Abstract
Lower termites express a unique form of eusocial polyphenism in that totipotent workers can differentiate into either soldier or reproductive caste phenotypes. In this initial effort using RNA interference in termites, we found that two hexamerin genes, Hex-1 and Hex-2, participate in the regulation of caste polyphenism. Our methodology involved a dual gene-silencing approach that used a single short-interfering RNA fragment to silence the two homologous hexamerin genes. We performed validation studies that evaluated effects on nontarget housekeeping genes, silencing of a nonhousekeeping control gene, and effects at the protein level. We found that the two hexamerin proteins, which are inducible by the morphogenetic juvenile hormone and which constitute a significant proportion of total termite protein, suppress juvenile-hormone-dependent worker differentiation to the soldier caste phenotype. This mechanism allows termite colonies to retain high proportions of altruistic worker caste members, thus apparently enhancing colony-inclusive fitness. These findings demonstrate a unique status quo regulatory mechanism for termite worker caste retention and provide an example of previously undescribed preadult developmental/caste-regulatory genes from any social insect.
Keywords: sociogenomics, RNA interference, short-interfering RNA, juvenile hormone, phenotypic plasticity
Termites (order Isoptera) are the only social insect group that undergoes hemimetabolous (incomplete) metamorphosis. The only other fully eusocial insects occur in the order Hymenoptera (bees, wasps, and ants) and undergo holometabolous (complete) metamorphosis. There are several other major differences between the Isoptera and Hymenoptera (1), most notably (i) isopteran workers can be either sex, whereas worker hymenopterans are always female; and (ii) immature isopterans resemble adults and are included in the worker caste, whereas immature hymenopterans require continual care by adult workers. For these reasons, it is believed that eusociality evolved independently in the Isoptera and Hymenoptera (2, 3). The order Isoptera is divided into the lower and higher termites on the basis of two distinguishing characteristics: the dependence of lower termites on protozoan symbionts for cellulose digestion and the adult status of workers in higher termites, which precludes further differentiation by workers. In lower termites such as the Reticulitermes, the worker caste is a highly plastic, totipotent immature stage.
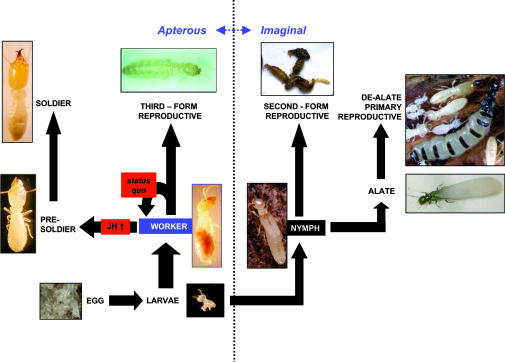
Termite caste differentiation is a postembryonic developmental process that is the result of differential gene expression (4–7). Lower termites such as the Reticulitermes undergo a caste differentiation process that is truly unique among the social insects (see Fig. 1 and ref. 8). The specific behaviors of the three termite caste phenotypes (worker, reproductive, and soldier) include foraging, tunneling, and brood tending (worker); the production of offspring (reproductive); and colony defense (soldier). Reticulitermes workers have three developmental potentials that include status quo molts in which they remain as workers, differentiation into the single-instar presoldier stage (followed immediately by molting into the soldier caste), or differentiation into apterous and eyeless neotenic reproductives. The morphogenesis of workers to presoldier and soldier caste phenotypes is induced by two- to fivefold increases in titers of the insect morphogenetic hormone, juvenile hormone (JH) III (9, 12). Therefore, socioregulatory factors would be expected to exist that maintain a status quo workforce via modulation of JH production [e.g., JH-suppressive allatostatins (13)], or that in some way buffer JH action downstream of its synthesis. In the present report, we focus on the latter factor.
Fig. 1.
Caste differentiation and development in Reticulitermes termites can follow either imaginal (winged) or apterous (wingless) routes (see also ref. 8). All phenotypes except alates and the three reproductive phenotypes are considered immature in lower termites. The initial developmental divergence point occurs in the last larval instar, in which workers or nymphs differentiate. Nymphs have two commonly observed developmental potentials: (i) differentiation into fully winged and eyed adult alates that disperse, mate, and become primary reproductives that head colonies or (ii) differentiation into rudimentary-winged and -eyed, nondispersive second-form reproductives that supplement the reproductive capacity within colonies. Workers have three developmental potentials: (i) status quo molts in which they remain as workers, (ii) differentiation into the single-instar presoldier stage (followed immediately by molting into the soldier caste), or (iii) differentiation into apterous and eyeless third-form reproductives that assume identical functions as second-form reproductives. JH plays a key role in presoldier differentiation and worker caste maintenance (9, 12). At high titers, JH induces presoldier differentiation from workers, whereas at lower titers status quo worker-to-worker molts take place.
