Abstract
Treatment of genetic disease such as the bleeding disorder hemophilia B [deficiency in blood coagulation factor IX (F.IX)] by gene replacement therapy is hampered by the risk of immune responses to the therapeutic gene product and to the gene transfer vector. Immune competent mice of two different strains were tolerized to human F.IX by hepatic gene transfer mediated by adenoassociated viral vector. These animals were subsequently challenged by systemic administration of an E1/E3-deleted adenoviral vector, which is known to induce a cytotoxic T lymphocyte response to the transgene product. Immune tolerance prevented cytotoxic T lymphocyte activation to F.IX and CD8+ cellular infiltrates in the liver. Moreover, a sustained and substantial increase in hepatic F.IX expression from the adenoviral vector was achieved despite in vitro T cell responses to adenoviral antigens. Cytolytic responses to therapeutic and to viral vector-derived antigens had been prevented in vivo by activation of regulatory CD4+ T cells, which mediated suppression of inflammatory lymphocyte responses to the liver. This result suggests that augmentation of regulatory T cell activation should provide new means to avoid destructive immune responses in gene transfer.
Keywords: adenoassociated virus, gene therapy, tolerance, hemophilia
Immune responses to vector-derived antigens or to the transgene product are a serious hurdle for gene replacement therapy. Upon in vivo gene transfer, signals derived from the procedure or from the presence of vector-associated molecular structures (such as viral particles) can cause activation of professional antigen-presenting cells. The ensuing adaptive immune response leads to production of neutralizing antibodies to the vector that abolish the ability to readminister. Antibodies to the transgene product may enhance clearance or interfere with the biological activity of the therapeutic protein. This issue is of particular concern in treatment of systemic protein deficiencies such as the X-linked bleeding diathesis hemophilia B, which is caused by mutations in the coagulation factor IX (F.IX) gene (1). Equally important, activation of CD8+ T cells can result in a cytotoxic T lymphocyte (CTL) response capable of eliminating cells that display transgene product- or vector-derived peptides on their surface. Antibody formation and efficient CTL responses typically depend on T help.
In general, the risk for immune responses is influenced by a number of factors, including the design of the vector, the target tissue, levels of gene expression, and genetic factors. Immune responses to the transgene product are more likely if a non-self protein is expressed (2). Gene transfer to the liver with an early-generation adenoviral vector often results in high but transient levels of expression because of CTL-mediated elimination of hepatocytes (3, 4). Transduced hepatocytes are killed mostly by Fas–Fas ligand-induced cell death mediated by CD8+ T cells, which are part of a lymphocytic inflammatory infiltrate and are specific to the transgene product or to adenoviral proteins expressed from the vector backbone (5–7). Additionally, antibodies to the transgene product are produced that eliminate systemic expression (8, 9). Adenoassociated viral (AAV) vectors have been more suitable for sustained transgene expression because of lower innate immunity, less efficient transduction of antigen-presenting cells, lack of viral coding sequences, and lower potential to cause inflammation (10–13). Nonetheless, cellular and humoral responses remain obstacles (14–16).
Upon in vivo gene transfer, hepatocyte-restricted transgene expression has the capacity to provide therapeutic circulating levels while avoiding deleterious immune responses and, moreover, inducing immune tolerance to the transgene product (9, 10, 17–24). Tolerized animals fail to form antibodies to this neoantigen after subsequent attempts to immunize with protein formulated in adjuvant (23–25). Deletion and anergy of CD4+ T cells with receptor to the transgene product has been described (22). Furthermore, activation of CD4+ regulatory T cells (Treg) capable of suppressing antibody formation after adoptive transfer has been documented (23). The significance of this discovery and effects on cellular responses had been unknown.
This study demonstrates that gene transfer to the liver not only results in a lack of CTL responses but also actively prevents subsequent cytolytic responses to the transgene product and to viral antigens expressed in the liver through an in vivo suppression mechanism that is mediated by CD4+ Treg (which had been activated by hepatic F.IX expression). Therefore, one should be able to develop protocols that abrogate undesired T cell responses to transduced cells by augmenting Treg responses to the transgene product.
Results
Sustained Expression After Secondary Gene Transfer with Adenoviral Vector.
