Abstract
Conventional agriculture has improved in crop yield but at large costs to the environment, particularly off-site pollution from mineral N fertilizers. In response to environmental concerns, organic agriculture has become an increasingly popular option. One component of organic agriculture that remains in question is whether it can reduce agricultural N losses to groundwater and the atmosphere relative to conventional agriculture. Here we report reduced N pollution from organic and integrated farming systems compared with a conventional farming system. We evaluated differences in denitrification potential and a suite of other soil biological and chemical properties in soil samples taken from organic, integrated, and conventional treatments in an experimental apple orchard. Organically farmed soils exhibited higher potential denitrification rates, greater denitrification efficiency, higher organic matter, and greater microbial activity than conventionally farmed soils. The observed differences in denitrifier function were then assessed under field conditions after fertilization. N2O emissions were not significantly different among treatments; however, N2 emissions were highest in organic plots. Annual nitrate leaching was 4.4–5.6 times higher in conventional plots than in organic plots, with the integrated plots in between. This study demonstrates that organic and integrated fertilization practices support more active and efficient denitrifier communities, shift the balance of N2 emissions and nitrate losses, and reduce environmentally damaging nitrate losses. Although this study specifically examines a perennial orchard system, the ecological and biogeochemical processes we evaluated are present in all agroecosystems, and the reductions in nitrate loss in this study could also be achievable in other cropping systems.
Keywords: denitrification, nitrogen, organic agriculture, sustainable agriculture, integrated farming
The intensification of agricultural production over the past 60 years and the subsequent increase in global synthetic N inputs have resulted in substantial N pollution and ecological damage (1). The primary source of N pollution comes from N-based agricultural fertilizers, whose use is forecasted to double or almost triple by 2050 (2). The application of N fertilizers has resulted in N leakage from agricultural systems into groundwater, rivers, coastal waters, and the atmosphere (3). Nitrate leaching and N2O emissions from agricultural soils are recognized as significant environmental threats by scientists, environmental groups, and agricultural policymakers (4, 5).
Nitrate leaching and runoff into rivers and estuarine ecosystems are responsible for algal blooms and eutrophication and also pose a public health risk (6, 7). For example, 9% of U.S. domestic wells sampled during 1993–2000 had nitrate concentrations exceeding the U.S. Environmental Protection Agency’s (EPA) maximum contaminant level of 10 mg·liter−1 as N (8). In the Yakima River Basin of Washington State, where this study was conducted, 13% of the samples taken from small-watershed sites exceeded the EPA’s maximum contaminant level, indicating a potential health risk to nearby residents with shallow wells (9).
N2O, a greenhouse gas nearly 300 times more effective at radiative warming than carbon dioxide (10), is produced mainly during the microbially mediated process of denitrification. There has been a marked increase in atmospheric N2O over the past 150 years, largely attributed to fertilized agriculture (11). In most unmanaged systems, the majority of the gas produced during denitrification is fully reduced N2, a nonreactive and environmentally benign gas. However, a variable portion of the nitrate that enters the process will escape as N2O before being fully reduced. In agricultural systems N2O emissions are enhanced after fertilization (12). The proportion of gas escaping as N2O [relative rate of N2O emissions (rN2O)] is highly dependent on environmental factors, with rN2O being lowest in high C environments (13, 14).
Given the environmental problems associated with the production and use of synthetic fertilizer, there is a great need for researchers concerned with global climate change and nitrate pollution to evaluate reduction strategies (1, 3, 4). Fertilization with organic wastes and composts is a means of recycling terrestrially available N, thereby reducing both N inputs to the biosphere and dependence on fossil fuels needed to produce synthetic fertilizers (15). It has also been suggested that the use of organic fertilizers, alone or in combination with synthetic fertilizers, may mitigate N pollution from agricultural systems (16, 17).
Here we investigate the role of the soil denitrifier community in mediating the magnitude and composition of gaseous N emissions and the relative balance of N losses through denitrification and leaching after fertilization in organic, integrated, and conventional apple orchards. For 9 years before this study, the organic system followed a regimen of organically certified practices, including the exclusion of synthetic agrochemicals, and the integrated system used a combination of organic and conventional fertilizers and techniques.
This study is unique because it compares denitrifier function in soils from organic, integrated, and conventional plots and then examines the field implications of observed functional differences after a fertilization experiment. Although numerous studies have compared N cycling in response to organic and synthetic fertilizer amendments (Table 4, which is published as supporting information on the PNAS web site), none has simultaneously quantified gaseous and leaching N losses after fertilization to our knowledge. In addition, comparative fertilization studies are frequently complicated by differences in N input intensity among the systems. This study examines denitrification and leaching from organic, integrated, and conventional systems receiving the same amount of N inputs but in different forms.
