Abstract
Neutrophilic airway inflammation is a hallmark of cystic fibrosis (CF). As high oxidant producers, airway neutrophils contribute largely to the systemic redox imbalance seen in CF. In turn, this chronic and profound imbalance can impact circulating neutrophils before their migration into airways. Indeed, in 18 CF patients with stable disease, blood neutrophils were readily deficient in the pivotal antioxidant glutathione (P = 0.003, compared with 9 healthy controls). In a phase 1 study, this deficiency was improved (P = 0.025) by the glutathione prodrug N-acetylcysteine, given orally in high doses (0.6 to 1.0 g three times daily, for 4 weeks). This treatment was safe and markedly decreased sputum elastase activity (P = 0.006), the strongest predictor of CF pulmonary function. Consistently, neutrophil burden in CF airways was decreased upon treatment (P = 0.003), as was the number of airway neutrophils actively releasing elastase-rich granules (P = 0.005), as measured by flow cytometry. Pulmonary function measures were not improved, as expected with short-term treatment. After excluding data from subjects without baseline airway inflammation, positive treatment effects were more pronounced and included decreased sputum IL-8 levels (P = 0.032). Thus, high-dose oral N-acetylcysteine has the potential to counter the intertwined redox and inflammatory imbalances in CF.
Keywords: pulmonary function, redox, neutrophil, elastase
Cystic fibrosis (CF) is the most frequent recessive disease in Caucasians, occurring in ≈1 in 2,500 live births (1). CF is caused by mutations of the CF transmembrane conductance regulator protein, a multifunctional protein that is chiefly, but not exclusively, expressed in exocrine epithelia. Although CF manifests as a multiorgan disease, chronic airway dysfunction is the main cause of morbidity and mortality among patients (2). A central feature of CF airway disease is persistent massive recruitment of neutrophils. Neutrophil counts in CF airway fluid are several hundred-fold higher than normal (3). Abnormal neutrophil recruitment to CF airways often starts in the neonatal period, due to, at least in part, excessive secretion of IL-8 by airway epithelial cells bearing mutant CF transmembrane conductance regulator proteins (4–6).
Once in the airways, neutrophils show multiple signs of dysfunction, culminating in their abnormal clearance and necrosis. CF airway neutrophils are the primary source of extracellular actin (7) and DNA (8), which contribute to mucus hyperviscosity. They also release, actively or upon death, massive amounts of effector molecules, including elastase and IL-8, which perpetuate tissue damage and neutrophil recruitment and contribute to create a more favorable environment for opportunistic pathogens (9). Sputum neutrophil count and elastase activity are very strong correlates to clinical measures of CF lung dysfunction, such as declining functional expiratory volume in 1 s (FEV1) or forced vital capacity (FVC) (10), which is consistent with neutrophils playing a central role in CF airway destruction.
Because neutrophilic inflammation is a major determinant in the progression of CF airway disease, this process must be targeted aggressively and effectively. The current standard of care utilizes palliative treatments as a primary means to stem airway inflammation in CF patients. Inhaled corticosteroid therapy, high-dose ibuprofen therapy, and azithromycin are medications commonly used (11). These medications are only partially effective and can cause major side effects (12). The development of safer and more effective therapies for CF airway inflammation rests in part on a better understanding of its relationship with systemic physiology.
Notably, CF is characterized by a state of systemic redox imbalance caused by the malabsorption of dietary antioxidants in the gut and the inability of cells bearing mutant CF transmembrane conductance regulator proteins to efflux glutathione (GSH), the most abundant cellular antioxidant, into the extracellular milieu (13, 14). Excessive oxidant release by inflammatory airway neutrophils also plays a major role in this systemic redox imbalance (15). Here, we provide evidence that, in turn, the systemic redox imbalance affects circulating neutrophils before their migration to CF airways, as evidenced by marked basal intracellular GSH deficiency. Because of a low basal activity of antioxidant enzymes (16), neutrophils are particularly at risk when facing significant GSH imbalance, leading to abnormal deformability, degranulation, and apoptosis (17–19).
Thus, we tested whether treatment with N-acetylcysteine (NAC), a well known antioxidant GSH prodrug (20), could improve the redox imbalance in circulating neutrophils and also possibly inhibit recruitment of neutrophils to CF airways and their subsequent dysfunction. NAC is an endogenous metabolic intermediate, which has long been used in CF (21) as an aerosolized mucus fluidifier, to break down disulfide bonds between mucin molecules. Unfortunately, the highly oxidizing CF airway environment consumes aerosolized antioxidants very rapidly (14). So far, NAC has never been prescribed to target circulating neutrophils in CF. To this end, we posit that the oral route would be most efficient, because it allows for rapid first-pass metabolism of NAC via the gut and liver and subsequent increase in circulating GSH. Defects in gut and liver function in CF patients (1), however, could hamper oral NAC efficiency.
We further postulated that several daily intakes of oral NAC, with relatively high doses, would be required to efficiently target circulating neutrophils. Indeed, these cells are characterized by their rapid turnover (4–8 h) and high daily production (up to 1012) (19). The findings we present here were collected during a short-term dose-escalation phase 1 trial in CF subjects of high-dose oral NAC treatment (0.6, 0.8, and 1.0 g per day, three times daily, for 4 weeks). We demonstrate the safety of this approach and its significant ability to modulate redox and inflammatory aspects of CF airway disease.
Results
Circulating Neutrophils from CF Patients in Stable Condition Are Deficient in the GSH Antioxidant System.
