Abstract
Antagonists of growth hormone-releasing hormone (GHRH) synthesized previously inhibit proliferation of various human cancers, but derivatisation with fatty acids could enhance their clinical efficacy. We synthesized a series of antagonists of GHRH(1-29)NH2 acylated at the N terminus with monocarboxylic or α,ω-dicarboxylic acids containing six to sixteen carbon atoms. These peptides are analogs of prior potent antagonists JV-1-36, JV-1-38, and JV-1-65 with phenylacetyl group at their N terminus. Several new analogs, including MZ-J-7-46 and MZ-J-7-30, more effectively inhibited GHRH-induced GH release in vitro in a superfused rat pituitary system than their parent compound JV-1-36 and had increased binding affinities to rat pituitary GHRH receptors, but they showed weaker inhibition of GH release in vivo than JV-1-36. All antagonists acylated with fatty acids containing 8–14 carbon atoms inhibited the proliferation of MiaPaCa-2 human pancreatic cancer cells in vitro better than JV-1-36 or JV-1-65. GHRH antagonist MZ-J-7-114 (5 μg/day) significantly suppressed the growth of PC-3 human androgen-independent prostate cancers xenografted into nude mice and reduced serum IGF-I levels, whereas antagonist JV-1-38 had no effect at the dose of 10 μg/day. GHRH antagonists including MZ-J-7-46 and MZ-J-7-114 acylated with octanoic acid and MZ-J-7-30 and MZ-J-7-110 acylated with 1,12-dodecanedicarboxylic acid represent relevant improvements over earlier antagonists. These and previous results suggest that this class of GHRH antagonists might be effective in the treatment of various cancers.
Keywords: antagonistic analogs, cancer therapy, proliferation
Antagonistic analogs of human growth hormone-releasing hormone (hGHRH) were designed to inhibit the secretion of GH from the pituitary by blocking the binding of hypothalamic GHRH to GHRH receptors (1–3). The first reported GHRH antagonist, or “standard antagonist,” has the chemical structure of [Ac-Tyr-1, d-Arg-2]hGHRH(1-29)NH2 (4). GHRH antagonists may find medical use in conditions such as acromegaly, diabetic retinopathy, or diabetic nephropathy (glomerulosclerosis). However, the main applications of GHRH antagonists would be in the field of cancer, in view of their ability to inhibit the proliferation of malignant cells dependent on GHRH or IGF-I and -II (1–3). By suppressing GH secretion from the pituitary GHRH antagonists diminish the synthesis of IGF-I in the liver and other tissues and reduce serum IGF-I levels, thus decreasing growth of IGF-I-dependent tumors (1–3). The proliferation of various experimental tumors can be also inhibited by a direct action of GHRH antagonists on the cancer cells, resulting in the reduction of IGF-I and -II levels in the tumor tissue or their secretion (1–3). Thus, GHRH antagonists previously developed in our laboratory strongly inhibited the growth of various human experimental tumors, including osteosarcomas, small-cell and non-small-cell lung carcinomas (SCLC and non-SCLC), and prostatic, colorectal, mammary, and renal cancers xenografted into nude mice (1–3). The effects were associated with a reduction in the levels of serum IGF-I, or tumoral IGF-I and/or IGF-II (1–3). Moreover, in other cancers, such as H-69 SCLC or HEC-1A endometrial tumors, in which GHRH itself acts as a growth factor, GHRH antagonists exert their antiproliferative effects by blocking the action of local autocrine/paracrine GHRH, without affecting the IGF system (1–3, 5). Direct inhibitory effects of GHRH antagonists appear to be mediated by the splice variants of GHRH receptors that have been found on various tumors (2, 3, 5, 6).
The therapeutic use of synthetic peptides is limited by their bioavailability. Strategies used to increase drug delivery in vivo include the enhancement of stability and circulation time in the bloodstream, targeting of specific tissues or cells, and facilitation of intracytoplasmic delivery (7). Among the modifications of peptides, an increase in lipophilicity by lipidation is a well accepted approach to enhance peptide–membrane interaction. Acylation with fatty acids allows the targeting of proteins and peptides to plasma membranes (8), to various subcellular organelles (9), and also to serum albumin, which has unique ligand binding properties and is abundant in the extracellular fluids (10). Lipopeptides are well defined, can be reproducibly prepared (11), exhibit long-term stability, and lack side effects and inflammatory reactions (12, 13). Unlike many small-peptide drugs, the lipidated peptides have long half-lives (14), probably because they penetrate cell membranes, where they become resistant to clearance and attack by proteases (15). N-terminal acylation of a somatostatin analog with long-chain fatty acids enhanced its stability and antiproliferative activity in human breast adenocarcinoma cells (16). Antagonists of human corticotropin releasing factor lipidated at the N terminus have also been synthesized, and it was found that antagonistic activity was independent of the type of N-terminal acylation (17).
Candidates of GHRH antagonists for clinical development should possess high binding affinities and exert biological effects on both the pituitary and the tumoral splice variant receptors for GHRH. To further elucidate the molecular mode of action of GHRH antagonists and to increase receptor binding affinities and biological activities, we synthesized a new series of GHRH antagonists. These peptides represent analogs of previously synthesized, highly effective GHRH antagonists JV-1-36 and JV-1-65 (1, 18) and are acylated with different monocarboxylic or α,ω-dicarboxylic acids at the N terminus. The length and hydrophobicity of the acylating moieties also were optimized. The new antagonists were then subjected to endocrine and oncological assays in vitro and in vivo to characterize their pharmacological properties.
Results
Synthesis.
