Abstract
Using a module exchange approach, we have tested a long-standing model for the role of Cro repressor in λ prophage induction. This epigenetic switch from lysogeny to the lytic state occurs on activation of the host SOS system, which leads to specific cleavage of CI repressor. It has been proposed that Cro repressor, which operates during lytic growth and which we shall term the lytic repressor, is crucial to prophage induction. In this view, Cro binds to the OR3 operator, thereby repressing the cI gene and making the switch irreversible. Here we tested this model by replacing λ Cro with a dimeric form of Lac repressor and adding several lac operators. This approach allowed us to regulate the function of the lytic repressor at will and to prevent it from repressing cI, because lac repressor could not repress PRM in our constructs. Repression of cI by the lytic repressor was not required for prophage induction to occur. However, our evidence suggests that this binding can make induction more efficient, particularly at intermediate levels of DNA damage that otherwise cause induction of only a fraction of the population. These results indicate that this strategy of module exchange will have broad applications for analysis of gene regulatory circuits.
Keywords: circuit design, epigenetic switch, gene regulation, systems biology, threshold behavior
A cell infected with λ can follow either of two exclusive pathways (1, 2). In the lytic pathway, a temporal pattern of gene expression occurs, the viral DNA replicates, and ≈100 new virions are made and released by cell lysis. In the alternative lysogenic pathway, the phage DNA is integrated into the host genome, and its lytic genes are repressed by CI repressor, resulting in a stable association with the host termed the lysogenic state. The initial choice between these two pathways is largely determined by the level of a viral regulatory protein, CII. If CII levels are high, it stimulates three promoters that act in various ways (3) to favor the lysogenic pathway. CII is unstable but is stabilized by another viral protein, CIII. Infection with several phages favors high CII levels and the lysogenic pathway (1, 4); poorly understood physiological factors also likely help to regulate CII levels.
The lysogenic state is highly stable; however, it can switch to the lytic state in an epigenetic switch called prophage induction (2, 5). Upon induction of the host SOS system (6) by DNA damage, such as from UV irradiation, RecA protein is activated to a form that mediates specific cleavage of CI (7, 8), inactivating CI and allowing expression of lytic genes and the lytic pathway.
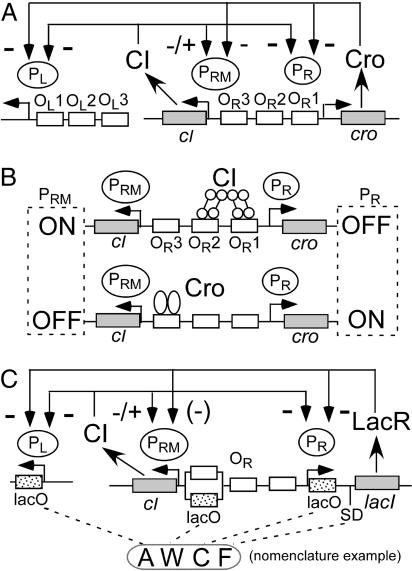
The central events that control and stabilize the regulatory states occur in the OR region (Fig. 1A). This region contains two promoters: the lytic promoter PR, from which cro and several early lytic genes are expressed, and PRM, from which cI is expressed in a lysogen. OR also contains three operator sites to which both Cro and CI bind. In addition, both proteins bind to three operators in the OL region; binding by either protein to OL1 or OL2 represses the early lytic promoter PL.
Fig. 1.
Design of λlacI. (A) Diagram of the phage λ regulatory circuit. (+) and (−) indicate activation and repression of promoter, respectively (LacR can repress PRM when a lacO variant is in the position of OR3). CIII and CII (not depicted) are expressed from PL and PR, respectively. Expression of PL is controlled by CI binding to two operators, OL1 and OL2, which lie to the right of PL in positions analogous to OR1 and OR2, respectively. (B) Patterns of occupancy by CI and Cro at moderate protein levels. CI stabilizes the lysogenic state by repressing PR and stimulating PRM. At higher levels, CI binds OR3 (cf. ref. 10), repressing PRM. Cro bound to OR3 represses PRM. At higher levels, Cro binds to OR2 and/or OR1, repressing PR. (C) Design of λlacIdim. Cro was replaced with lacIdim. Alleles of lacO were installed at PL and PR; at the position of OR3, some variants had a lacO allele, and some had OR3. The same set of lacO alleles was used at all three sites. Several alleles of the SD sequence for lacI were used. [Modified with permission from ref. 17 (Copyright 1992, Cold Spring Harbor Lab. Press).]
Each of these proteins binds with differing affinities and consequences to the three sites at OR (2). CI binds tightly to OR1 and weakly to OR2 and OR3; CI also binds cooperatively to OR1 and OR2. In the lysogenic state, CI bound to OR1 and/or OR2 represses expression of cro and lytic genes from PR, and CI bound to OR2 stimulates cI expression from PRM. CI also represses PL in the lysogenic state. Cro, by contrast, binds weakly to OR1 and OR2 and tightly to OR3. Cro is expressed from the PR promoter and, as its levels increase during the lytic cycle, it begins to bind to its operators. At relatively low concentrations, Cro binds to OR3; this represses PRM, although it is unclear whether this effect is important to normal lytic growth, because PRM is a weak promoter. At higher concentrations of Cro, such as those found late in infection, Cro also partially represses PR by binding to OR1 and/or OR2, so that early gene expression is reduced at later times (Fig. 1B; see also Fig. 5, which is published as supporting information on the PNAS web site). Cro also binds to three sites in the OL region, but this repression is probably only partial.
