviations
- tg
transgenic
- GTT
glucose tolerance test
- ITT
insulin tolerance test
- 2-DOG
2-deoxy-glucose
- EDL
extensor digitorum longis
- FFA
free fatty acid
- LPL
lipoprotein lipase.
Footnotes
Conflict of interest statement: No conflicts declared.
‖Zisman, A., Peroni, O. D., Abel, E. D., Michael, M. D., Ilany, J., Lowell, B. B., Kahn, C. R. & Kahn, B. B. (1999) Diabetes 48, Suppl. 1, A10 (abstr.).
References
- 1.Reynet C., Kahn C. R. Science. 1993;262:1441–1444. doi: 10.1126/science.8248782. [DOI] [PubMed] [Google Scholar]
- 2.Moller D. E., Bjørbæk C., Vidal-Puig A. Diabetes Care. 1996;19:396–400. doi: 10.2337/diacare.19.4.396. [DOI] [PubMed] [Google Scholar]
- 3.Garvey W. T., Maianu L., Kennedy A., Wallace P., Ganaway E., Hamacher L. L., Yarnall D. P., Lenhard J. M., Burns D. K. Diabetes. 1997;46:444–450. doi: 10.2337/diab.46.3.444. [DOI] [PubMed] [Google Scholar]
- 4.Paulik M. A., Hamacher L. L., Yarnall D. P., Simmons C. J., Maianu L., Pratley R. E., Garvey W. T., Burns D. K., Lenhard J. M. J. Cell Biochem. 1997;65:527–541. [PubMed] [Google Scholar]
- 5.Laville M., Auboeuf D., Khalfallah Y., Vega N., Riou J. P., Vidal H. J. Clin. Invest. 1996;98:43–49. doi: 10.1172/JCI118775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moyers J. S., Bilan P. J., Reynet C., Kahn C. R. J. Biol. Chem. 1996;271:23111–23116. doi: 10.1074/jbc.271.38.23111. [DOI] [PubMed] [Google Scholar]
- 7.Doria A., Caldwell J. S., Ji L., Reynet C., Rich S. S., Weremovicz S., Morton C. C., Warram J. H., Kahn C. R., Krolewski A. S. Diabetes. 1995;44:243–247. doi: 10.2337/diab.44.2.243. [DOI] [PubMed] [Google Scholar]
- 8.Yuan X., Yamada K., Ishiyama-Shigemoto S., Koyama W., Nonaka K. Metabolism. 1999;48:173–175. doi: 10.1016/s0026-0495(99)90029-x. [DOI] [PubMed] [Google Scholar]
- 9.Bilan P. J., Moyers J. S., Kahn C. R. Exp. Cell Res. 1998;242:391–400. doi: 10.1006/excr.1998.4092. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J., Bilan P. J., Moyers J. S., Antonetti D. A., Kahn C. R. J. Biol. Chem. 1996;271:768–773. doi: 10.1074/jbc.271.2.768. [DOI] [PubMed] [Google Scholar]
- 11.Moyers J. S., Bilan P. J., Zhu J., Kahn C. R. J. Biol. Chem. 1997;272:11832–11839. doi: 10.1074/jbc.272.18.11832. [DOI] [PubMed] [Google Scholar]
- 12.Fu M., Zhang J., Tseng Y. H., Cui T., Zhu X., Xiao Y., Mou Y., De Leon H., Chang M. M., Hamamori Y., et al. Cirulation. 2005;111:1071–1077. doi: 10.1161/01.CIR.0000156439.55349.AD. [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo R. A. Diabetes Rev. 1997;5:177–269. [Google Scholar]
- 14.Donoviel D. B., Shield M. A., Buskin J. N., Haugen H. S., Clegg C. H., Hauschka S. D. Mol. Cell. Biol. 1996;16:1649–1658. doi: 10.1128/mcb.16.4.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maguire J., Santoro T., Jensen P., Siebenlist U., Yewdell J., Kelly K. Science. 1994;265:241–244. doi: 10.1126/science.7912851. [DOI] [PubMed] [Google Scholar]
- 16.Chen H., Puhl H. L., III, Niu S. L., Mitchell D. C., Ikeda S. R. J. Neurosci. 2005;25:9762–9772. doi: 10.1523/JNEUROSCI.3111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orho M., Carlsson M., Kanninen T., Groop L. C. Diabetes. 1996;45:429–433. doi: 10.2337/diab.45.4.429. [DOI] [PubMed] [Google Scholar]
- 18.Wang G. Y., Niu T. H., Chen C. Z., Li Q. F., Xu X. P. Chin. Med. J. (Engl.) 2004;117:770–771. [PubMed] [Google Scholar]