In a search for superactive and long acting GHRH antagonists, 21 analogs of hGHRH(1-29)NH2 were prepared by solid-phase peptide synthesis and purified by reversed-phase HPLC (Table 1). All peptides contain d-Arg-2, para-chlorophenylalanine [Phe(pCl)6], α-aminobutyric acid (Abu15), norleucine (Nle27), d-Arg28, and homoarginine (Har29) substituents, because they are analogs of the previously reported highly potent GHRH antagonists JV-1-36 and JV-1-65 containing these structural features (1,18). Peptides 1 to 16 (Table 1) are derivatives of JV-1-36, which in addition to the above substitutions contains Arg-9 in the molecule (1). Peptides 1, 3, 5, 7, 9, 11 are acylated with monocarboxylic acids containing six to sixteen carbon atoms, such as hexanoic, octanoic, decanoic, lauric, myristic, and palmitic acids, whereas the other analogs (2, 4, 6, 8, 10, and 12) contain α,ω-dicarboxylic acids with six, eight, 10, 12, 14, and 16 carbon atoms, respectively. These latter molecules (2, 4, 6, 8, 10, and 12) have a negative charge at their N terminus. Analogs 13 and 14 contain an additional Phe and d-Phe, respectively, and are acylated with palmitic acid at the N terminus, whereas, in peptides 15 and 16, Arg and d-Arg acylated with phenylacetic acid (PhAc) were incorporated at the N terminus. Antagonist 17 has an extra positive charge in the molecule due to the free amino group at the N terminus.
Table 1.
Structure of GHRH antagonists acylated at the N-terminus with midchain substitutions in the sequence [X0-Tyr1, D-Arg2, Phe(pCl)6, Arg9, Abu15, Nle27, D-Arg28, Har29]hGHRH(1–29)NH2
| No. | Code no. | Substitutions at position |
|||
|---|---|---|---|---|---|
| X0- | 8 Asn | 9 Arg | 10 Tyr | ||
| 1 | MZ-J-7-50 | CH3-(CH2)4-CO- | |||
| 2 | MZ-J-7-52 | HOOC-(CH2)4-CO- | |||
| 3 | MZ-J-7-46 | CH3-(CH2)6-CO- | |||
| 4 | MZ-J-7-48 | HOOC-(CH2)6-CO- | |||
| 5 | MZ-J-7-42 | CH3-(CH2)8-CO- | |||
| 6 | MZ-J-7-44 | HOOC-(CH2)8-CO- | |||
| 7 | MZ-J-7-34 | CH3-(CH2)10-CO- | |||
| 8 | MZ-J-7-38 | HOOC-(CH2)10-CO- | |||
| 9 | MZ-J-7-26 | CH3-(CH2)12-CO- | |||
| 10 | MZ-J-7-30 | HOOC-(CH2)12-CO- | |||
| 11 | MZ-J-7-18 | CH3-(CH2)14-CO- | |||
| 12 | MZ-J-7-22 | HOOC-(CH2)14-CO- | |||
| 13 | MZ-J-7-54 | CH3-(CH2)14-CO-Phe- | |||
| 14 | MZ-J-7-56 | CH3-(CH2)14-CO-D-Phe- | |||
| 15 | MZ-J-7-62 | PhAc-Arg- | |||
| 16 | MZ-J-7-64 | PhAc-D-Arg- | |||
| 17 | MZ-J-7-102 | H- | |||
| 18 | MZ-J-7-112 | HOOC-(CH2)12-CO- | Cit | Cit | |
| 19 | MZ-J-7-114 | CH3-(CH2)6-CO- | Amp | Tyr(Me) | |
| 20 | MZ-J-7-116 | HOOC-(CH2)8-CO | Amp | Tyr(Me) | |
| 21 | MZ-J-7-110 | HOOC-(CH2)12-CO- | Amp | Tyr(Me) | |
| 22 | JV-1-36* | PhAc | Arg | ||
| 23 | JV-1-65* | PhAc | Amp | Tyr(Me) | |
| 24 | JV-1-38* | PhAc | Har | Tyr(Me) | |
Peptide 18, which is a derivative of MZ-J-7-78 (18), has citrulline (Cit) 8 and 9 (Cit8 and Cit9) substituents and, thus, differs in these two positions from JV-1-36 in addition to being acylated with 1,12-dodecanedicarboxylic acid. Compounds 19–21 have para-amidino-phenylalanine (Amp) at position 9 and O-methyltyrosine [Tyr(Me)] at position 10, and they are derivatives of JV-1-65 (18) acylated with mono- (octanoic acid, in peptide 19) or dicarboxylic acids (sebacic acid in peptide 20, and 1,12-dodecanedicarboxylic acid in peptide 21). Fatty acids applied in peptides 18–21 were chosen on the bases of in vitro results obtained with peptides 1–16 (see Tables 2, 3, and 5).
Table 2.
Inhibitory effects of GHRH antagonists on the GHRH-induced GH release in vitro in superfused rat pituitary cell system
| Antagonist |
Inhibition of GH release, % |
|||||
|---|---|---|---|---|---|---|
| No. | Code no. | 0 min | 30 min | 60 min | 90 min | 120 min |
| Standard antagonist* | 52 | 13 | 0 | 0 | 0 | |
| 1 | MZ-J-7-50 | 80 | 87 | 85 | 79 | 69 |
| 2 | MZ-J-7-52 | 48 | 39 | 44 | 30 | 22 |
| 3 | MZ-J-7-46 | 95 | 100 | 98 | 92 | 60 |
| 4 | MZ-J-7-48 | 78 | 80 | 77 | 74 | 73 |
| 5 | MZ-J-7-42 | 53 | 78 | 88 | 85 | 80 |
| 6 | MZ-J-7-44 | 85 | 82 | 86 | 76 | 66 |
| 7 | MZ-J-7-34 | 73 | 69 | 53 | 56 | 60 |
| 8 | MZ-J-7-38 | 53 | 57 | 34 | 37 | 45 |
| 9 | MZ-J-7-26 | 40 | 70 | 64 | 65 | 56 |
| 10 | MZ-J-7-30 | 87 | 84 | 70 | 64 | 65 |
| 11 | MZ-J-7-18 | 64 | 70 | 65 | 49 | 57 |
| 12 | MZ-J-7-22 | 13 | 23 | 24 | 29 | 27 |
| 13 | MZ-J-7-54 | 60 | 32 | 22 | 27 | 39 |
| 14 | MZ-J-7-56 | 75 | 49 | 32 | 27 | 39 |
| 15 | MZ-J-7-62 | 79 | 30 | 39 | 24 | 36 |
| 16 | MZ-J-7–64 | 38 | 36 | 34 | 18 | 26 |
| 17 | MZ-J-7-102 | 96 | 80 | 63 | 54 | 50 |
| 18 | MZ-J-7-112 | 58 | 83 | 73 | 56 | 56 |
| 19 | MZ-J-7-114 | 63 | 65 | 71 | 60 | 53 |
| 20 | MZ-J-7-116 | 72 | 64 | 46 | 53 | 44 |
| 21 | MZ-J-7-110 | 76 | 75 | 64 | 48 | 63 |
| 22 | JV-1-36* | 64 | 79 | 75 | 71 | 71 |
| 23 | JV-1-65* | 72 | 85 | 82 | 80 | 77 |
The antagonists were applied at a 30 nM concentration, except for the standard antagonist, which was applied at 100 nM.