During the process of prophage induction, CI levels fall over the course of 20–30 min (9) because of RecA-mediated cleavage. Cleavage of CI is counterbalanced in a complicated way by new CI synthesis from PRM. As CI levels drop, occupancy of the operators at OR and OL is progressively less favored, with several different consequences. The response of PRM activity to various levels of CI (10, 11), taken with other data, suggests that these events occur in the following order. First, negative autoregulation of PRM, conferred largely by CI-mediated looping between OL and OR (10), is progressively relieved as OR3 and OL3 become free. Derepression of PRM increases the rate of cI expression. Second, at lower CI levels, OR2 becomes free, and CI no longer positively autoregulates PRM, leading to a lower rate of cI expression. Eventually, CI no longer binds to OR1, allowing transcription from PR and expression of cro.
In this work we address whether Cro also plays a role in the process of prophage induction. As stated, Cro binds most tightly to OR3; when bound to this site, Cro represses PRM (12). Hence, it has been proposed (2, 13) that Cro plays a critical role by repressing PRM, thereby preventing further synthesis of CI and making the switch irreversible. One complication in testing this model is that CI also binds to OR3, and a mutated OR3 specifically blocking Cro binding has not been described (10). A recent approach to this problem used a deleted form of λ that can exist stably in the lytic or “antiimmune” state together with a cIts allele to allow partial loss of CI function at intermediate temperatures (14). This analysis suggested that Cro plays little or no role in switching to the lytic state. It is difficult, however, to relate this system to normal SOS-mediated prophage induction, with progressively lower levels of wild-type CI rather than a weakened mutant form of CI.
Here we describe another approach to testing the role of Cro in prophage induction. We replaced the λ cro gene with lacIdim, the gene for a dimeric mutant version of lac repressor (LacR) (15–17). In addition, we added binding sites for LacR that allowed it to repress PL and PR. LacR was chosen, first, because its activity can be modulated by addition of isopropyl-β-d-galactopyranoside (IPTG), and, second, because its binding site in the natural lac operon lies after the start-point of transcription. Importantly, this latter feature allowed us to leave intact the binding sites for CI without having LacR bind to them, removing the complication that both CI and Cro bind to the same sites in λ. We find that repression of PRM by the lytic repressor is not required for prophage induction. In addition, we delineate when the lytic repressor functions during lytic development and during prophage induction.
Results
Design and Isolation of λlacIdim Variants.
In a previous study (18), we replaced the λ cro gene with lacI, the gene for LacR. In addition, at PR, PL, and optionally at PRM, we added binding sites for LacR, allowing it to repress these promoters. We isolated phages with properties similar to those of wild-type λ, strongly suggesting that the λ circuitry is organized in a modular fashion, a prerequisite for this approach to work. Although the properties of these λlacI phages provided some insight into the roles of Cro, our evidence suggested that LacR, which is tetrameric, can support looping between lacO sites at PL and PR, an activity that Cro does not have. The consequences of looping made it difficult to predict and explain in detail the behavior of λlacI phages and to relate them to λ.
Here we have removed the possibility of looping by isolating a different type of λlacI with a mutant LacR that forms dimers but not tetramers, a property resulting from the deletion of 27 C-terminal residues (19). This change makes the behavior of LacR closer to that of Cro in another way. Dimerization of dimeric LacR is much weaker than the strong tetramerization of wild-type LacR. A dimer dissociation constant has not been measured for our construct, but its value for a dimeric mutant protein with a smaller deletion is ≈80 nM (20); our protein almost certainly does not dimerize more tightly than this, and dimerization may be weaker (K. S. Matthews, personal communication). Hence, its properties are closer to that of Cro, which dimerizes very weakly (≈1 μM dissociation constant) (21, 22). This feature confers nonlinearity to the binding curve at low protein concentrations, a feature that might change the system’s behavior. Accordingly, the behavior of these phages should more closely resemble that of λ.
The balance between the two states of the λ circuitry is likely to be influenced by the level of regulatory proteins and their affinity for their operators. Because we could not predict a priori which combinations of cis-acting sites would give a proper balance similar to that of λ, we used a combinatorial approach. We replaced cro with lacIdim and put lacO sites at PL and PR. Several alleles were allowed (Fig. 5) at each of four cis-acting sites: five lacO alleles with differing affinities at PL and PR, at OR3 the native λ OR3 site or one of five lacO alleles, and six alleles at the Shine–Dalgarno (SD) sequence of lacIdim. The lacO alleles are symbolized by “A”–“E” in order of decreasing affinity; SD alleles are termed “A”–“F” in order of decreasing strength. The design of variants was the same as in the previous study (18) except with the mutant form of lacI (Fig. 1; see also Fig. 6, which is published as supporting information on the PNAS web site), and the phage library was prepared as described in ref. 18. We denote these isolates by the alleles they carry at each of the four variable sites (Fig. 1C).