- 19.Shimizu-Nishikawa K., Tsuji S., Yoshizato K. Dev. Dyn. 2001;220:74–86. doi: 10.1002/1097-0177(20010101)220:1<74::AID-DVDY1090>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 20.Tseng Y. H., Vicent D., Zhu J., Niu Y., Adeyinka A., Moyers J. S., Watson P. H., Kahn C. R. Cancer Res. 2001;61:2071–2079. [PubMed] [Google Scholar]
- 21.Sonna L. A., Gaffin S. L., Pratt R. E., Cullivan M. L., Angel K. C., Lilly C. M. J. Appl. Physiol. 2002;92:2208–2220. doi: 10.1152/japplphysiol.01002.2001. [DOI] [PubMed] [Google Scholar]
- 22.Bruning J. C., Michael M. D., Winnay J. N., Hayashi T., Horsch D., Accili D., Goodyear L. J., Kahn C. R. Mol. Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 23.Eckel R. H. N. Engl. J. Med. 1989;320:1060–1068. doi: 10.1056/NEJM198904203201607. [DOI] [PubMed] [Google Scholar]
- 24.Jensen D. R., Schlaepfer I. R., Morin C. L., Pennington D. S., Marcell T., Ammon S. M., Gutierrez-Hartmann A., Eckel R. H. Am. J. Physiol. 1997;273:R683–R689. doi: 10.1152/ajpregu.1997.273.2.R683. [DOI] [PubMed] [Google Scholar]
- 25.Lewis G. F., Steiner G. Diabetes Care. 1996;19:390–393. doi: 10.2337/diacare.19.4.390. [DOI] [PubMed] [Google Scholar]
- 26.Wilkes J. J., Bonen A., Bell R. C. Am. J. Physiol. 1998;275:E679–E686. doi: 10.1152/ajpendo.1998.275.4.E679. [DOI] [PubMed] [Google Scholar]
- 27.Boden G. Diabetes Care. 1996;19:394–395. doi: 10.2337/diacare.19.4.394. [DOI] [PubMed] [Google Scholar]
- 28.Ren J. M., Marshall B. A., Mueckler M. M., McCaleb M., Amatruda J. M., Shulman G. I. J. Clin. Invest. 1995;95:429–432. doi: 10.1172/JCI117673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall B. A., Ren J. M., Johnson D. W., Gibbs E. M., Lillquist J. S., Soeller W. C., Holloszy J. O., Mueckler M. J. Biol. Chem. 1993;268:18442–18445. [PubMed] [Google Scholar]
- 30.Kim J. K., Fillmore J. J., Chen Y., Yu C., Moore I. K., Pypaert M., Lutz E. P., Kako Y., Velez-Carrasco W., Goldberg I. J., et al. Proc. Natl. Acad. Sci. USA. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beguin P., Mahalakshmi R. N., Nagashima K., Cher D. H., Kuwamura N., Yamada Y., Seino Y., Hunziker W. Biochem. J. 2005;390:67–75. doi: 10.1042/BJ20050414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J., Tseng Y. H., Kantor J. D., Rhodes C. J., Zetter B. R., Moyers J. S., Kahn C. R. Proc. Natl. Acad. Sci. USA. 1999;96:14911–14918. doi: 10.1073/pnas.96.26.14911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward Y., Yap S. F., Ravichandran V., Matsumura F., Ito M., Spinelli B., Kelly K. J. Cell Biol. 2002;157:291–302. doi: 10.1083/jcb.200111026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furukawa N., Ongusaha P., Jahng W. J., Araki K., Choi C. S., Kim H. J., Lee Y. H., Kaibuchi K., Kahn B. B., Masuzaki H., et al. Cell Metab. 2005;2:119–129. doi: 10.1016/j.cmet.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 35.JeBailey L., Rudich A., Huang X., Ciano-Oliveira C., Kapus A., Klip A. Mol. Endocrinol. 2004;18:359–372. doi: 10.1210/me.2003-0294. [DOI] [PubMed] [Google Scholar]
- 36.Kapur S., Bédard S., Marcotte B., Côte C., Marette A. Diabetes. 1997;46:1691–1700. doi: 10.2337/diab.46.11.1691. [DOI] [PubMed] [Google Scholar]
- 37.Guma A., Mora C., Santalucia T., Vinals F., Testar X., Palacin M., Zorzano A. FEBS Lett. 1992;310:51–54. doi: 10.1016/0014-5793(92)81144-b. [DOI] [PubMed] [Google Scholar]
- 38.Peterson J., Fujimoto W. Y., Brunzell J. D. J. Lipid. Res. 1992;33:1165–1170. [PubMed] [Google Scholar]