Asterisks indicate data taken from ref. 18.
Table 3.
Ki values and relative affinities of GHRH antagonists to membrane receptors on rat anterior pituitary cells
| Antagonist |
Ki, * nM | Relative affinity† | |
|---|---|---|---|
| No. | Code no. | ||
| Standard antagonist | 6.13 | 1 | |
| 1 | MZ-J-7-50 | 0.080 | 77 |
| 2 | MZ-J-7-52 | 0.122 | 50 |
| 3 | MZ-J-7-46 | 0.061 | 100 |
| 4 | MZ-J-7-48 | 0.089 | 69 |
| 5 | MZ-J-7-42 | 0.093 | 66 |
| 6 | MZ-J-7-44 | 0.094 | 65 |
| 7 | MZ-J-7–34 | 0.202 | 30 |
| 9 | MZ-J-7-26 | 0.114 | 54 |
| 10 | MZ-J-7-30 | 0.077 | 80 |
| 11 | MZ-J-7-18 | 0.113 | 54 |
| 17 | MZ-J-7-102 | 0.089 | 69 |
| 19 | MZ-J-7-114 | 0.069 | 89 |
| 20 | MZ-J-7-116 | 0.096 | 64 |
| 21 | MZ-J-7-110 | 0.071 | 86 |
| 22 | JV-1-36‡ | 0.091 | 67 |
| 23 | JV-1-65‡ | 0.112 | 55 |
*Dissociation constant of the inhibitor–receptor complex. Values were calculated from duplicate or triplicate determinations.
†Expressed relative to [Ac-Tyr1, d-Arg2]hGHRH(1–29)NH2 (standard antagonist) = 1.0.
‡Reference compounds.
Table 5.
Inhibitory effects of GHRH antagonists on the growth of MiaPaCa-2 human pancreatic carcinoma cell line in vitro
| GHRH antagonist no. | GHRH antagonist code no. | T/C % on MiaPaCa-2 cell line at the dose of |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10−6 M | Significance vs. |
3 × 10−6 M | Significance vs. |
10−5 M | Significance vs. |
||||||||
| Control | JV-1-36 | JV-1-65 | Control | JV-1-36 | JV-1-65 | Control | JV-1-36 | JV-1-65 | |||||
| Experiment 1 | |||||||||||||
| Control | 100.0 ± 1.43 | ||||||||||||
| 22 | JV-1-36* | 103.92 ± 2.26 | NS | 100.00 ± 2.26 | NS | 79.32 ± 0.83 | <0.001 | ||||||
| 3 | MZ-J-7-46 | 94.83 ± 3.35 | NS | NS | 93.09 ± 2.79 | NS | NS | 33.82 ± 0.98 | <0.001 | <0.001 | |||
| 4 | MZ-J-7-48 | 96.44 ± 1.95 | NS | NS | 90.86 ± 1.19 | <0.05 | <0.05 | 28.87 ± 0.91 | <0.001 | <0.001 | |||
| 6 | MZ-J-7-44 | 95.67 ± 1.67 | NS | NS | 92.25 ± 1.46 | NS | NS | 36.54 ± 0.63 | <0.001 | <0.001 | |||
| 10 | MZ-J-7-30 | 93.20 ± 1.43 | NS | <0.01 | 72.07 ± 1.66 | <0.001 | <0.001 | 19.24 ± 0.98 | <0.001 | <0.001 | |||
| Experiment 2 | |||||||||||||
| Control | 100.00 ± 0.90 | ||||||||||||
| 22 | JV-1-36* | 97.36 ± 1.46 | NS | 98.82 ± 1.17 | NS | 82.04 ± 1.22 | <0.001 | ||||||
| 1 | MZ-J-7-50 | 98.15 ± 1.39 | NS | NS | 95.99 ± 1.64 | NS | NS | 82.70 ± 2.82 | <0.001 | NS | |||
| 2 | MZ-J-7-52 | 97.12 ± 1.54 | NS | NS | 96.10 ± 1.28 | NS | NS | 87.58 ± 1.80 | <0.001 | NS | |||
| 5 | MZ-J-7-42 | 91.75 ± 0.70 | <0.01 | NS | 90.50 ± 1.60 | <0.001 | <0.01 | 40.72 ± 1.15 | <0.001 | <0.001 | |||
| 3 | MZ-J-7-46 | 95.40 ± 0.95 | NS | NS | 92.95 ± 1.90 | NS | NS | 48.32 ± 1.10 | <0.001 | <0.001 | |||
| 7 | MZ-J-7-34 | 94.68 ± 1.37 | NS | NS | 90.68 ± 1.27 | <0.001 | <0.01 | 37.65 ± 0.59 | <0.001 | <0.001 | |||
| 8 | MZ-J-7-38 | 95.30 ± 0.75 | NS | NS | 92.55 ± 0.85 | <0.05 | NS | 48.02 ± 0.60 | <0.001 | <0.001 | |||
| 9 | MZ-J-7-26 | 97.31 ± 0.98 | NS | NS | 97.12 ± 1.61 | NS | NS | 37.70 ± 0.63 | <0.001 | <0.001 | |||
| 18 | MZ-J-7-112 | 96.25 ± 0.56 | NS | NS | 89.63 ± 1.13 | <0.001 | <0.001 | 43.66 ± 0.87 | <0.001 | <0.001 | |||
| Experiment 3 | |||||||||||||
| Control | 100.00 ± 1.31 | ||||||||||||
| 22 | JV-1-36* | 96.99 ± 2.10 | NS | NS | 92.08 ± 1.39 | NS | NS | 61.7 ± 1.69 | <0.001 | NS | |||
| 23 | JV-1-65* | 98.17 ± 2.40 | NS | NS | 99.41 ± 1.89 | NS | NS | 62.4 ± 1.69 | <0.001 | NS | |||
| 21 | MZ-J-7-110 | 97.49 ± 1.71 | NS | NS | NS | 97.01 ± 1.68 | NS | NS | NS | 42.04 ± 1.82 | <0.001 | <0.001 | <0.001 |
| 19 | MZ-J-7-114 | 98.32 ± 2.25 | NS | NS | NS | 93.35 ± 2.25 | NS | NS | NS | 51.30 ± 1.00 | <0.001 | <0.01 | <0.01 |
*Reference compounds.