With respect to basal and other lower termites, the worker caste is suspended in a reversible, “temporarily altruistic” state (14) in which it can indirectly enhance the overall fitness of the colony through helping behaviors. However, because workers retain the ability to differentiate into reproductives, they have the potential to contribute genes directly to successive generations. Workers also retain the ability to differentiate to soldier caste members that are incapable of feeding and reproduction. Additionally, although the precise mechanism remains unknown, termite soldiers are capable of down-regulating the JH titers of nestmates (9, 12), possibly facilitating reproductive differentiation (12); thus, as postulated by Henderson (15), the soldier caste apparently does serve broader roles beyond defense alone. This plasticity suggests a unique need by termites for specialized physiological adaptations that suppress worker differentiation to other caste phenotypes. However, although efforts to identify specific termite caste-regulatory factors have met with some success (e.g., refs. 16 and 17), no specific genes that regulate caste polyphenism in termites, or in any other eusocial insects, have been reported to date.
Several tools and resources have become available in recent years to study the molecular basis of termite caste differentiation (5, 6, 10, 11, 18). Using these tools, we previously identified two JH-responsive and morphogenesis-associated hexamerin genes that are referred to as Hex-1 and Hex-2 (6, 11). Because hexamerins have well defined roles as JH-binding proteins (19, 20), because of the well established role of JH in termite caste differentiation (15), and on the basis of our observations that one of the hexamerins (Hex-2) is highly induced in response to rising JH titers during presoldier differentiation (11), we sought to further functionally characterize the Reticulitermes flavipes hexamerins. Here, our goal was to use RNA interference (RNAi) to investigate the roles of these two hexamerins in soldier caste differentiation by worker termites. In these studies, we had two alternate hypotheses: (i) presoldier differentiation would be inhibited by hexamerin silencing or (ii) presoldier differentiation would be enhanced. As a result of these efforts, we provide evidence as to how the proteins encoded by these genes may function as part of a major caste-regulatory mechanism.
Results and Discussion
Effects of RNAi on Target Gene Expression.
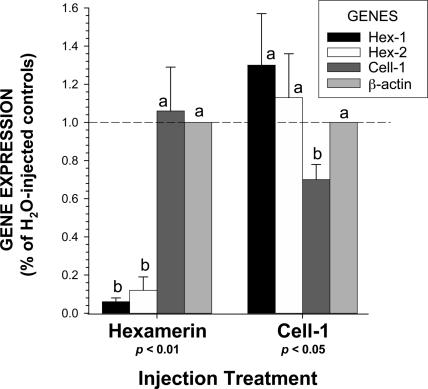
The molecular effects of RNAi on target and control gene expression were investigated by quantitative real-time PCR (qRT-PCR) in individuals sampled 24 h after injection with short-interfering RNA (siRNA) (Figs. 2 and 3). In all gene expression studies, results are reported as the proportion of gene expression in water-injected controls. All expression levels are normalized to β-actin, which has been found to be the least-variable reference gene (see Supporting Text and Table 1, which are published as supporting information on the PNAS web site). Significant silencing of target genes was observed by 24 h after injection (Fig. 3). In the hexamerin RNAi treatment, Hex-1 and Hex-2 were suppressed at statistically identical levels (94% and 88%, respectively); both genes were also unaffected in the Cell-1 control treatment. The control gene Cell-1 was silenced ≈30% in the Cell-1 RNAi treatment and was unaffected in the hexamerin RNAi treatment. The control housekeeping gene, β-actin, was unaffected in both RNAi treatments.
Fig. 2.
Termite worker receiving siRNA injection via the lateral thorax. No significant mortality was observed in association with the injection procedure (n = 48 replicates) relative to uninjected controls (n = 15 replicates; Kruskal–Wallis test; df = 1, P < 0.001).
Fig. 3.
Effects of RNAi on gene expression at 24 h after siRNA injection in the Indiana MSH colony. The graph compares silencing effects on the genes Hex-1, Hex-2, and Cell-1, as well as the control gene, β-actin, between hexamerin and Cell-1 RNAi treatments. Results are expressed as the percentage of gene expression relative to water-injected controls. Means labeled with the same letter (a or b) within each treatment are not significantly different by the Kruskal–Wallis test (n = 5; df = 1; P < 0.01 or P < 0.05). (Error bars represent SEM.)