These experiments were designed to test the hypothesis that animals tolerized to F.IX by hepatic gene transfer will continue to express the transgene after administration of a known activator of CTLs [an E1/E3-deleted adenoviral vector, Ad-hF.IX, expressing human F.IX (hF.IX) from the CMV immediate-early enhancer/promoter]. Male immune competent C3H/HeJ (H-2k) and BALB/c (H-2d) mice received portal vein injections of AAV-ApoE/hAAT-hF.IX for expression of hF.IX from a hepatocyte-specific promoter [1 × 1011 vector genomes (vg) per mouse; n = 4 per strain]. Two strains were used to eliminate strain-specific observations. In previous work we showed that hepatic gene transfer with this vector and dose directs sustained systemic expression of hF.IX in these strains at levels of up to 300 ng/ml (note that hepatic AAV gene transfer in C3H and BALB/c mice is less efficient compared with widely used C57BL/6 mice) (23).
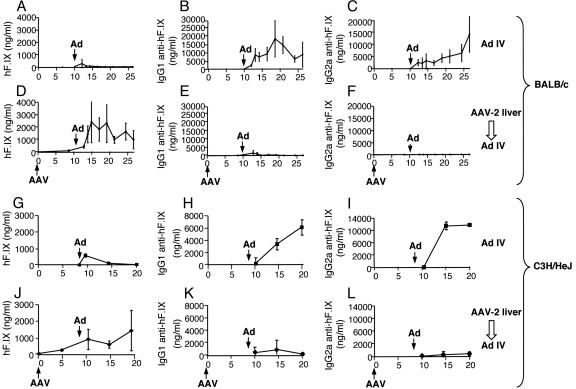
All animals showed systemic hF.IX levels (50–300 ng/ml) without evidence for anti-hF.IX IgG (Fig. 1D–F and J–L). Age- and sex-matched control mice (n = 4 per strain) did not receive AAV gene transfer. As expected, hF.IX expression in plasma of these control mice was transient after i.v. injection of Ad-hF.IX (4 × 1010 particles per mouse) and became undetectable within 4 weeks (Fig. 1 A and G). These mice formed high-titer IgG1 and IgG2a antibodies to hF.IX, indicating T helper 2- and T helper 1-dependent responses (Fig. 1 B, C, H, and I). No hF.IX expression was detectable in the liver when analyzed after 3–4 months (Fig. 2E and K).
Fig. 1.
Plasma levels of hF.IX (A, D, G, and J), IgG1 (B, E, H, and K), and IgG2a (C, F, I, and L) anti-hF.IX in BALB/c (A–F) or C3H/HeJ (G–L) mice as a function of time. All mice (n = 4 per group) received i.v. injection of Ad-hF.IX at week 10 (marked “Ad”; 4 × 1010 particles per mouse). Mice were naïve at this time point (A–C and G–I) or had previously received hepatic AAV-hF.IX gene transfer (marked “AAV”) at day 0 (1 × 1011 vg per mouse; D–F and J–L).
Fig. 2.
Liver sections of C3H/HeJ (A–D) and BALB/c (E–H) mice 2 weeks (A–D and G–J) or 3–4 months (E, F, K, and L) after i.v. infusion of Ad-hF.IX (4 × 1010 particles per mouse). Mice were naïve at the time of adenoviral gene transfer (A, C, E, G, I, and K) or had received hepatic AAV-hF.IX gene transfer 10 weeks earlier (B, D, F, H, J, and L). Immunostaining for hF.IX (red) and CD8 (green) is shown in A, B, E–H, K, and L. Hematoxylin and eosin staining is shown in C, D, I, and J. (Original magnification: ×100.)
In contrast, mice expressing hF.IX from prior hepatic AAV gene transfer showed an increase in hF.IX plasma levels to, on average, 1–2 μg/ml, when Ad-hF.IX vector was injected 9–10 weeks after the initial AAV administration. These levels (20–40% of normal levels in a human) would be curative of the disease. Expression was sustained for the duration of the experiment (3–4 months; Fig. 1 D and J). Immunostaining showed expression in 10–30% of hepatocytes in C3H/HeJ and in 40–60% of hepatocytes in BALB/c livers, respectively (3–4 months after Ad-hF.IX gene transfer; Fig. 2 F and L). Antibody formation to hF.IX was low-titer and transient (Fig. 1 E, F, K, and L).
Tolerized Mice Lack CD8+ Cellular Infiltrate in the Liver and Cytolytic Activity to hF.IX.