GHRH Antagonistic Activities in Vitro.
The antagonistic potencies of the analogs in vitro were determined by the superfusion assay using rat pituitary cell system. Inhibitory effects of the antagonists on GHRH-induced GH release are shown in Table 2. In the series of analogs of JV-1-36 and JV-1-65 modified with fatty acids at the N terminus, peptides 1, 3, 5, 6, and 10 showed the highest antagonistic potencies in vitro. Their inhibitory activities on GH release exceeded that of the reference peptide JV-1-36. Analog 3 (MZ-J-7-46), which was acylated with octanoic acid, caused a nearly complete blockade of GHRH induced GH release for the first 90 min, being the most potent antagonist in this assay.
Receptor Binding Affinities.
Most of the GHRH antagonists modified with fatty acids were also tested in receptor binding experiments on rat pituitary membrane fractions. The results of these experiments are given in Table 3. Ki values of the best antagonists (3, 10, 19, and 21) were in the 0.061–0.077 nM range and also exceeded the binding affinity of reference peptides JV-1-36 or JV-1-65. Peptide 3 (MZ-J-7-46), which had the strongest inhibitory activity on GH release in vitro, also showed the highest GHRH receptor binding affinity, its Ki value being 100 times lower than that of the standard antagonist.
GHRH Antagonistic Activities in Vivo.
Some of the antagonists with high activity in vitro were also evaluated in vivo to assess their potency and duration of action. The results of in vivo tests are presented in Table 4. Peptides 3 (MZ-J-7-46) and 19 (MZ-J-7-114) significantly inhibited the GHRH-evoked GH release in vivo. Although the inhibitory activity of these peptides did not reach that of the reference peptide JV-1-36, their effects were nevertheless long lasting. Compound 21 (MZ-J-7-110) showed a significant inhibition only at 5 min.
Table 4.
Inhibitory effects of GHRH antagonists in vivo on the GH release in rats induced by exogenous GHRH
| Antagonists |
Serum GH, ng/ml, and relative inhibition |
||||||
|---|---|---|---|---|---|---|---|
| No. | Code no. | Control | 5 min | 15 min | 30 min | 60 min | |
| 1 | MZ-J-7-50 | GHbaseline | 38.0 ± 6.5 | 37.6 ± 5.3 | 39.7 ± 11.8 | 64.6 ± 13.5 | 50.0 ± 12.7 |
| GHstimulated | 530 ± 101 | 448 ± 104 | 497 ± 129 | 683 ± 36 | 481 ± 65 | ||
| Inhibition, % | 17 | 7 | 0 | 10 | |||
| 3 | MZ-J-7-46 | GHbaseline | 41.6 ± 11.9 | 72.9 ± 17.6 | 11.0 ± 3.9 | 83.3 ± 25.4 | 29.5 ± 4.8 |
| GHstimulated | 1,215 ± 177 | 901 ± 181 | 715 ± 112∗ | 768 ± 60∗ | 945 ± 172 | ||
| Inhibition, % | 28 | 41 | 40 | 23 | |||
| 5 | MZ-J-7-42 | GHbaseline | 56.9 ± 14.4 | 50.6 ± 10.8 | 30.6 ± 11.4 | 68.4 ± 26.7 | 48.7 ± 13.0 |
| GHstimulated | 933 ± 350 | 1,210 ± 224 | 747 ± 134 | 1,186 ± 118 | 982 ± 96 | ||
| Inhibition, % | 0 | 21 | 0 | 0 | |||
| 6 | MZ-J-7-44 | GHbaseline | 50.4 ± 8.2 | 38.1 ± 19.2 | 39.7 ± 10.4 | 31.0 ± 6.2 | 37.4 ± 8.6 |
| GHstimulated | 1,390 ± 96 | 1,008 ± 225 | 1,368 ± 129 | 1,280 ± 204 | 1,253 ± 141 | ||
| Inhibition, % | 28 | 2 | 8 | 10 | |||
| 10 | MZ-J-7-30 | GHbaseline | 26.1 ± 10.9 | 16.4 ± 4.1 | 19.4 ± 5.3 | 29.5 ± 9.4 | 16.6 ± 3.0 |
| GHstimulated | 850 ± 159 | 863 ± 109 | 861 ± 77 | 812 ± 160 | 917 ± 106 | ||
| Inhibition, % | 0 | 0 | 4 | 0 | |||
| 19 | MZ-J-7-114 | GHbaseline | 48.7 ± 3.6 | 18.5 ± 4.4 | 33.5 ± 12.3 | 33.7 ± 7.8 | 25.0 ± 7.2 |
| GHstimulated | 1,379 ± 162 | 867 ± 116∗ | 788 ± 114∗ | 912 ± 88 | 809 ± 107∗ | ||
| Inhibition, % | 38 | 44 | 35 | 42 | |||
| 21 | MZ-J-7-110 | GHbaseline | 57.0 ± 10.8 | 26.7 ± 5.8 | 22.0 ± 10.9 | 49.2 ± 6.3 | 24.9 ± 4.7 |