Previous RNAi studies on the hemolymph protein vitellogenin in honey bees (21, 22) have shown similar levels of silencing to Hex-1 and -2, thus there is precedence for such high-level silencing of fat-body-synthesized hemolymph proteins by the hemocoel RNA injection approach. The modest, but significant, silencing of the Cell-1 gene likely relates to its site of expression. Specifically, the fact that Cell-1 is a protein produced in the salivary glands and secreted into the R. flavipes digestive tract (X.Z., unpublished data) may limit its amenability to silencing by hemocoel siRNA injection. Further studies will be necessary to elucidate such details. Most importantly, (i) substantial levels of silencing were observed for the two target hemolymph protein-encoding genes, Hex-1 and -2, and (ii) no readily apparent off-target effects occur in association with target gene silencing.
Effects of RNAi on Hexamerin Protein Expression.
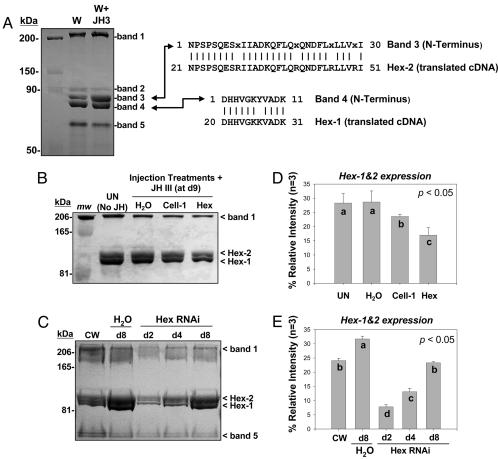
Five major hemolymph proteins are expressed in R. flavipes hemolymph (11). Using SDS/PAGE and N-terminal sequencing, we established the identity of Hex-1 and Hex-2 in the hemolymph (Fig. 4A). Hex-2 was formerly known as hemolymph protein band 3, and Hex-1 corresponds to hemolymph protein band 4 (11). Ongoing studies have shown that the hexamerins occur in both soluble and membrane protein fractions and make up a substantial proportion of total worker protein (32). Previously, we observed that the Hex-2 protein is most readily inducible in JH-treated workers that are undergoing presoldier differentiation (11). This observation led us to originally hypothesize that Hex-2 might participate in presoldier differentiation. The results of the present study, as described below, alternatively support the conclusion that both Hex-1 and Hex-2 induction suppress worker differentiation to other caste phenotypes.
Fig. 4.
SDS/PAGE and densitometric analyses of hemolymph protein expression. (A) The five major hemolymph proteins of workers (bands 1–5), from which band 3 is JH-inducible. Shown at the right are N-terminal peptide sequences aligned with translated Hex-2 and Hex-1 cDNA sequences, minus signal peptides. (B) RNAi effects on hemolymph protein expression in the Indiana MSH colony. Treatments shown are uninjected (UN) and water-injected (H2O) controls vs. RNAi treatments (Cell-1, Hex) after 9 d. The water, Cell-1, and Hex treatments were held with external JH; uninjected controls were not. (C) Time course of hexamerin protein silencing at 2, 4, and 8 d after hexamerin siRNA injection in the Florida CV-20 colony, relative to colony workers (CW) and water-injected controls (H2O). (D and E) Densitometric analyses of replicated gels from treatments shown in B and C, respectively. Bars labeled with the same letter (a, b, c, or d) are not significantly different by the Kruskal–Wallis test (n = 3; P < 0.05; df = 1). (Error bars represent SEM.)
Fig. 4 B and D show the effects of RNAi on hemolymph protein expression at 9 days after injection of hexamerin siRNA. The representative gel shows hexamerin expression among various controls and treatments relative to band 1, which is a putative vitellogenin (11). Fig. 4 C and E show the time course of hexamerin protein silencing and recovery at 2, 4, and 8 days after siRNA injection, relative to all major hemolymph proteins. Combined results from Fig. 4 B–E show pronounced effects on hexamerin protein reduction by hexamerin-targeted RNAi, an increase in hexamerin expression for JH-treated water-injected controls (as expected), but no significant differences between uninjected acetone controls and Cell-1 RNAi treatments. Most notably, these results show that hexamerin protein levels remain attenuated 8–9 days after injection, compared with water-injected controls. On the basis of these findings, as well as the fact that presoldier differentiation takes between 14 and 24 days to reach maximum levels (10, 11), we postulated that attenuated protein expression for >8 days after siRNA injection should be associated with clear phenotypic impacts on caste differentiation. To test this hypothesis, we conducted model caste-differentiation assays and quantified presoldier differentiation between 14 and 25 days after siRNA injection.