The experiment outlined above was repeated, except that mice were killed 2 weeks after Ad-hF.IX gene transfer (n = 4 per cohort). Livers were analyzed by immunohistochemistry by using a dual-antibody stain for hF.IX and CD8. Control mice (no prior AAV gene transfer) exhibited hF.IX expression by hepatocytes and, additionally, substantial CD8+ cellular infiltrates, consistent with CTL responses targeting the liver (Fig. 2 A and G). Livers of BALB/c mice generally showed stronger inflammation than those of C3H/HeJ mice (hematoxylin and eosin stains in Fig. 2 C and I). Although mice with prior AAV-hF.IX gene transfer also showed hF.IX expression by hepatocytes, CD8+ infiltrate was not detected or was limited to few cells (Fig. 2 B and H and data not shown), which correlated with sustained transgene expression shown in Fig. 1. Inflammation was limited in these livers and may reflect innate immunity to adenovirus (Fig. 2 D and J).
In vitro CTL assays demonstrated cytolytic activity against hF.IX-expressing MHC class I-matched target cells using effector cells (splenocytes) from control mice injected with the Ad-hF.IX vector, but not using cells from mice tolerized to hF.IX by prior hepatic AAV gene transfer (Fig. 3A; three independent experiments in C3H/HeJ mice). Nonetheless, the latter animals had detectable cytolytic activity to target cells transduced with an Ad-LacZ vector, indicating activation of CTLs to adenoviral vector-derived antigens (Fig. 3B). Analysis of genomic DNA by real-time PCR showed that livers of C3H/HeJ mice with prior hepatic AAV gene transfer maintained more Ad-hF.IX genomes (0.81 ± 0.28 copies per diploid genome at week 2 and 0.36 ± 0.11 at 3 months) than control mice (0.55 ± 0.13 at week 2 and 0.1 ± 0.01 at 3 months; n = 4 per group; data not shown). Compared with controls, mice tolerized to hF.IX had reduced frequencies of T cells reactive not only to hF.IX but also to adenoviral vector after injection of Ad-hF.IX, as determined by ELISpot assay (Table 1). Frequencies of IFN-γ- and IL-2-secreting cells in response to stimulation with adenoviral vector particles were 19–50% of those observed in controls.
Fig. 3.
In vitro CTL assay for hF.IX-specific (A) or adenovirus-specific (B) cytolytic activity of splenocytes isolated from C3H/HeJ mice that were naïve at the time of adenoviral gene transfer (squares in A) or had received hepatic AAV-hF.IX gene transfer 10 weeks earlier (circles in A and diamonds in B). Splenocytes were isolated 10 days after adenoviral gene transfer. C3H-derived fibroblasts expressing hF.IX (filled symbols in A) or no additional antigen (open symbols in A and B) or that had been infected with Ad-LacZ vector (filled squares in B) were used as targets. Data in A represent average results ± SD of three independent experiments (each involving splenocytes pooled from three animals per experimental group). B is the result of a single experiment using pooled splenocytes from three animals. Each effector:target ratio for each pool of effector cells was assayed in quadruplicate.
Table 1.
Frequency of cytokine-producing splenic C3H/HeJ T cells (spot-forming units per 106 cells) in response to in vitro stimulation with mock, Ad-hF.IX, Ad-LacZ, or hF.IX antigen by ELISpot
| Cytokine | Experimental group | Mock | Ad-hF.IX | Ad-LacZ | hF.IX |
|---|---|---|---|---|---|
| IFN-γ | AAV-hF.IX→Ad-hF.IX | 4 ± 2 | 329 ± 96∗ | 80 ± 46∗ | 9 ± 4 |
| Ad-hF.IX only | 17 ± 9 | >900∗ | 424 ± 111∗ | 50 ± 33 | |
| IL-2 | AAV-hF.IX→Ad-hF.IX | 3 ± 1 | 56 ± 19∗ | 15 ± 9∗ | 3 ± 1 |
| Ad-hF.IX only | 7 ± 6 | 114 ± 18∗ | 47 ± 11∗ | 20 ± 13 |
By using the identical vector administration protocol from Fig. 1, mice were naïve at the time of adenoviral gene transfer (Ad-hF.IX only) or had received hepatic AAV-hF.IX gene transfer 10 weeks earlier (AAV-hF.IX→Ad-hF.IX). Results are averages of four animals per experimental group ± SD.
∗, P < 0.01 (statistically significant difference between AAV-hF.IX-transduced group and control group).
Antigen Specificity and Requirements.