| GHstimulated | 1,197 ± 131 | 707 ± 61† | 927 ± 204 | 1,100 ± 110 | 906 ± 55 | ||
| Inhibition, % | 42 | 23 | 8 | 25 | |||
| 22 | JV-1-36‡ (Exp. 1) | GHbaseline | 158 ± 24 | 179 ± 14 | 139 ± 6 | 151 ± 9 | 120 ± 7 |
| GHstimulated | 565 ± 116 | 198 ± 22¶ | 261 ± 24† | 292 ± 19† | 479 ± 88 | ||
| Inhibition, % | 95 | 71 | 66 | 19 | |||
| 22 | JV-1-36‡ (Exp. 2) | GHbaseline | 48.0 ± 11.0 | 39.2 ± 12.2 | 73.1 ± 9.39 | 66.1 ± 9.67 | 73.2 ± 14.5 |
| GHstimulated | 1,307 ± 94.9 | 180 ± 28.1¶ | 689 ± 69.3¶ | 1,126 ± 101 | 1,337 ± 154 | ||
| Inhibition, % | 89 | 50 | 15 | 0 | |||
| 23 | JV-1-65‡ (Exp. 1) | GHbaseline | 56.5 ± 11.0 | 38.6 ± 5.86 | 112 ± 17.0 | 78.5 ± 32.1 | ND |
| GHstimulated | 906 ± 86.5 | 601 ± 64.2 | 919 ± 112 | 920 ± 123 | ND | ||
| Inhibition, % | 35 | 0 | 0 | ||||
| 23 | JV-1-65‡ (Exp. 2) | GHbaseline | 109 ± 20.2 | 79.0 ± 19.7 | 82.7 ± 24.4 | ND | ND |
| GHstimulated | 1,819 ± 191 | 1,551 ± 291 | 1,590 ± 264 | ND | ND | ||
| Inhibition, % | 15 | 13 | |||||
GH levels are mean ± SEM of five to seven animals per group.
∗, P < 0.05 vs. control;
†, P < 0.01 vs. control;
‡, reference compounds;
¶, P < 0.001 vs. control; ND, not determined.
Cell Proliferation Assay.
The inhibitory activities of the analogs, which were effective in superfusion assays, were also tested on the proliferation of MiaPaCa-2 human pancreatic cancer cell line in vitro at 10−6, 3 × 10−6, and 10−5 M concentrations (Table 5). Among the peptides tested, analog 5 (MZ-J-7-42) significantly inhibited the cell proliferation even at 10−6 M concentration. The proliferation of MiaPaCa-2 human pancreatic cancer cells were significantly inhibited by six peptides (4, 5, 7, 8, 10, and 18) at 3 × 10−6 M concentration, whereas reference peptides JV-1-36 and JV-1-65 did not show significant inhibition at this concentration. The inhibitory effects of antagonists 4, 5, 7, 10, and 18 at 3 × 10−6 M concentration were also significantly higher than that of parent peptide JV-1-36. All analogs significantly inhibited cell proliferation at 10−5 M concentration. The inhibitory effects of all new antagonists with the exception of peptides 1 and 2 were also significantly higher as compared to that of reference peptide JV-1-36 (Table 5) at 10−5 M concentration. The antiproliferative effect of compounds 19 (MZ-J-7-114) and 21 (MZ-J-7-110) also significantly (P < 0.01 and P < 0.001, respectively) exceeded that of their parent peptide JV-1-65 at 10−5 M concentration.
Oncological Study in Vivo.
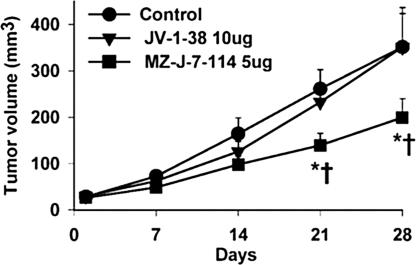
The antiproliferative effects of the new antagonist 19 (MZ-J-7-114) and its reference peptide JV-1-38 were investigated on s.c. implanted xenografts of PC-3 human androgen-independent prostate cancer in nude mice. After 4 weeks of treatment, peptide MZ-J-7-114 administered at 5 μg/day significantly inhibited the growth of PC-3 tumors in nude mice, and its effect was also significantly greater than that of its parent peptide JV-1-38 at the double dose of 10 μg/day (Table 6, Fig. 1). The new antagonist significantly inhibited tumor volume by 43% vs. control, whereas JV-1-38 was ineffective. The tumor weight was also significantly lowered in the group treated with MZ-J-7-114 by 58%, whereas antagonist JV-1-38 only caused 10% inhibition (Table 6). Body weights in treated animals did not differ from the control group (data not shown). Serum IGF-I levels were significantly (P < 0.001) inhibited by 33% after treatment with antagonist MZ-J-7-114, but not with JV-1-38 (Table 6). Tumoral IGF-II levels were not affected by the antagonists (data not shown).
Table 6.