Our goal was to isolate phage with behavior similar to that of λ, as judged by the ability to form plaques and stable lysogens and to undergo prophage induction. λ forms turbid plaques, because lysogens arise in the developing plaque. Hence, we isolated variants that could form turbid plaques. The pool was plated for plaques in the absence of IPTG or at 10−5 M IPTG. Turbid plaques were purified and analyzed. Twenty-one such isolates, termed λlacIdim, were characterized (Table 1; see also Table 2, which is published as supporting information on the PNAS web site). Sequence analysis showed 19 different variants. We focused on variants carrying a normal OR3, which we term the “W-variants” for brevity.
Table 1.
Behavior of selected λlacIdim variants
| [IPTG] | Plaque formation* |
Lysogenization† |
||
|---|---|---|---|---|
| 0 | 10−5 M | 10−4 M | 0 | |
| Name | ||||
| WT | + | + | + | + |
| AWAE | − | + | − | + |
| AWCA | + | + | − | + |
| AWCD | + | + | − | + |
| AWCF | + | + | − | + |
WT, wild type (λJL351).
*“+” indicates plaque formation. “−” indicates no plaque formation.
†“+” indicates lysogen formation.
Lytic Growth.
Cro protein is required for lytic growth of λ (23–26), operating by binding to sites that block the lytic promoters. In an infected cell, Cro favors the lytic pathway by inhibiting expression of CII and CIII; it does so by partially repressing PR and PL, respectively. In the absence of partial repression of the early lytic promoters, the infected cell cannot give a burst, probably for several different reasons. Among these reasons is that CII can block the lytic pathway by stimulating expression from the paQ promoter, thereby preventing functional levels of Q protein from accumulating (3). This growth defect can be suppressed (in a cI+ or cI857ts phage) by a cII mutation, indicating the importance of repression of CII by Cro. In the absence of CI function, a cro−cII− phage cannot grow (ref. 25; see also ref. 3), suggesting that a low level of CI function is needed to substitute for the partial repression function of Cro.
We tested whether LacR was similarly required for lytic growth of λlacIdim variants. Lytic growth of almost all variants was responsive to IPTG (Table 1; see also Table 2). The W-variants could not form plaques in the presence of 10−4 M IPTG, where the activity of LacR is very weak. We tested the effect of removing CII and found that cII− derivatives of AWCF and AWCA formed plaques with 10−4 M or 10−3 M IPTG, indicating that function of LacR was not required for the lytic pathway in the absence of CII. Possibly CI expressed at low levels from PRM can give partial repression of the lytic promoters. We conclude that repression of PL or PR is required to interrupt overproduction of CIII or CII, respectively, during the lytic pathway, as previously found for λ (3).
Formation of Stable Lysogens and the Lysis–Lysogeny Decision.
Six of the 19 λlacIdim variants were able to establish a stable lysogenic state. One might expect that any variant forming turbid plaques could lysogenize, but conditions differ in a plaque from those in an isolated cell, because lysogens in a plaque are often reinfected by other phages (see ref. 27). Among the six lysogenizing variants, the four W-variants could form single lysogens, as judged by a PCR test (see Materials and Methods). The other two isolates (ADED and BAAD, both bearing lacO at OR3) could not form stable single lysogens even in the presence of high levels of IPTG. We do not understand the basis of this finding.
We examined the frequency of lysogenization for the W-variants. The outcome of the lysis–lysogeny decision is thought to depend on a balance between sets of forces that favor each of the possible outcomes. Because the outcome depends in part on the level of CII, mechanisms that affect its level should alter the balance. The frequency of lysogenization depends on the multiplicity of infection (moi); after single infection, almost all cells follow the lytic pathway, whereas at high moi lysogenization becomes efficient (4). Presumably, the higher gene dosage at high moi increases the levels of CII and CIII.
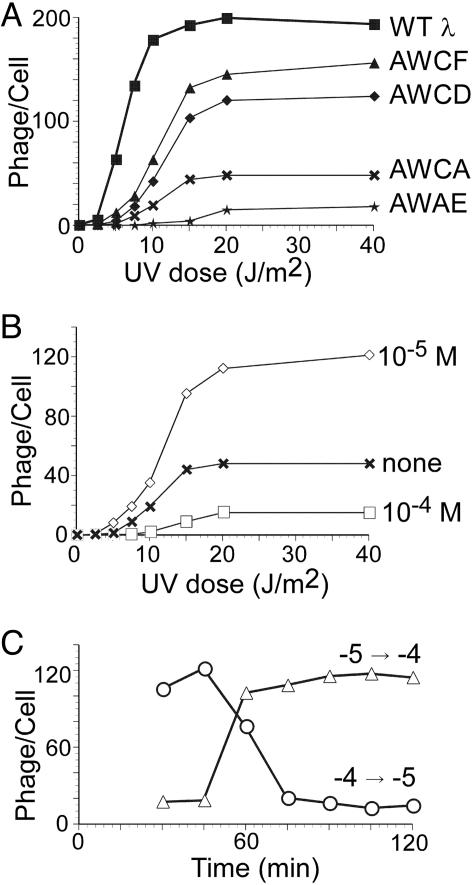
We compared the lysogenization frequency for several W-variants with that of λJL351 parent phage. After single infection, frequencies were low (Fig. 2A). At a moi of ≈6, AWCF and λJL351 gave values of 46 and 58% (data not shown). These values are similar to those reported (4) for wild-type λ as well. We conclude that AWCF closely resembles the λJL351 parent phage in its lysogenization frequency and response to multiplicity of infection.