Results and Discussion
Initially we compared a suite of fundamental ecological characteristics of the soils from the different treatment plots. Organically farmed soils had higher total N, organic matter content, microbial biomass C and N, nitrification potential, and l-asparaginase and β-glucosidase activity (enzymes indicative of microbial N and C cycling potential) compared with conventionally farmed soils (Table 1). These data are in agreement with other studies that have demonstrated that organically farmed soils support more active microbial populations then their conventional counterparts (18–20). Microbial activity and soil organic matter are important for soil nutrient cycling, a valuable ecosystem service (21), particularly when external inputs are not reliably available. Increased microbial activity and soil organic matter in the organically farmed soils results from a combination of enhanced C inputs during fertilization and increased grass cover relative to the integrated and conventional systems where glyphosate was used to keep the tree strips clean.
Table 1.
Soil biological and chemical properties
| Treatment | Total soil N, ppm | Organic matter, % | Microbial biomass c* | Microbial biomass N† | Nitrification potential‡ | l-asparaginase§ | β-Glucosidase¶ | DP‖ | Potential N2O** | Potential rN2O | DP:MBC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Organic | 1,955a | 3.40a | 512.7a | 61.0a | 0.77a | 91.9a | 192.9a | 113.92a | 43.08a | 0.38a | 0.25c |
| Integrated | 1,755a | 3.10a | 420.8a,b | 39.7b | 0.63a,b | 63.7b | 134.4b | 40.39b | 30.82b | 0.78b | 0.10b |
| Conventional | 1,242b | 2.23b | 357.7b | 34.0b | 0.49b | 59.8b | 131.3b | 12.21c | 8.68c | 0.73b | 0.04a |
Different superscript letters within rows indicate significant differences at the 0.05 level (least significant difference). MBC, microbial biomass carbon; DP, denitrification potential.
*mg of C pcr kg of soil.
†mg of N per kg of soil.
‡g of NO3− per g of soil per h.
§mg of N per g of soil per 2 h.
¶mg of p-nitrophenol per g of soil per h.
‖μmol N2O + N2 per h per g of soil.
**μmol of N2O per h per g of soil.
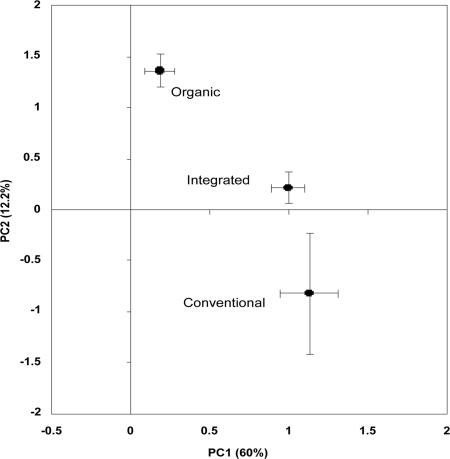
We then analyzed differences in microbial community composition using phospholipid fatty acid (PLFA) analysis, a standard microbial technique that quantifies fatty acids from microbiol cell walls. This technique can be used in conjunction with principal-components analysis to visualize similarities and differences in microbial communities. We found significant compositional differences between the microbial communities in the organic, integrated, and conventional systems (Fig. 1). The PLFA results indicate that the observed differences in microbial activity among the treatment soils may result from a combination of differences in microbial community size and composition.
Fig. 1.
PLFA analysis of soil samples from the organic, integrated, and conventional apple production systems. PLFA analysis, a standard microbial technique that can be used in conjunction with principal-components analysis to visualize similarities and differences in microbial communities, showed that there are significant differences between the microbial communities in the organic and the integrated and conventional systems. PLFA was performed in August 2002 on soils collected from 0- to 7.5-cm depth.
As the primary biological source of gaseous N emissions, the denitrifier community is of particular significance for those interested in mitigating N pollution. We assessed differences in denitrifier activity and efficiency among the systems using a denitrification potential assay. When carbon, nitrate, oxygen, and pH were adjusted to ideal denitrification conditions in the laboratory, we found that denitrifying communities were more active (higher overall gas emissions, denitrification potential) and more efficient (lower potential rN2O) in the organically farmed soils than in the conventionally farmed soils, with the integrated resembling the conventional in terms of efficiency and falling between the two treatments in terms of activity (Table 1). In addition, when denitrification potential was normalized to microbial biomass by calculating the ratio, the organic treatment had the highest ratio of denitrification potential to microbial biomass (Table 1), indicating that a higher proportion of the microbial community is able to denitrify in the organically farmed soils. Groffman and Tiedje (22) found that this ratio was a good predictor of annual denitrification N losses. In the field, differences in microbial function are mediated by environmental factors, and both activity and efficiency will vary in response to local conditions.