In the next set of experiments, we challenged AAV-hF.IX-transduced C3H/HeJ mice with Ad-LacZ vector and AAV-LacZ-transduced mice with Ad-hF.IX vector. AAV vector administration was again by portal vein infusion, and adenoviral vector was given 1.5 months later by tail vein injection. As expected, an additional cohort receiving AAV-hF.IX followed by Ad-hF.IX showed high levels of hF.IX expression with nearly undetectable levels of anti-hF.IX 1 month after adenoviral gene transfer (Fig. 4A). Mice that were injected with AAV-hF.IX followed by Ad-LacZ continued to express hF.IX (50–100 ng/ml) without anti-hF.IX formation (Fig. 4). Consistent with CTL responses expected for this vector, hepatic expression of β-galactosidase became undetectable by 1 month (data not shown). Superinfected hepatocytes expressing both hF.IX (from AAV) and LacZ (from adenovirus vector) were detectable at weeks 1–2 but not subsequently, indicating that the immune response targeted all Ad-LacZ-transduced cells (data not shown). In the reverse experiment, AAV-LacZ-transduced mice that subsequently received Ad-hF.IX vector lost hF.IX expression and developed antibodies to hF.IX within 1 month after Ad-hF.IX gene transfer (Fig. 4). These results show that hepatic AAV gene transfer did not cause a general unresponsiveness of the immune system. Induction of immune tolerance to hF.IX antigen was required for sustained expression from an otherwise immunogenic adenoviral vector (expressing the same transgene). Introduction of an independent set of antigens resulted in the expected loss of adenovirally transduced hepatocytes.
Fig. 4.
Antigen requirements for tolerance induction. C3H/HeJ mice received hepatic gene transfer with AAV vector-expressing hF.IX or LacZ transgenes (1 × 1011 vg per mouse, first vector) followed by i.v. injection of Ad-hF.IX or Ad-LacZ vector (4 × 1010 particles per mouse, second vector) 6 weeks later. Levels of systemic hF.IX (A) and anti-hF.IX (B) were measured 1 month after the second gene transfer.
Regulatory CD4+ T Cells Suppress Immune-Mediated Destruction of Hepatocytes in Vivo.
The fact that hF.IX expression from the Ad-hF.IX was sustained in tolerized mice despite T cell responses to adenovirus (detectable in vitro; Fig. 2B and Table 1) indicated an in vivo suppression mechanism. To test for activation of regulatory cells, splenocytes from C3H/HeJ mice tolerized to hF.IX by hepatic AAV gene transfer were adoptively transferred to naïve mice of the same strain (Fig. 5A). Controls were injected with cells from C3H/HeJ mice that had not received gene transfer. On the following day all recipient mice were given an i.v. injection of Ad-hF.IX vector.
Fig. 5.
Adoptive transfer of CD4+ and CD4-depleted splenocytes from hepatic AAV-hF.IX-transduced (hatched bars in graphs) or naïve control (open bars) C3H/HeJ mice. (A) Experimental outline. Six weeks after hepatic AAV-hF.IX gene transfer, cells were transferred by tail vein injection into naïve mice of the same strain. Control groups were given splenocytes from naïve mice. After 36 h, all animals received tail vein injection of Ad-hF.IX vector (4 × 1010 particles per mouse; n = 4 per experimental group). Systemic hF.IX levels were measured 1 month (B) and 2 months (C) after adenoviral gene transfer. D depicts anti-hF.IX levels at 2 months. Results in B–D are averages ± SD.
All recipients of CD4+ splenocytes from tolerized mice showed prolonged hF.IX expression (n = 4 per experimental group; Fig. 5 B and C). At 2 months, systemic expression was, on average, 0.7 μg/ml (14% of normal human levels and therefore well in the therapeutic range), and 1–2% of hepatocytes (as determined by image analysis) stained positive for hF.IX (Fig. 6A and B). Mice that received CD4+ cells from naïve controls without exception lost systemic and hepatic hF.IX expression by this time point (Figs. 5B and 6D). Animals injected with CD4-depleted cells from tolerized mice expressed undetectable to low levels of hF.IX in the circulation by 2 months (Fig. 5C) and had largely lost hF.IX expression in the liver (Fig. 6C; <0.1% hF.IX-positive hepatocytes). Animals from both of these control groups, in contrast to those injected with CD4+ cells from tolerized mice, developed higher-titer antibodies to hF.IX (Fig. 5D). Livers of mice that had received CD4+ cells from tolerized animals showed normal morphology and did not contain CD8+ cells at 2 months (Fig. 6 E and G), whereas livers from all other experimental groups had substantial inflammation and hepatic injury (Fig. 6 H–J). CD8+ infiltrate was still detectable in some livers of mice that received control cells (Fig. 6F). These data show that CD4+ Treg suppressed humoral and inflammatory immune responses to hF.IX and to Ad-hF.IX transduced hepatocytes.
Fig. 6.