Fig. 2.
Effect of IPTG on lysogenization frequency after single infection. (A) Lysogenization frequency without (open bar) or with IPTG (black bar, 10−5 M; gray bar, 10−4 M; stippled bar, 10−3 M). Cells were infected at an moi of ≈0.01; values are the percentage of infected cells giving rise to KanR colonies. (B) IPTG concentration-shift experiment (black bar, 10−7 M IPTG; hatched bar, 10−5 M IPTG). Frequencies observed at 10−7 and 10−5 M IPTG were approximately the same as seen without IPTG and with 10−3 M IPTG, respectively (see A).
To test whether the balance of the lysis–lysogeny decision could be perturbed by adding IPTG, we measured lysogenization frequency after single infection in the absence or presence of IPTG. In all cases, this value increased markedly (Fig. 2A), whereas IPTG had no effect on the λJL351 parent, as expected. This finding suggests that the lytic repressor acts during the lysis–lysogeny decision to favor the lytic response, as previously concluded for λ (3, 24).
To test when this action occurred, we applied 10-min pulses of IPTG at various times after infection and measured lysogenization frequencies (Fig. 2B). Cells were initially grown at 10−7 M IPTG after phage infection. Aliquots were then shifted to 10−5 M IPTG for 10-min pulses at various times. Although pulses before 15 min had no effect on the decision, those after 15 min increased the lysogenization efficiency. Pulses after 35 min also had no effect on the decision, indicating that LacR had to act before this time.
Prophage Induction.
We tested whether lysogens of the W-variants could carry out prophage induction (Fig. 3). Lysogens were irradiated with varying doses of UV light, and phage production was measured. As observed previously (9, 28), the wild type showed threshold behavior. Lysogens of the W-variants were also able to switch from the lysogenic to the lytic state upon induction of the SOS system (Fig. 3A) and also showed threshold behavior. The behavior of AWCF was closest to that of wild type. Because LacR cannot repress PRM in the W-variants (see also Lack of Effect of LacR on PRM), these findings showed that the lytic repressor (LacR) does not need to repress PRM during this process.
Fig. 3.
UV dose responses for prophage induction. Exponentially growing cultures of lysogens were centrifuged, suspended in TMG, and irradiated (see Methods) at the indicated doses; aliquots were diluted 10-fold in LBGM, shaken for 2 h at 37°C, treated with CHCl3, and titered. Values given are the average for three independent experiments. (A) Dose responses for wild type (λJL351) and the indicated variants. (B) Effect of IPTG on burst size. An AWCA lysogen was induced without IPTG (crosses) or with 10−5 M (open diamonds) or 10−4 M (open squares) IPTG. (C) Induction of AWCA lysogen in reciprocal IPTG-concentration-shift experiment. IPTG concentration shifted from 10−4 M to 10−5 M (open circles) and from 10−5 M to 10−4 M (open triangles) at indicated times after UV irradiation.
Three variants, AWCA, AWCD, and AWCF, differed only in the SD sequence of LacR, indicating that the strength of this site has an effect on burst size after prophage induction. Because phage with stronger SD sequences gave lower bursts, we infer that the burst defect resulted from overexpression of LacR, leading to overly strong repression of PR and/or PL.
To confirm that the burst size was sensitive to the amount of LacR function, we tested prophage induction of AWCA in the presence of IPTG. The burst size was increased with 10−5 M IPTG (Fig. 3B) but decreased again with 10−4 M IPTG. These results show that moderate repression of PR and PL is required to give a high burst size.
The finding that high levels of IPTG inhibited phage production by AWCA implies that LacR needs to act at some point during this process, presumably to inhibit expression of PL and/or PR. To delineate when this activity is required, we raised or lowered the concentration of IPTG at various times (Fig. 3C). First, lysogens of AWCA were grown with 10−4 M IPTG after UV irradiation; aliquots were then shifted to 10−5 M IPTG at intervals during the next 2 hours. Shifting to 10−5 M within 60 min resulted in high burst sizes. In the converse experiment, induced cells were shifted from 10−5 M to 10−4 M. This experiment gave the converse results; a shift after 60 min had no effect on burst size. We conclude that the lytic repressor is required between 30 and 60 min after irradiation. Because it takes ≈30 min after UV irradiation for most cells to reduce their level of CI by RecA-dependent cleavage and to switch their regulatory state (9), it is likely that the lytic repressor must be active during the first 30 min or so of the lytic cycle to allow a good burst size.