To examine field denitrification rates and their significance to overall N losses, we measured the relative magnitude of N losses as gaseous N2, N2O, and nitrate leaching for 1 month after fertilization in subplots in the experimental apple orchard. The conventional subplots were fertilized with Ca(NO3)2; the integrated subplots were fertilized with equal parts composted chicken manure and Ca(NO3)2; and in the organic plots two separate subplots were established to test two organic fertilizers: composted chicken manure and alfalfa meal. Nitrogen inputs were equal across treatments.
For the month after fall and spring fertilization, cumulative N2O emissions were roughly equivalent among the four fertilizer treatments and significantly above controls in the fall (Table 2) (for time series data see Figs. 4 and 5, which are published as supporting information on the PNAS web site). Nitrous oxide emission rates ranged from 1 to 9 ng of N2O-N cm−2·h−1, higher than observed rates in most unfertilized systems and similar to observed rates in most fertilized annual cropping systems (12, 23) (for comparison to other systems see Table 5, which is published as supporting information on the PNAS web site). Overall N2O losses in the month after fertilization were <1% of the applied fertilizer N in both the fall and the spring.
Table 2.
N2O emissions and NO3− leaching
| Treatment and subplots | NO3 leaching (fall), μg of NO3-N at 100 cm | N2O (fall), g/ha N2O-N | NO3 leaching (spring), μg of NO3-N at 100 cm | N2O (spring), g/ha N2O-N | Annual NO3 leaching, μg of NO3-N at 100 cm | Leaf N, % |
|---|---|---|---|---|---|---|
| Organic | ||||||
| Compost | 9.66a,b | 88.57b,c | 180.13a | 330.83b | 241.26a | 2.59a |
| Alfalfa | 9.38a,b | 55.65b | 234.11a | 316.10b | 309.84a | 2.68a |
| Control | 3.73a | 16.83a | 68.06a | 282.28a,b | 108.47a | 2.51a |
| Integrated | ||||||
| CaNO3 + compost | 14.08b | 124.57c | 608.26b | 327.25b | 772.83b | 2.40a |
| Control | 4.43a | 19.24a | 97.50a | 269.03a,b | 154.85a | 2.67a |
| Conventional | ||||||
| CaNO3 | 13.08b | 125.87c | 1,092.24b | 325.98b | 1,352.52c | 2.55a |
| Control | 3.41a | 30.24a | 73.38a | 175.70a | 130.96a | 2.56a |
All data were log-transformed for analysis. Significant differences at the 0.05 level (least significant difference) are indicated by different letters within rows. Fall and spring measurements of N2O and nitrate teaching are for 1 month after fertilization.
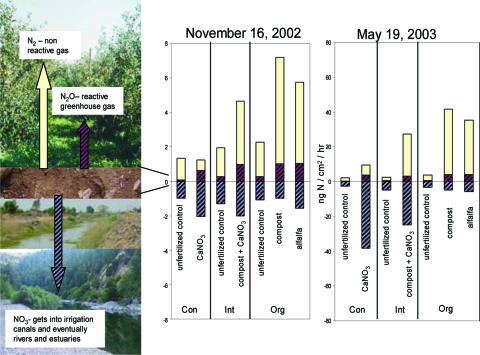
On Nov. 16, 2002, and May 19, 2003, N2 and N2O emission rates were simultaneously assessed by using intact cores and acetylene to determine whether soils receiving organic fertilizer amendments showed enhanced N2 emission rates. N2 loss rates were significantly higher from both organic treatments compared with the conventional treatment on both dates, mirroring the differences in function seen in the laboratory. Rates of N2O emissions were similar among all four fertilizer treatments on both dates, implying enhanced denitrification efficiency in the organic and, to a lesser extent, the integrated treatments relative to the conventional (Fig. 2 and Table 3). This difference in efficiency can be attributed to number of factors including (i) increased C inputs from grass roots and fertilizer in the organic treatments; (ii) higher soil C and N in the organic and integrated treatments than the conventional treatment; (iii) larger, more active microbial communities in the organic treatments; and (iv) the observed differences in the functioning of the denitrifier communities.