Effects of GHRH antagonists MZ-J-7-114 and JV-1-38 on the growth of human PC-3 androgen-independent prostate cancer in nude mice and serum IGF-levels
| Treatment | Tumor volume, mm3 |
Tumor volume doubling time, days | Tumor weight, mg (% inhibition) | Serum IGF-I, ng/ml (% inhibition) | |
|---|---|---|---|---|---|
| Initial | Final (% inhibition) | ||||
| Control | 28.4 ± 4.2 | 351.1 ± 84.9 | 8.3 ± 0.7 | 282.7 ± 72.1 | 262 ± 18.1 |
| JV-1-38* (10 μg/day) | 29.3 ± 3.8 | 351.0 ± 72.9 | 8.7 ± 0.7 | 254.3 ± 52.0 (10) | 293 ± 28.0 |
| MZ-J-7-114 (5 μg/day) | 26.5 ± 3.7 | 199.3 ± 40.4 (43)†‡ | 12.6 ± 2.5† | 119.1 ± 25.3 (58)†‡ | 175 ± 12.9 (33%)¶ |
*Reference compound;
†, P < 0.05 vs. control;
‡, P < 0.05 vs. JV-1-38;
¶, P < 0.001 vs. control and JV-1-38.
Fig. 1.
Inhibitory effects of GHRH antagonists on the tumor growth of PC-3 human androgen-independent prostate cancers xenografted into nude mice. ∗, P < 0.05 vs. control; †, P < 0.05 vs. JV-1-38.
Discussion
Considerable efforts have been devoted in our laboratory to the synthesis of GHRH antagonists with improved activities (1–3, 18). In this study, a new series of GHRH antagonists was synthesized and modified at the N terminus with fatty acids and tested in vitro and in vivo. The design of these compounds was based on the incorporation of monocarboxylic and α,ω-dicarboxylic acids into the molecules for the enhancement of peptide-membrane interaction, long-term stability against enzymes, and circulation time of peptides in bloodstream. This approach was previously used successfully for the synthesis of insulin analogs and glucagon-like peptide analogs with protracted activities in vivo due to an enhanced binding to serum albumin, which protects the peptides from enzymatic degradation and also reduces their clearance from the blood (10, 19).
Of the 14 analogs of JV-1-36 acylated with fatty acids at the N terminus, two peptides (4 and 7) were as active in the superfusion tests in vitro as the parent antagonist JV-1-36, and five analogs (1, 3, 5, 6, and 10) were more potent. These latter peptides were acylated at their N terminus with hexanoic, octanoic, decanoic, sebacic, and 1,12-dodecanedicarboxylic acids, respectively. Interestingly, antagonist 17 having free amino group at the N terminus also strongly inhibited GHRH-induced GH release at 0 min, but this effect was of a short duration. The stability of peptide 17 against enzymatic degradation is probably decreased due to its free N terminus. In studies on binding to GHRH receptors on rat pituitary membrane fractions, the relative affinities of analogs 1, 3, and 10 were also higher than that of JV-1-36, whereas compounds 5 and 6 showed similar affinities to JV-1-36. Peptide 3 (MZ-J-7-46), having octanoyl group at the N terminus, showed the highest binding affinity among GHRH antagonists, its value being 100 times greater than that of the standard antagonist. The binding affinities of analogs of JV-1-36 acylated with fatty acids were in good correlation with their inhibitory effects in the rat superfusion system.
In addition to the N-acyl analogs of JV-1-36, we synthesized three GHRH antagonists (19–21) based on the sequence of JV-1-65, because recent results demonstrated that this antagonist has increased antitumor activity despite a weaker inhibitory effect on the release of GH in vivo (2,3,18). Although analogs 19–21 were less active in the rat superfusion assay than JV-1-65, their binding affinities to rat pituitary GHRH receptors exceeded that of reference peptide JV-1-65, indicating the possibility of enhanced peptide–membrane interaction due to the presence of fatty acids at the N termini of these peptides.
Analogs that were effective in the superfusion and receptor binding assay in vitro were also tested for the inhibition of GH release in vivo. In the series of antagonists (peptides 1, 3, 5, 6, and 10), based on the structure of JV-1-36, the acylation caused a decrease in the inhibitory activity in vivo. Only the effect of analog 3 (MZ-J-7-46) was significant up to 30 min. However, peptides 19 (MZ-J-7-114) and 21 (MZ-J-7-110) showed increased inhibitory effects compared to their parent peptide JV-1-65. Antagonist 19 (MZ-J-7-114) significantly inhibited GH release in vivo even at 60 min after administration. Thus, N-acylation with fatty acids decreased the activity in vivo of analogs of JV-1-36, but enhanced the antagonistic effect of analogs of JV-1-65. The reason for the different effect of N-acylation with fatty acids in the two series of peptides is not known. In previous work, it was also found that inhibitory effects on GH release in vivo did not always parallel the activities in vitro (18).
Because the main applications of GHRH antagonists would be in the area of oncology, we tested the new analogs for inhibition of cancer cell proliferation in vitro. MiaPaCa-2 human pancreatic cancer line was chosen for the initial oncological evaluation of GHRH antagonists, because this cell line is a particularly appropriate model for testing the direct effects of GHRH antagonists. MiaPaCa-2 cells express the tumoral splice variants of GHRH receptors, whereas other receptors related to GHRH such as those for vasoactive intestinal peptide, pituitary adenylate cyclase activating peptide, secretin, or glucagon, that could interfere in the proliferation assays, are not present (6, 20). Our results indicate that all antagonistic analogs of GHRH acylated with mono- or dicarboxylic acids containing 8–14 carbon atoms have increased antiproliferative effect in vitro as compared to their parent peptides JV-1-36 and JV-1-65. However, analogs 1 and 2, acylated with the shorter hexanoic or adipic acids containing six carbon atoms, were less potent.
Some of the peptides described in this paper have been and continue to be tested in various tumor models in vivo to more completely characterize oncological activities. Thus, in the present study, we found that GHRH antagonist 19 (MZ-J-7-114) given at 5 μg/day significantly inhibited the growth of PC-3 human androgen-independent prostate cancers xenografted into nude mice and reduced serum IGF-I levels, whereas antagonist JV-1-38 had no effect at the dose of 10 μg/day. Our group also demonstrated that MZ-J-7-114 at 10 μg doses very strongly inhibits the growth of H460 and A549 human non-SCLC tumors in nude mice (2, 3). In another study, in which nude mice bearing H-69 human SCLC tumors were treated with GHRH antagonists JV-1-65 and MZ-J-7-110 at doses of 10 μg/day, it was found that both antagonists significantly decreased tumor volume and weight, the inhibitory effect of MZ-J-7-110 being greater than that of the parent peptide JV-1-65 (21).