Phenotypic Effects of RNAi on Caste Differentiation.
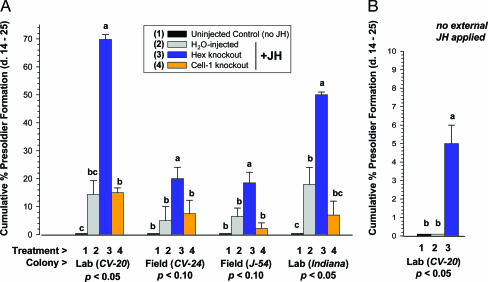
Fig. 5 shows the effects of RNAi on presoldier differentiation for workers from four different colonies. In the phenotypic experiments, we evaluated the same RNAi treatments and controls that were compared above (water, Hex-1 + -2, and Cell-1), as well as uninjected controls. With our injection approach, no significant differences in mortality (P < 0.001) occurred between pooled injected treatments (10.7 ± 2.4% mortality; n = 48 replicates) and pooled noninjected controls (9.9 ± 0.82% mortality; n = 15 replicates). No presoldier differentiation occurred for all four colonies in uninjected controls held without JH (Fig. 5A). For water-injected controls and the control gene (Cell-1) RNAi treatments, all held with external JH, presoldier differentiation occurred at natural levels (i.e., 5–15%). Most notably, significant three- to fivefold elevations of presoldier differentiation occurred in Hex-1 + -2 RNAi treatments relative to all other injection treatments. The presoldier induction levels in the hexamerin RNAi treatments far exceed constitutive soldier caste ratios observed in termite field colonies (e.g., ref. 23). Additionally, all presoldiers induced in association with hexamerin silencing were indistinguishable from wild types; they were morphometrically similar to natural presoldiers and were observed to readily engage in trophallaxis behaviors with workers.
Fig. 5.
Phenotypic effects of RNAi on JH-induced presoldier differentiation by termite workers. These results indicate that the hexamerins modulate or buffer, rather than mediate, the JH signal. (A) Average cumulative presoldier induction between days 14 and 25 after siRNA injection. (B) Hexamerin silencing in workers held without external JH still results in significant presoldier differentiation, but at natural soldier caste proportions. Note differences in scale of y axis between A and B. Within colonies, bars labeled with the same letter (a, b, or c) are not significantly different by the Kruskal–Wallis test (n = 3; df = 1; P < 0.05 or P < 0.10). (Error bars represent SEM.)
Identical trends in JH-induced presoldier differentiation were noted for multiple colonies, including laboratory and field colonies, as well as colonies originating from geographically separate areas (Florida and Indiana). In hexamerin RNAi treatments held without external JH (Fig. 5B), presoldier differentiation was also significantly elevated, but at naturally observed levels (23). This latter finding suggests how the hexamerins may function in caste regulation under natural colony conditions and constitutive JH titers. These findings support that Hex-1 and -2 are part of a suppressive caste-regulatory mechanism, and not an inductive factor, as originally hypothesized.
Summary and Conclusions.
Insect hexamerins are part of a large family of hexameric proteins that are closely linked with diversifying evolution in arthropods (24). Strong evidence suggests that hexamerin-like proteins of terrestrial insects evolved from oxygen-carrying hemocyanins of ancestral aquatic insects and crustaceans (24, 25). In terrestrial insects, the hexamerins have acquired diverse storage (26) and hormone-binding functions (19). Our data suggest a previously undescribed function for termite hexameric storage proteins, which classically occur at high levels during preimaginal stages in most other insects (26). Specifically, the termite hexamerins have been exploited/co-opted during social evolution to inhibit the final worker molt to a terminally developed soldier phenotype. This caste-regulatory mechanism results in colony individuals being temporally arrested in an immature stage wherein they can function as workers. From the perspective of cellulase expression alone (5, 6) (X.Z., unpublished data), maximizing worker numbers would clearly enhance colony nutritional status, and thus overall colony fitness. Moreover, if soldier caste members are truly capable of regulating reproductive differentiation (12, 15), the hexamerin mechanism would take on even broader importance for maintaining status quo colony conditions.