Fig. 2.
Relative N loss rates from conventional (Con), integrated (Int), and organic (Org) orchard treatments on Nov. 16, 2002, and May 19, 2003. Nitrate leaching rates are based on the assumption that the 1-cm2 resin bag absorbed nitrate from a soil column not >10 cm2 and not <1 cm2, giving a range for nitrate leaching. The values shown are the average of minimum and maximum leaching rates. See Table 3 for numeric values and statistical significance.
Table 3.
Relative daily N loss rates and soil nitrate pools
| Treatment and subplots | November 16, 2002 |
May 19, 2003 |
||||||
|---|---|---|---|---|---|---|---|---|
| Nitrate leaching | N2O emissions | N2 emissions | Soil nitrate | Nitrate leaching | N2O emissions | N2 emissions | Soil nitrate | |
| Organic | ||||||||
| Compost | 0.021a | 0.024b | 0.148b | 2.18a | 0.118a | 0.096b | 0.906c | 2.18a |
| Alfalfa | 0.038a | 0.025b | 0.113b | 1.40a | 0.135a | 0.099b | 0.753c | 2.47a |
| Control | 0.026a | 0.006a | 0.048a,b | 1.06a | 0.075a | 0.014a | 0.077a | 1.82a |
| Integrated | ||||||||
| CaNO3 + compost | 0.048a | 0.024b | 0.088a,b | 6.67b | 0.593b | 0.079b | 0.577b,c | 3.18a,b |
| Control | 0.031a | 0.007a,b | 0.040a | 1.14a | 0.112a | 0.013a | 0.049a | 0.58a |
| Conventional | ||||||||
| CaNO3 | 0.049a | 0.015a,b | 0.015a | 8.87c | 0.916b | 0.095b | 0.133a,b | 5.43b |
| Control | 0.024a | 0.002a | 0.030a,b | 1.20a | 0.064a | 0.004a | 0.049a | 1.30a |
All loss rates are expressed in ng of N per cm2 per h. Soil nitrate is expressed as kg/ha N (for mean daily nitrate pools for 1 month after fall and spring fertilizations see Fig. 7, which is published as supporting information on the PNAS web site). Nitrate leaching rates are based on the assumption that the 1-cm2 resin bag absorbed nitrate from a soil column not >10 cm2 and not <1 cm2, giving a range for nitrate leaching. The values shown are the average of minimum and maximum leaching rates. All data were log-transformed for analysis. Different superscript letters within rows represent significant differences at P < 0.05 (least significant difference).
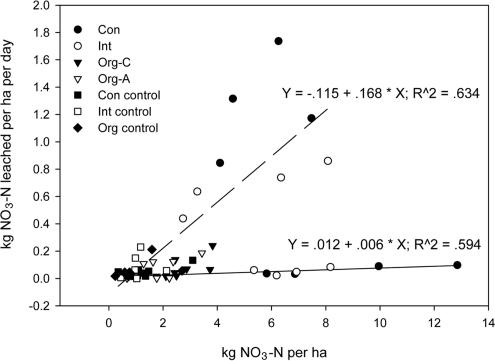
Nitrate leaching was determined by using ion exchange resin bags placed below the rooting zone. Cumulative nitrate leaching was highest from the conventionally fertilized treatment after spring fertilization, followed by the integrated and then the two organic treatments, where leaching rates were not significantly higher than controls. Spring nitrate losses were an order of magnitude higher than losses after fall fertilization (Table 2). This seasonal difference likely resulted from weekly irrigation during the growing season and from build-up of nitrate during the winter, when plant uptake was slow and rainfall was insignificant. Nitrate is a mobile anion that does not bind to soil particles. In this orchard nitrate leaching was highly correlated with soil nitrate pools that were significantly higher in the conventional treatment after fertilization (Fig. 3). Increased water flow and increased mineralization of soil organic matter in response to warm soils during the spring and summer greatly increase the potential for nitrate leaching.
Fig. 3.
Relationship between soil nitrate and daily nitrate leaching rate on Nov. 16, 2002 (solid line), and May 19, 2003 (dashed line). Different symbols represent various treatments. Both regressions are significant at P < 0.0001.