Histochemical analyses of liver cross sections from C3H/HeJ mice that had received adoptive splenocyte transfer and Ad-hF.IX vector (2-month time point; animals are identical to those used for Fig. 5). (A–D) Immunostaining for hF.IX (red). (E and F) Immunostaining for CD8 (green). (F–J) Hematoxylin and eosin staining. Mice had received adoptive transfer of CD4+ cells from AAV-hF.IX-transduced mice (A, B, E, and G) or from naïve controls (D, F, and J) or CD4-depleted cells from AAV-hF.IX-transduced mice (C and H) or from naïve controls (I). (Original magnification: ×100.)
We used flow cytometry to determine changes in the CD4+ splenocyte population after gene transfer. There was an increase in CD4+ lymphocytes of Ad-hF.IX transduced C3H/HeJ mice compared to animals that had not been subjected to gene transfer. This rise was largely due to an increase in CD4+CD62L+ cells and was greater in tolerized animals (i.e., mice that had received prior AAV-hF.IX gene transfer; Fig. 7A). This result indicates homing of CD4+ T cells to this lymphoid organ. In all groups, ≈90% of CD4+CD62L+ cells were CD25− (data not shown). Only CD4+ splenocytes from tolerized mice showed a measurable (1.8-fold) increase in mRNA levels of the transcription factor FoxP3, which is expressed by naturally occurring CD4+ Treg (Fig. 7C). Such Treg constitutively express CD25 and glucocorticoid-induced TNF receptor (GITR) on the cell surface, and we indeed found a correlating increase in CD4+CD25+GITR+ splenocytes in tolerized mice (Fig. 7B).
Fig. 7.
Analyses of splenocyte populations in C3H/HeJ mice, which were naïve (open bars), had received Ad-hF.IX only (shaded bars), or had received hepatic AAV-hF.IX gene transfer 10 weeks before Ad-hF.IX administration (filled bars). Splenocytes were isolated 10 days after adenoviral gene transfer. Shown are the percentage of CD4+CD62L+ of total splenocytes (A) and the percentage of CD4+CD25+GITR+ of total splenocytes (B) as determined by flow cytometry. (C) Levels of FoxP3 mRNA in CD4+ splenocytes relative to naïve controls (which were set as 100%) as measured by quantitative RT-PCR. Results are average ± SD for n = 4 mice per experimental group.
Discussion
Treatment of inherited protein deficiency by gene therapy may be limited by immune responses to the therapeutic transgene product, which may represent a neoantigen. Recent studies demonstrate induction of immune tolerance to the transgene product by restricting expression to hepatocytes (9, 10, 17–20, 24). Our study reveals an in vivo suppression mechanism that is mediated by CD4+ Treg and that is capable of blocking the development of an inflammatory CD8+ T cell response that otherwise leads to immune-mediated destruction of hepatocytes.
Tolerance induction to hF.IX by AAV-mediated hepatic gene transfer blocked subsequent activation of CTL responses to hF.IX and allowed for systemic administration of a viral vector that normally causes hepatocellular destruction. Earlier studies suggested that CTL responses to adenoviral vector-derived antigens were sufficient to eliminate transgene expression (7). However, our study demonstrates that it is possible to prevent at least adaptive immune responses to vg-encoded antigens by prior induction of tolerance to the transgene product. Because inflammatory signals derived from infection, injury, or repeat administration of viral vector can cause activation of T cells, it is important that hyporesponsiveness among cytolytic T cells is established. Despite the well documented immunogenicity of proteins expressed from the adenoviral genome (5), we achieved sustained increases in hF.IX expression levels upon secondary gene transfer with this vector.
Although we observed in vitro cytokine production and cytolytic activity to adenoviral antigens, this response did not cause T cell infiltrates in the liver or loss of transgene expression in vivo (“split tolerance”). These results pointed toward an in vivo suppression mechanism, which we have been able to confirm by adoptive transfer experiments. Transfer of CD4+ cells, which contained hF.IX antigen-experienced cells (but which were naïve to adenoviral antigens), was sufficient to suppress elimination of adenovirus-derived hF.IX expression and limit inflammation in the liver (Figs. 5 and 6). Additional analyses of splenocyte populations (Fig. 7) suggest a complex pattern of CD4+ T cell activation, which includes an increase in Treg with a phenotype comparable to that of naturally occurring CD4+CD25+ Treg. Future experiments should address the relative contributions of CD25+ and CD25− cells to the regulatory response. Thus far we have obtained only low levels of IL-10 or TGF-β secretion after in vitro stimulation with hF.IX in cytokine release assays, suggesting that type 1 T regulatory cells, T helper 2, or T helper 3 cells do not play a major role here (data not shown and refs. 15 and 22).