As described above, after infection, LacR plays a critical role in limiting the amount of CII made. We tested whether its role in prophage induction was likewise to give partial repression of CII by comparing the burst sizes of AWCA with those of AWCA cII− after prophage induction. The cII− derivative gave the same burst sizes as AWCA: at 0, 10−5, and 10−4 M IPTG and a UV dose of 40 J/m2, respective burst sizes were 48, 115, and 15 for AWCA, and 45, 113, and 14 for AWCA cII−. This finding implies that CII does not act after prophage induction of λlacIdim to counteract the switch to the lytic pathway and that the role of LacR in prophage induction differs from its role after infection. It is likely that the role in induction is to confer partial repression of PL and/or PR, as discussed above for lytic growth after infection. Our data suggest that overexpression from these promoters late in the prophage induction process did not impair phage production substantially.
Lack of Effect of LacR on PRM.
Because the W-variants have a normal OR3, the ability of these phages to undergo prophage induction suggests that repression of PRM by the lytic repressor (LacR in this case) is not essential for prophage induction (see also Discussion). However, it remained possible that LacR could somehow repress PRM by binding to the lacO site at PR and acting on PRM at a distance.
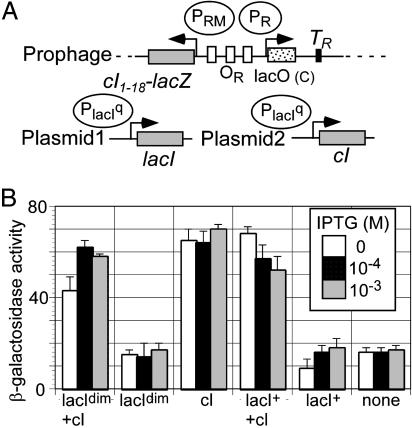
We tested this possibility by using a PRM::lacZ protein fusion (Fig. 4A). We provided LacR from a constitutive promoter, the lacIq promoter, on a multicopy plasmid. CI was similarly supplied from a lacIq::cI fusion on a separate plasmid. Fig. 4 shows the activity of the PRM promoter from AWCF in the presence and absence of IPTG, LacR, and CI. The presence of dimeric LacR had little or no effect on activity, either in the presence or absence of CI. We also tested the effect of the wild-type LacR. This protein likely can form a DNA loop between the lacO site at OR and a naturally occurring secondary operator early in the lacZ reporter (29). Perhaps for this reason, wild-type LacR gave partial repression of PRM. These results strongly suggest that the dimeric LacR does not repress PRM. We conclude from these data and those above that repression of PRM by the lytic repressor is not an essential feature of prophage induction.
Fig. 4.
Response of PRM from AWCF to presence of dimeric and wild-type LacR. (A) Design of the assay. The PRM promoter from AWCF was fused to lacZ. The PRM::lacZ protein fusion was carried on a prophage. Strains contained two plasmids. The first was either pBR322 or carried a fusion of the lacIq promoter (an up-promoter mutation of the natural lacI promoter) driving lacIdim or lacI+. The second plasmid was pGB2 or a lacIq::cI fusion. (B) Cells were grown in the absence (open bar) or presence (black bar, 10−4 M; gray bar, 10−3 M) of IPTG, and β-galactosidase levels were measured as described in Materials and Methods. None, no plasmids were present. Values given are the average of three separate experiments.
Discussion
Relevance of These Phages to λ.
The λlacIdim phage behaved in several ways like λ. We list these similarities for AWCF, the phage whose behavior most closely resembles that of the λJL351 parent we used as wild type. First, AWCF and λJL351 showed similar lysogenization frequencies after single infection and at high multiplicities. Second, both AWCF and λJL351 could form stable single lysogens. Third, AWCF and λJL351 underwent prophage induction with threshold behavior. One difference, discussed in detail below, is that AWCF had a higher set point for this process. Finally, both λ+ and AWCF required function of the lytic repressor for lytic growth after infection, and loss of repressor activity could be suppressed by a cII− mutation. We conclude that AWCF resembles λ closely enough that its behavior can provide insights into that of λ.
Lysis–Lysogeny Decision.
Transient inactivation of LacR between 15 and 35 min after infection favored entry into the lysogenic pathway (Fig. 2B). A mechanism to account for this behavior is suggested by studies with λ that examined directly the function of CII and Q proteins (3). In the absence of Cro, a persistently high level of CII activity was seen, and this prevented Q activity. Our results suggest that CII could reach levels high enough to favor lysogeny if the activity of lytic repressor is inhibited briefly after 15 min. This finding is consistent with results that the peak of CII expression in λ is at ≈20 min (3).
Inactivation of LacR function before 15 min did not increase the lysogenization frequency. Perhaps DNA replication must raise the gene dosage to a point at which overexpression of CII can activate the paQ promoter sufficiently to block the lytic pathway. It may also take a period of time for LacR levels to build up to a level required for partial repression of PL and PR.