It has been hypothesized that caste differentiation in paper wasps evolved through exploitation of a developmental diapause switch (27), and that the foraging division of labor in the honey bee has evolved through co-option of a reproductive genetic network that includes the vitellogenin protein (28). Our data show that the developmental trait of hexamerin expression/accumulation specifically has been co-opted in termites to regulate phenotypic plasticity, and as a result, directly influences caste formation and composition. In light of our findings, it would be highly interesting to test whether hexamerins have been recruited as JH-binding, caste-regulatory factors in the hymenopteran social insects noted above, or as polyphenism-regulatory factors in other nonsocial insects (29, 30).
Although the exact mechanism by which the hexamerins attenuate JH-dependent morphogenesis remains unknown, we propose two possibilities relating to JH sequestration and nutrition for further consideration. First, based on the finding that a closely related locust hexamerin serves as a JH-binding protein (19), it is possible that the termite hexamerins are capable of JH sequestration. In agreement with this, we have observed that the Hex-1 protein is recognized by the anti-JH antiserum described by Goodman et al. (31) and that immunoreactivity is reduced in the presence of competing JH and after stripping with apolar solvents (32). These observations agree with the earlier finding that RNAi-based silencing of honey bee vitellogenin, a putative JH-binding hemolymph protein, leads to increases in JH titers (22). Further, in higher insects, hexamerins have the ability to interact with homologous receptor proteins and enter fat body cells via receptor-mediated endocytosis (26). Thus, one hypothesis is that the R. flavipes hexamerins are part of a mechanism that sequesters JH in the fat body, away from membrane/nuclear JH receptors that activate morphogenesis. To conclusively test the JH sequestration hypothesis, additional research will be necessary. For this purpose, we propose a testable model whereby (i) presoldier morphogenesis ensues when JH titers exceed the binding/sequestration capacity of the hexamerin mechanism or (ii) status quo worker-to-worker molts occur when the hexamerin mechanism can successfully sequester all available JH (see Fig. 1). To take the JH model one step further, it would be interesting to test whether the hexamerins play a role in regulating third-form reproductive differentiation from workers, or nymphal differentiation from larvae (see Fig. 1 for pathways).
A second hypothesis is that the hexamerins serve as a signaling mechanism for nutritional status, and that presoldier caste differentiation is suppressed when certain nutritional requirements are met. This hypothesis is also well supported because most insect hexamerins identified to date are up-regulated during periods of active larval feeding (i.e., storage) and also participate in nutrient release during periods of metamorphosis and/or diapause (26). Studies are under way to test both the JH-sequestration and nutritional hypotheses.
In conclusion, we have reported here on the elucidation of the hexamerin genes Hex-1 and Hex-2 as examples of caste-regulatory genes from a termite. A mechanism of this kind has not been previously described in a social insect. Our findings are highly significant from two perspectives. First, these findings provide insight into how termites have answered the significant eusocial challenge of worker caste maximization/retention. Without question, workforce maximization enhances inclusive fitness (14) and, therefore, must be centrally important to the evolution of present-day termite eusociality (3). Second, these findings represent the transition into a new era of sociogenomics research (33) in the highly underrepresented eusocial insect order Isoptera. This demonstration of RNAi as a viable, functional genomic tool for use in termites suggests that it will be relatively straightforward to conduct future characterization of dozens of genes identified in our laboratory (5, 6, 18) (X.Z., unpublished data), as well as in other laboratories worldwide (e.g., refs. 4–7, 18, 34, 35). In coming years, our understanding of the molecular basis of termite caste differentiation and caste polyphenism is almost certain to grow by leaps and bounds.
Materials and Methods
Termites.
Termites designated as field colonies were collected from field sites within 1–2 weeks of use in our experiments; those designated as laboratory colonies were maintained in the laboratory for >6 months before inclusion in the experiments. The three Florida colonies (CV-20, CV-24, and J-54) were collected within 1–3 miles (1.6–4.8 km) of each other on the University of Florida campus (Gainesville). The Indiana colony (MSH) was collected ≈1,000 miles (1,609 km) distant on the Purdue University campus (West Lafayette, IN). All termites were held in darkness at 24°C and 70 ± 5% relative humidity.
RNAi.