In the course of synthesis of previous series of GHRH antagonists we began to observe a dissociation between the endocrine and oncological activities. Among the older GHRH antagonists, JV-1-36 and JV-1-38 inhibited the growth of PC-3 and other human cancers xenografted into nude mice at a dose of 20 μg/day, and newer and more potent antagonist JV-1-65 was effective at 10 μg/day (1–3, 18, 21). Antagonist JV-1-36 strongly inhibited GH release in rats, whereas antagonists JV-1-38 and JV-1-65 had only weak and nonsignificant inhibitory effects on GH release in vivo (18). Although the new analogs MZ-J-7-114 and MZ-J-7-110, acylated with fatty acids, show a modest enhancement of inhibitory actions on GH release in vivo as compared to their parent peptides JV-1-65 and JV-1-38, their endocrine activity on the pituitary in vivo is weaker than that of JV-1-36 or of other antagonists with outstanding inhibitory effects on GH release, such as JV-1-63 (18). However, the oncological activity of the new antagonists is substantially increased, as demonstrated by the significant inhibitory effect of MZ-J-7-114 and MZ-J-7-110 in small dose on the growth of PC-3 and other tumors as cited above. This finding indicates an augmentation of the direct effects on tumors, mediated by tumoral GHRH receptors.
In conclusion, our results demonstrate the beneficial effect of modification at the N terminus with octanoic or 1,12-dodecanedicarboxylic acids on the inhibitory activity of GHRH antagonists. The enhanced antiproliferative activities of GHRH antagonists acylated with fatty acids at their N terminus indicate marked improvements over earlier antagonists. Some of the analogs of this type could be considered for clinical development for treatment of various cancers.
Materials and Methods
Peptide Synthesis.
GHRH antagonists with amidated C termini were prepared by solid-phase methodology on p-methylbenzhydrylamine resin using Boc-chemistry, as described (18). All amino acid derivatives, resins, and reagents used were obtained from Bachem (Torrance, CA), RSP Amino Acids DBA (Shirley, MA), or Sigma. N-terminal acylation of peptides was performed after completion of the synthesis and removal of the N-α-Boc protecting group from Tyr-1. Resin-bound peptides were acylated with mono- or α,ω-dicarboxylic acids using 10 equivalents of preformed anhydrides of the appropriate fatty acids. Final deprotection and cleavage of the peptides from the resin with anhydrous hydrogen fluoride, as well as their purification and analysis by semipreparative and analytical HPLC, were done as described (18).
Evaluation of GHRH Antagonistic Activity in Vitro.
Antagonistic activities of the peptides on GH release were determined by using a superfused rat pituitary cell system as described (18). Briefly, the antagonists were perfused through the cells at 30 nM concentration, followed by stimuli of 1 nM hGHRH(1-29)NH2 applied 0, 30, 60, 90, and 120 min later. The amount of GH secreted by the cells was determined by RIA. The inhibitory activities of antagonists were expressed as % inhibition of the reference GH response induced by GHRH in the absence of antagonist.
Receptor Binding.
Preparation of rat pituitary membrane fractions and receptor binding of GHRH were performed as previously described (18, 22), using a sensitive in vitro ligand competition assay based on binding of 125I-labeled [His1, Nle27]hGHRH(1-32)NH2 to rat anterior pituitary membrane homogenates.
GHRH Antagonistic Activity in Vivo.
The inhibitory effect of antagonists (80 μg/kg i.v.) on the GH release induced by hGHRH(1-29)NH2 (3 μg/kg i.v.) in rats was tested as described (18). Separate groups (five to seven rats per group) received the GHRH stimulus at varying time intervals (5, 15, 30, or 60 min) after the antagonist. Serum GH levels before the administration of the antagonist (GHbaseline) and 5 min after the injection of GHRH (GHstimulated) were measured by RIA. Controls received no antagonist before the GHRH stimulus. Percent inhibition of GH release was calculated as described (18).
All experiments in vivo were performed in accordance with institutional ethical guidelines for the care and use of experimental animals.
Cell Proliferation Assay.
MiaPaCa-2 human pancreatic cell line was obtained from the American Type Culture Collection (Manassas, VA) and maintained in culture as described (20). For evaluation of the inhibitory activity of the analogs on the proliferation of this cell line in vitro, MiaPaCa-2 cells were seeded into 96-well plates and exposed to 10−6, 3 × 10−6, and 10−5 M concentrations of the antagonists, in octuplicate wells each, for 70–72 h. At the end of treatment, cell proliferation was measured by the tetrazolium dye [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) assay as described (20).
Oncological Study in Vivo.
PC-3 human androgen-independent prostate cancer cell line was obtained from the American Type Culture Collection. Male athymic (Ncr nu/nu) nude mice, ≈6 weeks old on arrival, were obtained from the National Cancer Institute (Frederick Cancer Research and Development Center, Frederick, MD). S.c. xenografts of PC-3 tumor tissue were performed as described (23). The animals (8–10 mice per group) were then treated s.c. with GHRH antagonists JV-1-38 (10 μg/day) and MZ-J-7-114 (5 μg/day). Tumors were evaluated after 4 weeks of treatment, and serum IGF-I and tumoral IGF-II were also measured as described (23).
RIA for GH.
Rat GH levels in aliquots of superfusion samples and in serum were measured by double-antibody RIA using materials supplied by the National Hormone and Pituitary Program, Rockville, MD (rat GH-RP-2/AFP-3190B, rat GH-I-6/AFP-5676B, and anti-rat GH-RIA-5/AFP-411S). Interassay variation was <15%, and intraassay variation was <10%.
Statistical Analysis.