Resin bags remained in the soil for 1 year, allowing for comparison of annual relative nitrate losses. Differences among the treatments in nitrate leaching were most pronounced on an annual basis, with leaching from conventionally fertilized plots 4.4 and 5.6 times higher than that in the two organic treatments, with the integrated treatment in between (Table 2). Lower annual nitrate leaching from the organic and integrated treatments can be explained by a combination of factors that resulted in reduced nitrate levels in the organic treatments and, to a lesser extent, the integrated treatment relative to the conventional treatment. Most importantly, the conventional treatment was fertilized with Ca(NO3)2, whereas the majority of the N applied to the organic treatments and some of the N applied to the integrated treatment during fertilization was incorporated in organic matter, which must be mineralized and nitrified before contributing to the soil nitrate pool. Another factor reducing nitrate losses from the organic treatments was increased denitrification that enhanced gaseous N losses. Although increased grass cover can also reduce leaching losses, the similar amounts of nitrate leached from the control plots (no fertilizer added) in all three treatments (Table 2) indicate that the greater grass cover in organic treatments did not significantly influence nitrate losses.
We determined a conservative range for nitrate leaching rates based on the assumption that the 1-cm2 resin bag absorbed nitrate from a soil column not >10 cm2 and not <1 cm2. Leaching rates in the conventional treatment were of similar magnitude to N2 emissions rates in either of the two organic treatments at spring sampling (Fig. 2). In this orchard, organic fertilization practices shift the relative balance of gaseous and leaching losses such that proportionately more N is lost as N2 from the organically farmed soils and as leachate from the conventionally farmed soils.
This study does not establish a direct causal link between enhanced gas emissions and reduced leaching. However, when gas emissions are high as on May 19, 2003, when denitrification as N2 losses in the organic treatments were equal to 42% of the soil nitrate pool (Table 3), denitrifiers can effectively reduce the amount of nitrate in the soil profile susceptible to leaching. Given the significant correlation (P < 0.0001) between the soil nitrate pool and daily nitrate leaching rates on Nov. 16, 2002, and May 19, 2003 (Fig. 3), a substantial reduction in the soil nitrate pool would be expected to reduce nitrate leaching. It is also notable that enhanced gas emissions in the organic treatments occur without a problematic increase in N2O emissions.
The notion that enhanced gaseous losses may reduce leaching losses is not new. Strategies aimed at mitigation of nitrate leaching often involve the use of wetlands, where rapid denitrification prevents excess nitrate losses, thereby protecting surface water and aquifers (24). Denitrifiers provide an important ecosystem service by removing excess nitrate from terrestrial and aquatic ecosystems (21). Our results suggest that organic, and to a lesser extent integrated, management practices can foster active and efficient denitrifier communities, thereby serving a similar purpose on working farmlands.
Although not the focus of this study, crop yield is a primary concern for farmers. We assessed crop N status by evaluating leaf N levels at harvest. All four subplot fertilization treatments resulted in statistically similar leaf N levels (Table 2), which were within the critical nutrient range for optimal fruit growth and productivity (25), indicating that organic fertilization, alone or in combination with synthetic fertilizers, may provide a viable alternative to conventional fertilization practices. These results are supported by previous research, which showed that the three apple production systems, when receiving the same amount of N fertilizer, had similar cumulative yields in the first 6 years of the study (18).
Conclusions
This study is significant in that it examines both N2O and N2 emissions in the lab and in the field. Although several field studies have compared both gaseous loss pathways after inputs of organic or mineral fertilizer, none has examined the implications of enhanced N2 from organic systems for nitrate leaching or overall agricultural N pollution to our knowledge. Most comparative studies of N losses from organic and conventional systems are complicated by differences in N application rates and timing among treatments (26) (Table 4). In our study equal amounts of N were simultaneously applied in different forms to each of the treatments. The study was designed to highlight the ecological mechanisms that underlie agricultural N losses from different management systems. Although the mechanisms and processes described in this study are ubiquitous in agroecosystems, actual N losses from organic and conventional farms will vary depending on the specific management and ecology of the system.
The results of our study indicate that use of organic fertilizers in orchards significantly reduces harmful nitrate leaching and enhances denitrifier activity and efficiency. The microbial processes described in these Washington apple orchards operate in all soil ecosystems, and, as such, the observed reductions in environmentally damaging nitrate losses are theoretically achievable in other cropping systems, such as vegetable and grain systems, where denitrifier activity is enhanced through C inputs as organic fertilizers, crop residues, or root exudates from cover crops.
Given the problems associated with global N enrichment caused by agricultural practices, the observed reduction in environmentally damaging nitrate losses from the organic and integrated systems in this study is of important practical significance for both public health and the environment. It is critical for scientists, farmers, and policymakers interested in addressing N pollution problems to look for agricultural systems where reduction in synthetic fertilizer use is possible. Apples and other high-value perennial food crops, which constitute ≈21% of the total value of U.S. food crops (27), are good candidates for consideration because of their reduced N demand.