Double-stranded siRNA was synthesized by using a commercially available kit (Silencer; Ambion, Austin, TX). Although extensive studies have shown that siRNAs are highly specific (36, 37), we minimized the possibility of nontarget effects by designing siRNAs to have virtually no sequence similarity to ≈300 known genes/ESTs from R. flavipes, or to other nonhexamerin genes deposited in GenBank. dsRNA templates corresponded to nonhomologous, ≈500-bp portions from the ORFs of the two genes Hex-2 and Cell-1 (see Fig. 6, which is published as supporting information on the PNAS web site). Because there is ≈50% sequence identity between Hex-1 and Hex-2 in the RNAi-targeted region, the Hex-2 RNAi fragment also allowed for the concurrent silencing of Hex-1. The template cDNAs were amplified by PCR primers that had T7 RNA polymerase sequences (TAATACGACTCACTATAGGG) appended to their 5′ ends. The dsRNA-template PCR primers (shown 5′ to 3′) were as follows: Hex-2 (forward, ATACGCCAATGGACAGGAAG; reverse, GCGCTTGAGGATTTGGTAGT) and Cell-1 (forward, AGACATGACGATGTCCAGACC; reverse, GACCCTTGGGTGTCTTCTTCT).
After synthesis, the 500-bp dsRNAs were digested into siRNAs (15–25 bp) with RNase III. siRNAs corresponding to Hex-1 + -2 and Cell-1 were diluted individually in nuclease-free water to 15 pg/nl for injection into worker termites. The siRNAs (500 pg) were injected into the side of the thorax by using a microinjector fitted with a custom-pulled borosilicate glass needle, and with the assistance of a custom vacuum manifold that facilitated termite immobilization (Fig. 2). Controls were injected with an equivalent volume of nanopure water alone.
JH Bioassays.
After siRNA injection, replicated groups of 15 termites were subjected to model JH bioassays as described previously (10, 11). Briefly, termites were placed on filter papers treated with 150 μg of JH III in acetone (Sigma) and 150 μl of deionized water, within 30-mm Petri dishes. It is customary that presoldier emergence begins on bioassay days 14–15 and plateaus by day 25; thus, assays are typically run for 25 d. Mortality and presoldier formation were scored every 5th day, and data were summarized as cumulative presoldier differentiation between days 14 and 25. Three injection replicates (n = 15 workers per replicate) were performed per treatment, per colony, on 3 successive days. Within-colony cumulative means were analyzed after 25 days by the Kruskal–Wallis test (n = 3; df = 1; P < 0.05 or P < 0.10).
qRT-PCR and Reference Gene Selection.
qRT-PCR was performed with an iCycler iQ real-time PCR detection system with iQ SYBR Green Supermix (Bio-Rad). cDNA, which served as the template for qRT-PCR, was synthesized from the total RNA of each of five individuals at 24 h after injection. Total RNA and cDNA were obtained by using the SV Total RNA isolation system (Promega) and the iScript cDNA synthesis kit (Bio-Rad), respectively, in accordance with manufacturer protocols. The suitability of the three reference/control genes, β-actin, HSP-70, and NADH-dh, was evaluated with bestkeeper (38) and normfinder (39) software. Both programs were developed to find the least-variable reference genes, to enable accurate and reliable normalization of qRT-PCR data (40). We used these programs not only to evaluate potential reference genes, but also to assess the effects of RNAi on nontarget genes. Primer sets for reference genes, shown 5′ to 3′, were as follows: β-actin (forward, AGAGGGAAATCGTGCGTGAC; reverse, CAATAGTGATGACCTGGCCGT), HSP-70 (forward, GAAGACAAGGTGAAGGC; reverse, TGCCGTGGATTGACTCTAGC), and NADH-dh (forward, GCTGGGGGTGTTATTCATTCCTA; reverse, GGCATACCACAAAGAGCAAAA). Upon direct sequencing of the β-actin qRT-PCR product, we verified that the qRT-PCR product shares 100% identity with the R. flavipes β-actin sequence (GenBank accession nos. AY572863 and DQ206832). Positions of the qRT-PCR primers for the Hex-1, Hex-2, and Cell-1 genes are shown in Fig. 6. Relative expression levels for specific genes, in relation to the most reliable reference gene (β-actin), were calculated by the 2−ΔΔCT method (41). Mean expression levels for five individuals, each assayed in triplicate, were analyzed by the Kruskal–Wallis test (n = 5; df = 1; P < 0.01 or P < 0.05).
SDS/PAGE and N-Terminal Sequencing.
Hemolymph proteins were collected in ice-cold PBS, quantified, and compared by 8% SDS/PAGE, in accordance with procedures described previously (11). Hemolymph was collected from pooled groups of 15 water-injected control workers or siRNA-injected workers at 2, 4, 8, or 9 days after injection. Colony workers were bled immediately upon removal from laboratory colonies. Electrophoresis and transfer were conducted with minigel and miniwet transfer cell systems (Bio-Rad). N-terminal protein sequencing was performed at the University of Florida Protein Core Facility, by using the Edmann degradation, on proteins transferred to Immobilon-P poly(vinylidene difluoride) membrane (Millipore). PAGE band intensities were quantified from three replicate gels per experiment by using quantity-one software interfaced with a Gel-Doc 2000 imager (Bio-Rad).