Expression of the same transgene by both (AAV and adenoviral) vectors was important to achieve a sustained increase in expression while avoiding detrimental immune responses. The reduction in the frequency of T cells reactive to adenovirus may be explained by a “bystander” suppression-effect Treg with reactivity to hF.IX (26). Alternatively, T cell responses to adenoviral antigens could have been amplified by the response to hF.IX in control animals. In tolerized mice, only transient hF.IX expression was observed in other tissues such as lung or spleen (data not shown) or if secondary adenoviral gene transfer was directed to skeletal muscle (unpublished observations). Thus, the liver likely has distinct properties with regard to antigen presentation or homing and function of Treg.
Recent clinical studies have shown that hepatic AAV-hF.IX gene transfer in human subjects with hemophilia B can be associated with loss of transgene expression due to a memory CD8+ T cell response to AAV capsid (14). Therefore, one has to caution that the activity of Treg may be less effective in the context of memory T cells to viral antigens that were originally generated by natural infection. Nonetheless, our current study provides proof-of-principle that the immune system has a mechanism in place that can be exploited to overcome CD8+ T cell responses in gene transfer. CD4+ Treg play a role in promoting sustained transgene expression by suppressing T cell responses to the transgene product and to viral antigens. Tolerance induction protocols to the transgene product that expand Treg may therefore hold the key to sustained therapeutic expression.
Methods
Viral Vectors.
AAV vector expressing an hF.IX minigene from the apolipoprotein A enhancer linked to the human α1-antitrypsin promoter (ApoE/hAAT) was as described (23). AAV (serotype 2) vector was produced by triple transfection of HEK-293 cells and purified by repeated CsCl centrifugation as published (27). Vector titers were determined by quantitative slot blot hybridization. AAV2-LacZ vector for expression of β-galactosidase from the EF1α promoter was kindly provided by H. Nakai (University of Pittsburgh, Pittsburgh). E1/E3-deleted adenoviral vector expressing hF.IX or β-galactosidase from the CMV immediate-early enhancer/promoter was as described earlier (8, 28). This vector was produced by infection of HEK-293 cells and purified by CsCl centrifugation by using standard protocols.
Animal Experiments.
Male C3H/HeJ and BALB/c mice (4–6 weeks old) were purchased from The Jackson Laboratory. AAV vector (1 × 1011 vg per animal) was infused into the portal vein as described (29). Adenoviral gene transfer (4 × 1010 particles per mouse) was through tail vein injection. Blood was collected from mice by retroorbital bleeding into heparinized capillary tubes. Plasma levels of hF.IX and anti-hF.IX IgG were measured by ELISA as published (8).
In Vitro CTL Assay.
Splenocytes (pooled from three mice per experimental group) were cocultured with irradiated, C3H-derived C3H/10T fibroblasts (American Type Culture Collection) stably transfected with an hF.IX-expressing plasmid. In vitro stimulation was carried out for 5 days as published (27). Cytolytic activity against hF.IX-expressing C3H/10T or untransfected C3H/10T (mock) target cells was determined with the CytoTox96 nonradioactive cytotoxicity assay from Promega, which is based on colorimetric detection of lactate dehydrogenase, a stable cytosolic enzyme that is released upon cell lysis (16). To measure CTL responses to adenoviral antigens, splenocytes were stimulated with Ad-LacZ vector (multiplicity of infection = 0.1 infectious particles per cell) as described above, and target cells for lysis were C3H/10T infected with Ad-LacZ (multiplicity of infection = 10) 24 h earlier. For each effector:target cell ratio (ranging from 100:1 to 0.4:1), specific target cell lysis was calculated as follows: [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. Each effector:target ratio was measured in quadruplicate.
ELISpot for Cytokine-Secreting Cells.
ELISpot kits for mouse IFN-γ and IL-2 cytokines from BD Biosciences were used (15, 16). Splenocytes from individual animals (isolated 10 days after Ad-hF.IX injection; n = 4 per experimental group) were applied in duplicate at 106 cells per well (coated with cytokine-specific antibody) in RPMI medium 1640/10% FBS and incubated for 3 days. Media additionally contained 10 μg/ml hF.IX-derived peptide containing an immunodominant CD4+ T cell epitope (kindly provided by E. Armstrong, Children’s Hospital of Philadelphia, Philadelphia), heat-inactivated Ad-hF.IX or Ad-LacZ (250 particles per cell), or mock. Spots were detected with 3-amino-9-ethylcarbazole substrate after incubation with horseradish peroxidase-conjugated anti-mouse IFN-γ or IL-2 and counted with the ImmunoSpot Analyzer (Hightech Instruments, Edgemont, PA). Results were calculated as spot-forming units per 106 total cells.