Materials and Methods
Study Area.
The experimental site covered 1.7 hectares (ha) of four replicate plots for each of three apple production systems in a randomized complete block design. The organic treatment followed the U.S. Department of Agriculture National Organic Program (www.ams.usda.gov/nop/NOP/NOPhomeNetscape.html, accessed November 6, 2005) and the Washington State Department of Agriculture Organic Food Program (http://agr.wa.gov/FoodAnimal/Organic/default.htm#OrganicFoodProgram, accessed November 6, 2005) certification guidelines. The conventional treatment followed practices reflecting the management of typical conventional, commercial apple orchards in Washington State. The integrated treatment combined soil, horticultural, and pest management practices from the organic and conventional systems. Details of the experimental design and farming practices are described elsewhere (18, 19). Details of fertilizer management between 1994 and 2003 are shown in Table 6, which is published as supporting information on the PNAS web site.
Soil Analyses.
On Aug. 10, 2002, we collected soil samples (0- to 7.5-cm depth) from the 12 organic, integrated, and conventional plots and analyzed them in the laboratory within 3 days for potential denitrification, potential N2O fluxes, and rN2O by using the soil slurry method described by Cavigelli and Robertson (28). Nitrification potential was assessed by using the method described by Hart et al. (29). Potential assays were used to measure microbial function independent of environmental variability among the treatment soils and can be viewed as a long-term, integrative product of multiple physical and biological factors. We analyzed l-asparaginase and β-glucosidase as described by Tabatabai (30). PLFA analysis was performed according to the techniques described by Bossio et al. (31). Details of analytical procedures for soil organic matter, total N, microbial biomass C and N, and mineralizable N are described elsewhere (19).
Fertilization Subplots.
Fertilizer recommendations and timing were determined in cooperation with apple orchard managers and professional horticultural consultants in the region to ensure that input rates were reasonably representative of farmer practices. Within each of the 12 plots of the experimental orchard, three 4-m2 subplots were established and fertilized in different forms according to farm management system at a rate of 67.3 kg· ha−1 N on Oct. 22, 2002, and 44.9 kg·ha−1 N on May 1, 2003 (Fig. 6, which is published as supporting information on the PNAS web site). These split application rates are typical for young grafted trees in the Yakima Valley area, where the experimental orchard was located. The conventional subplots were fertilized with Ca(NO3)2; the integrated subplots were fertilized with equal parts composted chicken manure and Ca(NO3)2; and in the organic plots two separate subplots were established to test two organic fertilizers: composted chicken manure and alfalfa meal. Nitrogen inputs were held constant across treatments to facilitate a mechanistic interpretation of the results without the added complication of differences in input intensity. One unfertilized control subplot was also established in each of the 12 plots to determine baseline N cycling data.
Nitrogen Loss Measurements.
We installed cation–anion exchange resin bags at 100-cm depths to estimate relative nitrate mobility in the soil profile as a proxy for nitrate leaching. Nitrate at 100-cm soil depth was used as an estimate of leaching because this depth was below most roots of the trees in the orchard. After removal, we returned resin bags to the laboratory and extracted nitrate by shaking bags in 50 ml of 2 M KCl. We analyzed KCl extracts for inorganic N by using an Alpkem RFA/2. Resin bags remained in the soil from Oct. 19, 2002, through Oct. 19, 2003; we replaced and analyzed them monthly.
We monitored N2O emissions and soil nitrate availability 10 times from Oct. 19 through Nov. 22, 2002 (fall fertilization), and 5 times from May 1 through June 2, 2003 (spring fertilization). We used static chambers to measure fluxes of N2O as described by Matson et al. (12). After removal of the chamber lid, soil temperature was measured and one soil core (0–15 cm) was removed from within the plot for analysis of inorganic N and soil moisture. Inorganic N was immediately extracted from the soil cores by placing a 10-g subsample of soil in 100 ml of 2 M KCl, shaking for 1 min, and then allowing to equilibrate overnight. The supernatant was removed and stored at 4°C until analysis by using an Alpkem RFA/2. Soil moisture was determined by taking a 10-g subsample from each core, weighing it fresh, and then drying it at 105°C for 2 days before reweighing it to determine gravimetric water content. Cumulative N2O emissions in the month after fertilization were calculated by extrapolating measured rates during periods between sampling dates.