Supplementary Material
Acknowledgments
We thank Michael Pfaffl and Aleksandar Radonic for discussions and advice regarding real-time PCR normalization; Drion Boucias for use of his custom injection platform; colleagues from Purdue University (G. W. Bennett, C. R. Ratliff, B. R. Pittendrigh, G. Buczkowski, and W. Sun) for assistance with earlier phases of this research; as well as the University of Florida Protein Core Facility (Ran Zhang and Scott McClung) for N-terminal sequencing. We also thank Matthew Tarver, Dan Hahn, Diana Wheeler, Claudia Husseneder, Barbara Thorne, and Prof. Charles Noirot for critical review of manuscript drafts and helpful discussions on altruism. Some photos were taken by Lyle Buss (primary reproductive, egg, nymph), Jody Green (larva, third-form reproductive), and Dean Brad (second-form reproductive). This work was supported by Procter and Gamble, Inc., and the Florida Agricultural Experiment Station, and was approved for publication as Journal Series R-11019.
Abbreviations
- JH
juvenile hormone
- RNAi
RNA interference
- qRT-PCR
quantitative real-time PCR
- siRNA
short-interfering RNA.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. AY572858 (Hexamerin-1, Hex-1), AY572859 (Hexamerin-2, Hex-2), AY572862 (Cellulase-1, Cell-1), AY572863 (beta-actin, β-actin), DQ206832 (beta-actin, β-actin), CK906362 (70-kDa cognate heat shock protein, HSP-70), and BQ788185 (NADH dehydrogenase, NADH-dh)].
References
- 1.Wilson E. O. The Insect Societies. Cambridge, MA: Belknap Press of Harvard Univ; 1971. [Google Scholar]
- 2.Thorne B. L. Annu. Rev. Ecol. Syst. 1997;28:27–54. [Google Scholar]
- 3.Thorne B. L., Traniello J. F. A. Annu. Rev. Entomol. 2003;48:283–306. doi: 10.1146/annurev.ento.48.091801.112611. [DOI] [PubMed] [Google Scholar]
- 4.Miura T., Kamikouchi A., Sawata M., Takeuchi H., Natori S., Kubo T., Matsumoto T. Proc. Natl. Acad. Sci. USA. 1999;96:13874–13879. doi: 10.1073/pnas.96.24.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scharf M. E., Wu-Scharf D., Pittendrigh B. R., Bennett G. W. Genome Biol. 2003;4:R62. doi: 10.1186/gb-2003-4-10-r62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scharf M. E., Wu-Scharf D., Zhou X., Pittendrigh B. R., Bennett G. W. Insect Mol. Biol. 2005;14:31–44. doi: 10.1111/j.1365-2583.2004.00527.x. [DOI] [PubMed] [Google Scholar]
- 7.Koshikawa S., Cornette R., Hojo M., Maekawa K., Matsumoto T., Miura T. FEBS Lett. 2005;579:1365–1370. doi: 10.1016/j.febslet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 8.Lainé L. V., Wright D. J. Bull. Entomol. Res. 2003;93:267–278. doi: 10.1079/ber2003238. [DOI] [PubMed] [Google Scholar]
- 9.Park Y. I., Raina A. K. J. Insect Physiol. 2005;51:385–391. doi: 10.1016/j.jinsphys.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Scharf M. E., Ratliff C. R., Hoteling J. T., Bennett G. W. Insectes Sociaux. 2003;50:346–354. [Google Scholar]
- 11.Scharf M. E., Ratliff C. R., Wu-Scharf D., Zhou X., Pittendrigh B. R., Bennett G. W. Insect Biochem. Mol. Biol. 2005;35:207–215. doi: 10.1016/j.ibmb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Mao L., Henderson G., Liu Y., Laine R. Ann. Entomol. Soc. Am. 2005;98:340–345. [Google Scholar]
- 13.Yagi K. J., Kwok R., Chan K. K., Setter R. R., Myles T. G., Tobe S. S., Stay B. J. Insect Physiol. 2005;51:357–365. doi: 10.1016/j.jinsphys.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Myles T. G., Nutting W. L. Q. Rev. Biol. 1988;63:1–23. [Google Scholar]
- 15.Henderson G. In: Pheromone Communication in Social Insects. Vander Meer R. K., Breed M. D., Espelie K. E., Winston M. L., editors. Boulder, CO: Westview; 1998. pp. 314–329. [Google Scholar]