The inability to stimulate lysogeny at later times (>35 min) is consistent with findings (3, 30) that functional levels of Q accumulate at later times. The cell has reached a point of no return and is committed to late gene expression; at this time, providing CII function is too late to halt the lytic pathway.
Cro Binding to OR3 Is Not Required for Efficient Prophage Induction but Modulates This Process.
The initial proposal (13) that Cro is critical for prophage induction was based on evidence with a phage carrying a doubly mutant OR3. These data were later reinterpreted in light of the finding that CI-mediated looping between OL and OR confers far more negative autoregulation than originally believed (see ref. 10). In a recent study using a prophage with deletions of most lytic genes and a cI857ts allele, the presence of Cro made little difference in the fraction of switched cells in cultures grown at temperatures ≤37°C and had a slight effect at 39°C (14). As argued above, it is unclear whether this result bears directly on SOS-mediated prophage induction. Hence, the question of whether Cro plays a role in SOS-mediated prophage induction has remained open.
Our evidence shows directly that a dimeric form of LacR had at most a small effect on expression of the PRM promoter from the AWCF isolate. This isolate also was able to carry out prophage induction. We conclude that repression of PRM by the lytic repressor is not necessary for prophage induction to occur in λlacIdim phages. In addition, we argue below that this conclusion is likely to apply as well to λ.
At the same time, our data suggest a more subtle role for Cro in this process. We first describe our current model for the threshold behavior of prophage induction. We believe that a major contributor to threshold behavior is the timing with which RecA is activated by DNA damage (cf. ref. 27). CI cleavage is a slow process, taking ≈20–30 min to be complete (9). If RecA does not remain activated long enough to reduce CI levels below a critical value, the likelihood of switching is low. The set point for prophage induction is the level of DNA damage at which approximately half the cells switch and half do not.
Importantly, the set point for AWCF was markedly increased relative to that of the λJL351 parental phage (Fig. 3A). Previous evidence (27, 31) indicates that the set point is determined in part by the strength of PRM; presumably, this affects the level of CI and the ability of PRM to provide more CI as CI levels fall and negative autoregulation is relieved. In AWCF, however, PRM is the same as that of wild type, and its expression is not markedly affected by dimeric LacR. If the switching process involves only activated RecA and CI (if the lytic repressor has no role in switching) one would expect the set point to be the same for AWCF and its parent. What then can account for the difference in set point?
Our evidence suggests that LacR itself plays little or no role in determining the set point, because this value is the same for AWCF, AWCD, and AWCA, which probably have different levels of LacR. The simplest interpretation of the differences in set point between λJL351 and AWCF is that, in the former, Cro protein acts to reduce the set point. In this view, at intermediate doses of DNA damage (5–10 J/m2 of UV), a substantial fraction of the cells would remain in the lysogenic state in the absence of Cro action, but Cro pushes these cells toward the lytic state, presumably by binding to OR3 and repressing PRM. Accordingly, Cro does play a role in prophage induction, but the role is to modulate the decision to switch in cells that are otherwise “on the edge” and can go either way.
Other evidence indicates that this conclusion is likely to be true in λ as well. We analyzed a mutation (termed z8) (32) lying just before the start codon of cro (A. Celovsky, C. B. Michalowski, and J.W.L., unpublished data). This mutation reduces expression of cro to ≈1/3 the level of wild type, as judged by the expression of a PR::cro::lacZ protein fusion. In prophage induction experiments, λ z8 also showed an increase in the set point, although the increase was less than that seen here with AWCF. The simplest interpretation of these findings is that reducing the level of Cro makes switching less likely at intermediate doses of DNA damage, as seen here with AWCF.
Usefulness of Module Exchange in Systems Biology.
A major goal of systems biology is to describe complex regulatory circuits well enough to predict systems behavior (30). Disentangling the circuitry is more difficult when mutations are pleiotropic or when various regulatory elements have partially redundant functions or use a common set of cis-acting sites, as in the λ case. Our data indicate that the present approach can complement other methods for dissecting circuits. It partially uncouples the circuit, but, importantly, the analysis takes place in a functional system with behaviors resembling those of the natural system. We believe that it will be useful in the analysis of other circuits, provided that they have a modular organization similar to that of λ.
Materials and Methods
Media.
Tryptone broth and LB were prepared as described in ref. 33 and were supplemented with antibiotics as appropriate; kanamycin was at 10 μg/ml. LBGM, LBMM, and Tris-Mg-gelatin (TMG) were prepared as described in ref. 28.
Phage and Bacterial Strains and Plasmids.
Phage strain λJL351 was used as wild type (27). It has a set point for prophage induction roughly half that of wild-type λ due to a silent XhoI site early in cI. This site is present in the phage analyzed here. λJL387 was λJL351 v1 v3 and was used as the cloning vector for the OR region as described (18, 27). A cII− derivative of AWCF was isolated by crossing AWCF with pJWL848 (see Supporting Text, which is published as supporting information on the PNAS web site), isolating clear plaques, and verifying the presence of the cII mutation by sequencing. P1 clr100 Cm (34) was obtained from Lee Rosner (National Institutes of Health, Bethesda). Bacterial strain JL6142 Δ(lacIPOZYA) (18) was used as wild type. JL7223 was an hfl− derivative of JL6142 made by transduction with P1 clr100 Cm by using JL6039 hflA150 (linked to Tn10) as donor (27), selecting for tetracycline resistance and screening for the Hfl phenotype as described in ref. 27.