Overall denitrification and rN2O were assessed according to Mosier and Klemedtsson (32) by using structurally intact paired cores on Nov. 16, 2002, and May 19, 2003. Two intact cores (0–15 cm) were obtained from each plot, and cores were immediately sealed with a septum-fitted lid for gas analysis. Within 2 h of collection, jars were flushed with air to remove any N2O accumulated, and then one of the cores from each ring site was injected with CaC2-generated acetylene to a final volume of 15–20% to block N2O reductase. The remaining cores were not treated with acetylene, so they could be used to assess N2O production and rN2O. Gas samples were collected at 2, 6, and 18 h and stored in Wheaton vials until analysis with a gas chromatograph.
To determine the relative magnitude of the different N loss pathways for the organic, integrated, and conventional plots, N loss rates on Nov. 16, 2002, and May 19, 2003, were converted to ng of N cm−2·h−1. Resin bags could not be used to precisely quantify nitrate leaching on a per-area basis because it was not possible to determine the exact diameter of the soil column that drains through the bag. For these calculations it was assumed that the 1-cm2 resin bag absorbed resin from a soil column not >10 cm2 and not <1 cm2, giving a range for nitrate leaching (minimum and maximum, respectively).
Leaf Nitrogen.
We collected 20 leaf samples at harvest in August 2003 from each of 10 trees per treatment block. Leaves from each treatment were pooled, air-dried, ground up, and analyzed by using an elemental analyzer from Carlo Erba Instruments (Milan).
Statistical Analyses.
Soil property, N loss, and leaf measurements for treatments were statistically analyzed by using sas software (SAS Institute, Cary, NC) for a randomized complete block design. The least significant difference mean separation procedure was used to determine differences at the 0.05 level of significance.
Supplementary Material
Acknowledgments
We thank A. Dolph, E. Dolph, and Stemilt Growers, Inc., for the use of their farm; T. Crews, J. Galloway, D. Huggins, L. Klein, R. Koenig, W. Schlesinger, and P. Vitousek for many insightful comments on earlier drafts of the manuscript; P. Matson for providing advice, laboratory space, and equipment for this project; and Z. Moskovitz, A. Sullivan, T. Gagnolet, R. Alldredge, G. Peck, and R. Rupp for technical assistance. This work was supported by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (Managed Ecosystems), the U.S. National Science Foundation Graduate Research Fellowship Program, The Land Institute Graduate Research Fellowship Program, and the Teresa Heinz Environmental Science and Policy Fellowship Program.
Abbreviations
- rN2O
relative rate of N2O emissions
- PLFA
phospholipid fatty acid
- ha
hectare.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Alcamo J., Ash N. J., Butler C. D., Callicott J. B., Capistrano D., Carpenter S. R., Castilla J. C., Chambers R., Chopra K., Cropper A., et al. Millenium Ecosystem Assessment: Ecosystems and Human Well-Being: A Framework for Assessment. Washington, DC: Island; 2005. [Google Scholar]
- 2.Tilman D., Fargione J., Wolff B., D’Antonio C., Dobson A., Howarth R., Schindler D., Schlesinger W. H., Simberloff D., Swackhamer D. Science. 2001;292:281–284. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- 3.Galloway J., Aber J., Erisman J., Seitzinger S., Howarth R., Cowling E., Cosby B. BioScience. 2003;53:341–356. [Google Scholar]
- 4.Tilman D., Cassman K., Matson P., Naylor R., Polasky S. Nature. 2002;418:671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- 5.Environmental Defense Fund . The Case for Reforming Farm Programs to Preserve the Environment and Help Family Farmers, Ranchers, and Foresters. Washington, DC: Environmental Defense Fund; 2001. [Google Scholar]
- 6.Beman J., Arrigo K., Matson P. Nature. 2005;434:211–214. doi: 10.1038/nature03370. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe A. H., Patz J. A. Ambio. 2002;31:120–125. doi: 10.1579/0044-7447-31.2.120. [DOI] [PubMed] [Google Scholar]