- 16.Lefevue P., Bordereau C. Proc. Natl. Acad. Sci. USA. 1984;81:7665–7668. doi: 10.1073/pnas.81.23.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roux E. A., Korb J. J. Evol. Biol. 2004;17:869–875. doi: 10.1111/j.1420-9101.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 18.Wu-Scharf D., Scharf M. E., Pittendrigh B. R., Bennett G. W. Sociobiology. 2003;41:479–489. [Google Scholar]
- 19.Braun R. P., Wyatt G. W. J. Biol. Chem. 1996;6:31756–31762. doi: 10.1074/jbc.271.49.31756. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert L. I., Granger N. A., Roe R. M. Insect Biochem. Mol. Biol. 2000;30:617–644. doi: 10.1016/s0965-1748(00)00034-5. [DOI] [PubMed] [Google Scholar]
- 21.Amdam G. V., Simões Z. L. P., Guidugli K. R., Norberg K., Omholt S. W. BMC Biotechnol. 2003;3:1. doi: 10.1186/1472-6750-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guidulgi K. R., Nascimento A. M., Amdam G. V., Barchuk A. R., Omholt S., Simões Z. L. P., Hartfelder K. FEBS Lett. 2005;579:4961–4965. doi: 10.1016/j.febslet.2005.07.085. [DOI] [PubMed] [Google Scholar]
- 23.Haverty M. I. Sociobiology. 1977;2:199–216. [Google Scholar]
- 24.Burmester T., Massey H. C., Zakharkin S. O., Benes H. J. Mol. Evol. 1998;47:93–108. doi: 10.1007/pl00006366. [DOI] [PubMed] [Google Scholar]
- 25.Hagner-Holler S., Schoen A., Erker W., Marden J. H., Rupprecht R., Decker H., Burmester T. Proc. Natl. Acad. Sci. USA. 2004;101:871–874. doi: 10.1073/pnas.0305872101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burmester T., Scheller K. Naturwissenschaften. 1999;86:468–474. doi: 10.1007/s001140050656. [DOI] [PubMed] [Google Scholar]
- 27.Hunt J. H., Amdam G. V. Science. 2005;308:264–267. doi: 10.1126/science.1109724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amdam G. V., Norberg K., Hagen A., Omholt S. O. Proc. Natl. Acad. Sci. USA. 2004;100:1799–1802. doi: 10.1073/pnas.0333979100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zera A. J., Denno R. F. Annu. Rev. Entomol. 1997;42:207–230. doi: 10.1146/annurev.ento.42.1.207. [DOI] [PubMed] [Google Scholar]
- 30.Kang L., Chen X. Y., Zhou Y., Liu B. W., Zheng W., Li R. Q., Wang J., Yu J. Proc. Natl. Acad. Sci. USA. 2004;101:17611–17615. doi: 10.1073/pnas.0407753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman W. G., Orth A. P., Toong Y. C., Ebersohl R., Hiruma K., Granger N. A. Arch. Insect Biochem. Physiol. 1995;30:295–306. doi: 10.1002/arch.940300215. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X., Tarver M., Bennett G. W., Oi F. M., Scharf M. E. Gene. 2006 doi: 10.1016/j.gene.2006.02.002. in press. [DOI] [PubMed] [Google Scholar]
- 33.Robinson G. E., Grozinger C. M., Whitfield C. W. Nat. Rev. Genet. 2005;6:257–270. doi: 10.1038/nrg1575. [DOI] [PubMed] [Google Scholar]
- 34.Thompson G. J., Crozier Y. C., Crozier R. H. Insect Mol. Biol. 2003;12:1–7. doi: 10.1046/j.1365-2583.2003.00381.x. [DOI] [PubMed] [Google Scholar]
- 35.Hojo M., Morioka M., Matsumoto T., Miura T. Insect Biochem. Mol. Biol. 2005;35:347–354. doi: 10.1016/j.ibmb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Novina C. D., Sharp P. A. Nature. 2004;430:161–164. doi: 10.1038/430161a. [DOI] [PubMed] [Google Scholar]
- 37.Elbashir S. M., Landeckel W., Tuschl T. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfaffl M. W., Tichopad A., Prgomet C., Neuvians T. P. Biotechnol. Lett. 2004;26:509–515. doi: 10.1023/b:bile.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 39.Andersen C. L., Ledet-Jensen J., Ørntoft T. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 40.Huggett J., Dheda K., Bustin S., Zumula A. Genes Immun. 2005;6:279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- 41.Livak K. J., Schmittgen T. D. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.