Maps and construction of plasmids used in this work are shown in Fig. 7, which is published as supporting information on the PNAS web site. pJWL614 and 839 are derivatives of pBR322 and AmpR and carry the lacIq promoter driving lacI+ and lacIdim, respectively. pJWL843, a derivative of pGB2, carries the lacIq promoter driving cI; it is Spcr and compatible with pACYC184- and ColE1-derived plasmids. On cells carrying pJWL843, λJL351 and AWCF plated with low efficiency and made tiny plaques, indicating that the cells had partial immunity. pJWL614 and pJWL839 made cells immune to AWCF but not to λJL351. These results indicate that CI and LacR are expressed from these plasmids.
Screening of λlacI.
Preparation of the phage library and screening were as described (18) and detailed in Supporting Text, using oligonucleotides listed in Table 3, which is published as supporting information on the PNAS web site, except that we used a mutant lacI gene coding for a shortened LacR protein (lacIdim) of 333 residues. Numbers of recombinants were estimated from a control experiment (Table 4, which is published as supporting information on the PNAS web site). Phages of this library were plated on JL6142 without or with IPTG at 10−5 M or 10−4 M. Turbid plaques were purified and analyzed by PCR; isolates with a fragment of the expected size were further characterized. Segments of the λlacIdim phages were amplified by PCR of plaques as described in ref. 18. PCR products (PL-OL and CI-PRM-OR-PR-lacI-λ NsiI) were sequenced by automated cycle sequencing at the Division of Biotechnology, University of Arizona.
Test for Single Lysogens.
This test was carried out as described in ref. 35. A single lysogen gives a single PCR product; the presence of a second product indicates either the presence of multiple prophages or the presence of replicating λ DNA, resulting from spontaneous switching of a relatively unstable lysogen.
Lysogenization Frequency.
JL6142 was grown in LBMM to 2 × 108 per ml, centrifuged, concentrated 10-fold in TMG, and mixed with phage at a moi of ≈0.01 (for single infection) or ≈6, as indicated; after 20 min at room temperature, aliquots were diluted into LBGM containing 0, 10−5, 10−4, or 10−3 M IPTG, shaken for 30 min at 37°C, and plated in 3 ml of top agar plus 300 μl LB on tryptone plates with 10 μg/ml kanamycin. For the IPTG concentration-shift assay (Fig. 2B), after LBGM was added as described above, IPTG was added at the indicated times, and the solution was diluted 100-fold with LBGM after another 10 min.
Prophage Induction.
Prophage induction was done as described (27). For the IPTG concentration-shift assay, after UV irradiation, IPTG concentration was shifted by adding IPTG or by diluting the culture 10-fold with LBGM at indicated times.
Reporter Assays.
λJL611 (27) was crossed with a derivative of pRS414 (36) bearing the OR region of AWCF to make the reporter construct (Fig. 4A), which was used as a single lysogen. In each strain, except the control lacking cI and lacI, cells carried two plasmids. The first plasmid carried lacIdim (pJWL839) or lacIwt (pJWL614), or was the vector (pBR322). The second plasmid carried cI (pJWL843) or was the vector (pGB2). β-Galactosidase assays were as described (27).
Supplementary Material
Acknowledgments
We thank Kathleen Matthews for helpful discussions; Kim Giese, Andy Celovsky, Don Court, and Sankar Adhya for comments on the manuscript; and Lee Rosner for strains. This work was supported by National Institutes of Health Grant GM24178.