- 8.Nolan B., Hitt K., Ruddy B. Environ. Sci. Technol. 2002;36:2138–2145. doi: 10.1021/es0113854. [DOI] [PubMed] [Google Scholar]
- 9.Fuhrer G. J., Morace J. L., Johnson H. M., Rinella J. F., Ebbert J. C., Embrey S. S., Waite I. R., Carpenter K. D., Wise D. R., Hughes C. A. Water Quality in the Yakima River Basin, Washington, 1999–2000, Circular 1237. Reston, VA: U.S. Geological Survey; 2004. [Google Scholar]
- 10.Ramaswamy V., Boucher O., Haigh J. In: Climate Change 2001: The Scientific Basis, Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Houghton J. T., Meira Filho L. G., Bruce J., Lee H., Callender B. A., Haites E., Harris N., Maskell K., editors. Cambridge, U.K: Cambridge Univ. Press; 2001. pp. 349–416. [Google Scholar]
- 11.Mosier A., Kroeze C., Nevison C., Oenema O., Seitzinger S., van Cleemput O. Nutrient Cycling Agroecosystems. 1998;52:225–248. [Google Scholar]
- 12.Matson P., Billow S., Hall S., Zachariassen J. J. Geophys. Res. 1996;101:18533–18545. [Google Scholar]
- 13.Firestone M., Firestone R., Tiedje J. Science. 1980;208:749–751. doi: 10.1126/science.208.4445.749. [DOI] [PubMed] [Google Scholar]
- 14.Weier K. L., Doran J. W., Power J. F., Walters D. T. Soil Sci. Soc. Am. J. 1993;57:66–72. [Google Scholar]
- 15.Ceotto E. Bioresource Tech. 2005;96:191–196. doi: 10.1016/j.biortech.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Drinkwater L., Wagoner P., Sarrantonio M. Nature. 1998;396:262–265. [Google Scholar]
- 17.Nosengo N. Nature. 2003;425:894–895. doi: 10.1038/425894a. [DOI] [PubMed] [Google Scholar]
- 18.Reganold J., Glover J., Andrews P., Hinman H. Nature. 2001;410:926–930. doi: 10.1038/35073574. [DOI] [PubMed] [Google Scholar]
- 19.Glover J., Reganold J., Andrews P. Agric. Ecosystem Env. 2000;80:29–45. [Google Scholar]
- 20.Mader P., Fliessbach A., Dubois D., Gunst L., Fried P., Niggli U. Science. 2002;296:1694–1697. doi: 10.1126/science.1071148. [DOI] [PubMed] [Google Scholar]
- 21.Ewel K. C. In: Nature’s Services: Societal Dependence on Natural Ecosystems. Daily G., editor. Washington, DC: Island; 1997. pp. 329–344. [Google Scholar]
- 22.Groffman P., Tiedje J. Soil Biol. Biochem. 1989;21:621–626. [Google Scholar]
- 23.Roelandt C., Van Wesemael B., Rounsevell M. Global Change Biol. 2005;11:1701–1711. doi: 10.1111/j.1365-2486.2005.01052.x. [DOI] [PubMed] [Google Scholar]
- 24.Jordan T., Correll D., Weller D. J. Environ. Qual. 1993;22:467–473. [Google Scholar]
- 25.Gavlak R. G., Horneck D. A., Miller R. O. Plant, Soil, and Water Reference Methods for the Western Region, Western Regional Extension Publication 125. Fairbanks: Univ. of Alaska Cooperative Extension Service; 1994. [Google Scholar]
- 26.Kirchmann H., Bergstrom L. Commun. Soil Sci. Plant Anal. 2001;32:997–1028. [Google Scholar]
- 27.U.S. Department of Agriculture National Agricultural Statistics Service . Agricultural Statistics 2004. Washington, DC: U.S. Government Printing Office; 2004. [Google Scholar]
- 28.Cavigelli M., Robertson G. P. Ecology. 2000;81:1402–1414. [Google Scholar]
- 29.Hart S. C., Stark J. M., Davidson E. A., Firestone M. K. In: Methods of Soil Analysis Part II: Microbiological and Biochemical Properties. Weaver R. W., Angle J. S., Bottomley P. J., editors. Madison, WI: Soil Sci. Soc. Am; 1994. pp. 985–1018. [Google Scholar]
- 30.Tabatabai M. A. In: Methods of Soil Analysis Part II: Microbiological and Biochemical Properties. Weaver R. W., Angle J. S., Bottomley P. J., editors. Madison, WI: Soil Sci. Soc. Am; 1994. pp. 775–853. [Google Scholar]
- 31.Bossio D., Scow K., Gunapala N., Graham K. Microbial Ecol. 1998;36:1–12. doi: 10.1007/s002489900087. [DOI] [PubMed] [Google Scholar]
- 32.Mosier A. R., Klemedtsson L. In: Methods of Soil Analysis Part II: Microbiological and Biochemical Properties. Weaver R. W., Angle J. S., Bottomley P. J., editors. Madison, WI: Soil Sci. Soc. Am; 1994. pp. 1047–1065. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.