Immunostaining of Hepatic Tissue.
Cryosections of liver tissue were processed as published (15). Anti-CD8β (Pharmingen) and goat anti-hF.IX (Affinity Biologicals, Ontario, Canada) were applied (each at 1:100 dilution) for dual stain by using secondary antibody conjugated to FITC (Pharmingen) for CD8 stain and tetramethylrhodamine B isothiocyanate (TRITC) for hF.IX (Caltag; 1:100 dilution). Sections were viewed with the Eclipse E800 microscope (Nikon). At least 10 slides containing two sections each were analyzed per animal (n = 4 per experimental group). Images were captured with a Cool Snap-Pro camera and analyzed with image pro-plus software (both from Media Cybernetics, Silver Spring, MD).
Adoptive Lymphocyte Transfer.
Splenocytes were isolated from C3H/HeJ mice (naïve or 1.5 months after hepatic AAV-hF.IX administration). CD4+ T cells were purified from splenocytes (pooled from four mice per experimental group) by magnetic cell sorting by using a column for positive selection with anti-murine CD4 (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions (23). Analysis by flow cytometry showed >90% purity of CD4+ T cells. CD4+ cells (1 × 106 per recipient mouse) or CD4+ cell-depleted splenocytes (1 × 107 per recipient) were adoptively transferred to naïve C3H/HeJ mice by means of tail injection (n = 4 per cell type and experimental group). Ad-hF.IX vector (4 × 1010 per animal) was injected into tail veins of recipient mice 36 h later.
Flow Cytometry and RT-PCR.
Splenocytes were analyzed for cell-surface markers by flow cytometry by using triple color stains with combinations of antibodies to murine CD4 and CD25 (Caltag), CD62L (Pharmingen), and GITR (R & D Systems). Results were analyzed with cellquest software (BD Biosciences). Quantitative RT-PCR (TaqMan; Applied Biosystems) for FoxP3 expression (normalized for hypoxanthine phosphoribosyltransferase transcript) in magnetically sorted CD4+ cells was done following the published protocol by Hori et al. (30).
Acknowledgments
This work was supported by National Institutes of Health Grants R01 AI/HL51390-01 and P01 HL078810-01 (to R.W.H.).
Abbreviations
- AAV
adenoassociated virus
- CTL
cytotoxic T lymphocyte
- F.IX
factor IX
- hF.IX
human F.IX
- GITR
glucocorticoid-induced TNF receptor
- Treg
regulatory T cell
- vg
vector genome.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Herzog R. W., Dobrzynski E. Semin. Thromb. Hemostasis. 2004;30:215–226. doi: 10.1055/s-2004-825635. [DOI] [PubMed] [Google Scholar]
- 2.Sabatino D. E., Armstrong E., Edmonson S., Liu Y. L., Pleimes M., Schuettrumpf J., Fitzgerald J., Herzog R. W., Arruda V. R., High K. A. Blood. 2004;104:2767–2774. doi: 10.1182/blood-2004-03-1028. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y., Ertl H. C., Wilson J. M. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y., Xiang Z., Ertl H. C., Wilson J. M. Proc. Natl. Acad. Sci. USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jooss K., Ertl H. C., Wilson J. M. J. Virol. 1998;72:2945–2954. doi: 10.1128/jvi.72.4.2945-2954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z. X., Govindarajan S., Okamoto S., Dennert G. J. Immunol. 2001;166:3035–3041. doi: 10.4049/jimmunol.166.5.3035. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y., Jooss K. U., Su Q., Ertl H. C., Wilson J. M. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]
- 8.Fields P. A., Kowalczyk D. W., Arruda V. R., Armstrong E., McCleland M. L., Hagstrom J. N., Pasi K. J., Ertl H. C., Herzog R. W., High K. A. Mol. Ther. 2000;1:225–235. doi: 10.1006/mthe.2000.0032. [DOI] [PubMed] [Google Scholar]
- 9.Pastore L., Morral N., Zhou H., Garcia R., Parks R. J., Kochanek S., Graham F. L., Lee B., Beaudet A. L. Hum. Gene Ther. 1999;10:1773–1781. doi: 10.1089/10430349950017455. [DOI] [PubMed] [Google Scholar]