Abbreviations
- IPTG
isopropyl-β-d-galactopyranoside
- LacR
lac repressor
- moi
multiplicity of infection
- SD
Shine–Dalgarno
- W-variant
variant carrying a normal OR3.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Herskowitz I., Hagen D. Annu. Rev. Genet. 1980;14:399–445. doi: 10.1146/annurev.ge.14.120180.002151. [DOI] [PubMed] [Google Scholar]
- 2.Ptashne M. A Genetic Switch: Phage Lambda Revisited. Woodbury, NY: Cold Spring Harbor Lab. Press; 2004. [Google Scholar]
- 3.Kobiler O., Rokney A., Friedman N., Court D. L., Stavans J., Oppenheim A. B. Proc. Natl. Acad. Sci. USA. 2005;102:4470–4475. doi: 10.1073/pnas.0500670102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kourilsky P. Mol. Gen. Genet. 1973;122:183–195. doi: 10.1007/BF00435190. [DOI] [PubMed] [Google Scholar]
- 5.Roberts J. W., Devoret R. In: Lambda II. Hendrix R. W., Roberts J. W., Stahl F. W., Weisberg R. A., editors. Woodbury, NY: Cold Spring Harbor Lab. Press; 1983. pp. 123–144. [Google Scholar]
- 6.Little J. W., Mount D. W. Cell. 1982;29:11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- 7.Little J. W. Proc. Natl. Acad. Sci. USA. 1984;81:1375–1379. [Google Scholar]
- 8.Little J. W. J. Bacteriol. 1993;175:4943–4950. doi: 10.1128/jb.175.16.4943-4950.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailone A., Levine A., Devoret R. J. Mol. Biol. 1979;131:553–572. doi: 10.1016/0022-2836(79)90007-x. [DOI] [PubMed] [Google Scholar]
- 10.Dodd I. B., Perkins A. J., Tsemitsidis D., Egan J. B. Genes Dev. 2001;15:3013–3022. doi: 10.1101/gad.937301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodd I. B., Shearwin K. E., Perkins A. J., Burr T., Hochschild A., Egan J. B. Genes Dev. 2004;18:344–354. doi: 10.1101/gad.1167904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer B. J., Maurer R., Ptashne M. J. Mol. Biol. 1980;139:163–194. doi: 10.1016/0022-2836(80)90303-4. [DOI] [PubMed] [Google Scholar]
- 13.Johnson A. D., Poteete A. R., Lauer G., Sauer R. T., Ackers G. K., Ptashne M. Nature. 1981;294:217–223. doi: 10.1038/294217a0. [DOI] [PubMed] [Google Scholar]
- 14.Svenningsen S. L., Constantino N., Court D. L., Adhya S. Proc. Natl. Acad. Sci. USA. 2005;102:4465–4469. doi: 10.1073/pnas.0409839102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenowitz M., Mandal N., Pickar A., Jamison E., Adhya S. J. Biol. Chem. 1991;266:1281–1288. [PubMed] [Google Scholar]
- 16.Alberti S., Oehler S., Von Wilcken-Bergmann B., Krämer H., Müller-Hill B. New Biol. 1991;3:57–62. [PubMed] [Google Scholar]
- 17.Chen J., Matthews K. S. J. Biol. Chem. 1992;267:13843–13850. [PubMed] [Google Scholar]
- 18.Atsumi S., Little J. W. Genes Dev. 2004;18:2086–2094. doi: 10.1101/gad.1226004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell C. E., Lewis M. Nat. Struct. Biol. 2000;7:209–214. doi: 10.1038/73317. [DOI] [PubMed] [Google Scholar]
- 20.Chen J., Matthews K. S. Biochemistry. 1994;33:8728–8735. doi: 10.1021/bi00195a014. [DOI] [PubMed] [Google Scholar]
- 21.Darling P. J., Holt J. M., Ackers G. K. Biochemistry. 2000;39:11500–11507. doi: 10.1021/bi000935s. [DOI] [PubMed] [Google Scholar]
- 22.LeFevre K. R., Cordes M. H. J. Proc. Natl. Acad. Sci. USA. 2003;100:2345–2350. doi: 10.1073/pnas.0537925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisen H., Brachet P., Pereira da Silva L., Jacob F. Proc. Natl. Acad. Sci. USA. 1970;66:855–862. doi: 10.1073/pnas.66.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Echols H., Green L., Oppenheim A. B., Oppenheim A., Honigman A. J. Mol. Biol. 1973;80:203–216. doi: 10.1016/0022-2836(73)90167-8. [DOI] [PubMed] [Google Scholar]
- 25.Folkmanis A., Maltzman W., Mellon P., Skalka A., Echols H. Virology. 1977;81:352–362. doi: 10.1016/0042-6822(77)90151-9. [DOI] [PubMed] [Google Scholar]
- 26.Eisen H., Georgiou M., Georgopoulos C. P., Selzer G., Gussin G., Herskowitz I. Virology. 1975;68:266–269. doi: 10.1016/0042-6822(75)90168-3. [DOI] [PubMed] [Google Scholar]
- 27.Michalowski C. B., Short M. D., Little J. W. J. Bacteriol. 2004;186:7988–7999. doi: 10.1128/JB.186.23.7988-7999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Little J. W., Shepley D. P., Wert D. W. EMBO J. 1999;18:4299–4307. doi: 10.1093/emboj/18.15.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oehler S., Eismann E. R., Krämer H., Müller-Hill B. EMBO J. 1990;9:973–979. doi: 10.1002/j.1460-2075.1990.tb08199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Little J. W. Proc. Natl. Acad. Sci. USA. 2005;102:5310–5311. doi: 10.1073/pnas.0501645102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michalowski C. B., Little J. W. J. Bacteriol. 2005;187:6430–6442. doi: 10.1128/JB.187.18.6430-6442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pakula A. A., Young V. B., Sauer R. T. Proc. Natl. Acad. Sci. USA. 1986;83:8829–8833. doi: 10.1073/pnas.83.23.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller J. H. Experiments in Molecular Genetics. Woodbury, NY: Cold Spring Harbor Lab. Press; 1972. [Google Scholar]
- 34.Rosner J. L. Virology. 1972;49:679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- 35.Powell B. S., Court D. L., Nakamura Y., Rivas M. P., Turnbough C. L., Jr. Nucleic Acids Res. 1994;22:5765–5766. doi: 10.1093/nar/22.25.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons R. W., Houman F., Kleckner N. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.