Data are expressed as means ± SEM. Statistical evaluation of the results was done by Student’s two-tailed t test or one-way ANOVA followed by Bonferroni’s test.
Acknowledgments
The gifts of materials from the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases and Dr. A. F. Parlow (Harbor–UCLA Medical Center, Los Angeles) used in the RIA are appreciated. We thank Elena Glotser for her excellent technical assistance and Dudley Callais for editorial help. This work was supported by the Medical Research Service of the Veterans Affairs Department (to A.V.S.) and by a grant from Zentaris AG (Frankfurt am Main, Germany) to Tulane University School of Medicine (to A.V.S.).
Abbreviations
- GHRH
growth hormone-releasing hormone
- SCLC
small-cell lung carcinoma.
Footnotes
Conflict of interest statement: Tulane University has applied for a patent on the GHRH antagonists listed in this paper, and M.Z., J.L.V., and A.V.S. are coinventors on that patent.
References
- 1.Schally A. V., Varga J. L. Trends Endocrinol. Metab. 1999;10:383–391. doi: 10.1016/s1043-2760(99)00209-x. [DOI] [PubMed] [Google Scholar]
- 2.Schally A. V., Varga J. L. Comb. Chem. High Throughput Screen. 2006 doi: 10.2174/138620706776055449. in press. [DOI] [PubMed] [Google Scholar]
- 3.Varga J. L., Schally A. V. In: Handbook of Peptides. Kastin A., editor. London: Elsevier; 2006. in press. [Google Scholar]
- 4.Robberecht P., Coy D. H., Waelbroeck M., Heiman M. L., deNeef P., Camus J.-C., Christophe J. Endocrinology. 1985;117:1759–1764. doi: 10.1210/endo-117-5-1759. [DOI] [PubMed] [Google Scholar]
- 5.Engel J. B., Keller G., Schally A. V., Toller G. L., Groot K., Havt A., Armatis P., Zarandi M., Varga J. L., Halmos G. J. Clin. Endocrinol. Metab. 2005;90:3614–3621. doi: 10.1210/jc.2004-2179. [DOI] [PubMed] [Google Scholar]
- 6.Havt A., Schally A. V., Halmos G., Varga J. L., Toller G. L., Horvath J. E., Szepeshazi K., Koster F., Kovitz K., Groot K., et al. Proc. Natl. Acad. Sci. USA. 2005;102:17424–17429. doi: 10.1073/pnas.0506844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller M. R., Wiesmüller K. H., Jung G., Loop T., Humar M., Pfannes S. D., Bessler W. G., Mittenbühler K. Int. Immunopharmacol. 2002;2:1065–1077. doi: 10.1016/s1567-5769(02)00030-9. [DOI] [PubMed] [Google Scholar]
- 8.Resh M. D. Biochim. Biophys. Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 9.Liu J., Hughes T. E., Sessa W. C. J. Cell Biol. 1997;137:1525–1535. doi: 10.1083/jcb.137.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtzhals P., Havelund S., Jonassen I., Kiehr B., Larsen U. D., Ribel U., Markussen J. Biochem. J. 1995;312:725–731. doi: 10.1042/bj3120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung G., Beck-Sickinger A. G. Angew. Chem. Int. Ed. Engl. 1992;31:367–383. [Google Scholar]
- 12.Mittenbühler K., Baier W., Esche U. V. D., Heinevetter L., Wiesmüller K-H., Jung G. Curr. Top. Pept. Prot. Res. 1997;2:125–135. [Google Scholar]
- 13.Erhard M. H., Schmidt P., Hofman A., Bergmann J., Mittermeier P., Kaufmann P. Altern. Lab. Anim. 1997;25:173–181. [Google Scholar]
- 14.Kurtzhals P., Havelund S., Jonassen I., Markussen J. J. Pharmacol. Sci. 1997;86:1365–1368. doi: 10.1021/js9701768. [DOI] [PubMed] [Google Scholar]
- 15.Loing E., Thiam K., Gilles F., Verwaerde C., Quatannens B., Auriault C., Gras-Masse H. Lett. Pept. Sci. 1997;4:397–402. [Google Scholar]
- 16.Dasgupta P., Singh A., Mukherjee R. Biol. Pharm. Bull. 2002;25:29–36. doi: 10.1248/bpb.25.29. [DOI] [PubMed] [Google Scholar]
- 17.Rijkers D. T. S., den Hartog J. A. J., Liskamp R. M. J. Bioorg. Med. Chem. 2004;12:5099–5106. doi: 10.1016/j.bmc.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Varga J. L., Schally A. V., Horvath J. E., Kovacs M., Halmos G., Groot K., Toller G. L., Rekasi Z., Zarandi M. Proc. Natl. Acad. Sci. USA. 2004;101:1708–1713. doi: 10.1073/pnas.0307288101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knudsen L. B., Nielsen P. F., Huusfeldt P. O., Johansen N. L., Madsen K., Pedersen F. Z., Thogersen H., Wilken M., Agerso H. J. Med. Chem. 2000;43:1664–1669. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- 20.Rekasi Z., Varga J. L., Schally A. V., Plonowski A., Halmos G., Csernus B., Armatis P., Groot K. Peptides. 2001;22:879–886. doi: 10.1016/s0196-9781(01)00413-2. [DOI] [PubMed] [Google Scholar]
- 21.Kanashiro C. A., Schally A. V., Zarandi M., Hammann B. D., Varga J. L. Int. J. Cancer. 2004;112:570–576. doi: 10.1002/ijc.20437. [DOI] [PubMed] [Google Scholar]
- 22.Halmos G., Rekasi Z., Szoke B., Schally A. V. Receptor. 1993;3:87–97. [PubMed] [Google Scholar]
- 23.Stangelberger A., Schally A. V., Varga J. L., Hammann B. D., Groot K., Halmos G., Cai R.-Z., Zarandi M. Prostate. 2005;64:303–315. doi: 10.1002/pros.20262. [DOI] [PubMed] [Google Scholar]