- 10.De Geest B. R., Van Linthout S. A., Collen D. Blood. 2003;101:2551–2556. doi: 10.1182/blood-2002-07-2146. [DOI] [PubMed] [Google Scholar]
- 11.Jooss K., Yang Y., Fisher K. J., Wilson J. M. J. Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaiss A. K., Liu Q., Bowen G. P., Wong N. C., Bartlett J. S., Muruve D. A. J. Virol. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manno C. S., Chew A. J., Hutchison S., Larson P. J., Herzog R. W., Arruda V. R., Tai S. J., Ragni M. V., Thompson A., Ozelo M., et al. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 14.Manno C. S., Pierce G. F., Glader B., Arruda V. R., Ragni M. V., Rasko J., Ozelo M., Hoots K., Blatt P., Konkle B., et al. Nat. Med. 2006 Feb 12; doi: 10.1038/hm1358. [DOI] [PubMed] [Google Scholar]
- 15.Wang L., Cao O., Swalm B., Dobrzynski E., Herzog R. W. Gene Ther. 2005;12:1453–1464. doi: 10.1038/sj.gt.3302539. [DOI] [PubMed] [Google Scholar]
- 16.Wang L., Dobrzynski E., Schlachterman A., Cao O., Herzog R. W. Blood. 2005;105:4226–4234. doi: 10.1182/blood-2004-03-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Follenzi A., Battaglia M., Lombardo A., Annoni A., Roncarolo M. G., Naldini L. Blood. 2004;103:3700–3709. doi: 10.1182/blood-2003-09-3217. [DOI] [PubMed] [Google Scholar]
- 18.Franco L. M., Sun B., Yang X., Bird A., Zhang H., Schneider A., Brown T., Young S. P., Clay T. M., Amalfitano A., et al. Mol. Ther. 2005;12:876–884. doi: 10.1016/j.ymthe.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 19.Mount J. D., Herzog R. W., Tillson D. M., Goodman S. A., Robinson N., McCleland M. L., Bellinger D., Nichols T. C., Arruda V. R., Lothrop C. D., High K. A. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- 20.Nathwani A. C., Davidoff A., Hanawa H., Zhou J., Vanin E. F., Nienhuis A. W. Blood. 2001;97:1258–1265. doi: 10.1182/blood.v97.5.1258. [DOI] [PubMed] [Google Scholar]
- 21.Schiedner G., Hertel S., Johnston M., Dries V., van Rooijen N., Kochanek S. Mol. Ther. 2003;7:35–43. doi: 10.1016/s1525-0016(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 22.Dobrzynski E., Mingozzi F., Liu Y.-L., Cao O., Wang L., Herzog R. W. Blood. 2004;104:969–977. doi: 10.1182/blood-2004-03-0847. [DOI] [PubMed] [Google Scholar]
- 23.Mingozzi F., Liu Y.-L., Dobrzynski E., Kaufhold A., Liu J. H., Wang Y. Q., Arruda V. R., High K. A., Herzog R. W. J. Clin. Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziegler R. J., Lonning S. M., Armentano D., Li C., Souza D. W., Cherry M., Ford C., Barbon C. M., Desnick R. J., Gao G. P., et al. Mol. Ther. 2004;9:231–240. doi: 10.1016/j.ymthe.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J., Xu L. F., Haskins M. E., Ponder K. P. Blood. 2004;103:143–151. doi: 10.1182/blood-2003-06-2181. [DOI] [PubMed] [Google Scholar]
- 26.Homann D., Holz A., Bot A., Coon B., Wolfe T., Petersen J., Dyrberg T. P., Grusby M. J., von Herrath M. G. Immunity. 1999;11:463–472. doi: 10.1016/s1074-7613(00)80121-1. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y. L., Wagner K., Robinson N., Sabatino D., Margaritis P., Xiao W., Herzog R. W. BioTechniques. 2003;34:184–189. doi: 10.2144/03341dd07. [DOI] [PubMed] [Google Scholar]
- 28.Walter J., You Q., Hagstrom J. N., Sands M., High K. A. Proc. Natl. Acad. Sci. USA. 1996;93:3056–3061. doi: 10.1073/pnas.93.7.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakai H., Herzog R. W., Hagstrom J. N., Walter J., Kung S. H., Yang E. Y., Tai S. J., Iwaki Y., Kurtzman G. J., Fisher K. J., et al. Blood. 1998;91:4600–4607. [PubMed] [Google Scholar]
- 30.Hori S., Nomura T., Sakaguchi S. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]