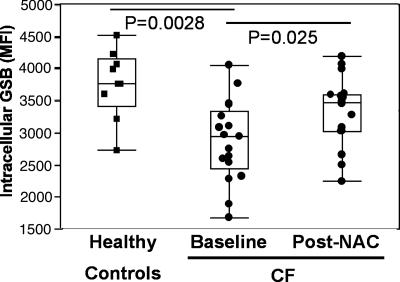
A direct semiquantitative FACS-based method for measuring neutrophil GSH, which is applicable to unprocessed whole blood samples, was used, coupled with the analytical gating of neutrophils (see Materials and Methods) (22, 23). This method was used because neutrophils are very sensitive to external stimulation (19, 24). Usual neutrophil purification procedures (e.g., centrifugation through Percoll gradients) stimulate neutrophils (25, 26), even if minimal agitation occurs, and thus are insufficient to properly measure GSH. Hence, although GSH measurement was performed by high-performance liquid chromatography (HPLC) on whole blood (see Materials and Methods), we could not resort to this procedure for neutrophils. With the FACS-based method, intracellular GSH levels in neutrophils quantified by fluorescent glutathione-S-bimane (GSB) adducts were markedly decreased in CF compared with healthy subjects (Fig. 1).
Fig. 1.
The basal GSH deficiency in blood neutrophils from stable CF patients is ameliorated by short-term high-dose oral NAC. Blood neutrophils from 18 CF patients in stable condition were assessed at baseline for fluorescent GSB (shown in median fluorescence intensity, MFI), revealing a significant decrease compared with 9 healthy controls. CF patients were reassessed after 4-week treatment with high-dose oral NAC, showing a significantly increase in GSB MFI. Individual data are shown in box plot (median line in box delimited by 25th and 75th quantiles, ± 1.5 × interquartile range, delimited by whiskers; see Materials and Methods).
The GSH Prodrug NAC Is Safe to Use at High Oral Doses in CF and Efficient at Countering GSH Deficiency in Blood Neutrophils.
We conducted a phase 1 (unblinded dose-escalation tolerability and exploratory efficacy) clinical trial, in which high-dose oral NAC treatment was tested in 18 CF subjects over a 4-week period. The treatment proved to be safe at all three doses (0.6, 0.8, and 1.0 g per day, three times daily). Treatment compliance was high (93 ± 1%), with no adverse effect identified on clinical examination, complete blood count, laboratory tests, and CF quality of life questionnaire. Subjects reported very mild and infrequent drug-related adverse effects (Table 2, which is published as supporting information on the PNAS web site). In terms of efficacy, short-term high-dose oral NAC treatment significantly increased GSH levels in CF blood neutrophils (Fig. 1). In addition, the treatment significantly increased whole blood GSH levels in CF patients, as measured independently by HPLC (Table 1). Results obtained with the three doses were not statistically different.
Table 1.
Effect of short-term high-dose oral NAC treatment on chosen endpoints measured in CF patients
| Subjects | Statistics | Redox imbalance |
Inflammatory imbalance |
Spirometry |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Blood neutrophil GSH | Whole blood GSH | Sputum neutrophil count | Sputum elastase activity | Sputum CD63hi neutrophils | Sputum IL-8 levels | FEV1, % predicted | FVC, % predicted | ||
| n = 18, all patients | % change or CIB/N | +23% | +12% | CIB: 16;88 | CIB: 28;93 | CIB: 11;77 | — | — | — |
| CIN: 8;34 | CIN: 16;50 | CIN: 6;26 | |||||||
| P value∗ | 0.025 | 0.031 | 0.003 | 0.006 | 0.005 | NS | NS | NS | |
| n = 15, 3 excluded† | % change or CIB/N | +30% | +16% | CIB: 20;103 | CIB: 31;106 | CIB: 14;91 | CIB: 67;38 | — | — |
| CIN: 9;34 | CIN: 18;57 | CIN: 7;31 | CIN: 52;27 | ||||||
| P value | 0.003 | 0.017 | 0.002 | 0.013 | 0.003 | 0.032 | NS | NS | |
NS, not significant.
∗Differences between baseline data (B) and post-NAC treatment data (N) are expressed in % change for normally distributed variables and with 95% confidence intervals (CIB and CIN, respectively) for nonnormally distributed variables (see Materials and Methods).
†The three excluded patients had their baseline airway neutrophil count in the normal range (see Fig. 2 and text).
Short-Term High-Dose Oral NAC Treatment Significantly Decreases Neutrophil Count in CF Airways.
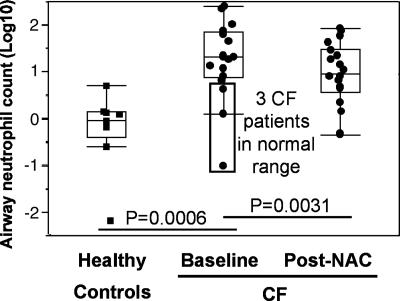
We tested whether the amelioration of the GSH imbalance in circulating neutrophils by NAC treatment would be associated with decreased migration into the airways, as suggested by previous studies (27, 28). Consistent with the notion that neutrophilic inflammation is a self-amplifying process in CF airways, baseline airway neutrophil count (as measured in induced sputum) was highly variable in our CF cohort and followed a logarithmic distribution. As demonstrated in ref. 10, baseline airway neutrophil count was a strong predictor of pulmonary function (data not shown). Upon NAC treatment, the airway neutrophil count was significantly reduced (Fig. 2). This reduction was even more pronounced when data from three CF patients with baseline sputum neutrophil values within the normal range were excluded (Table 1). Interestingly, sputum IL-8 levels (which may originate from neutrophils, as well as epithelial cells) were also significantly reduced by treatment when excluding the same three subjects (Table 1).
Fig. 2.
Short-term high-dose oral NAC decreases neutrophil count in CF airways. Airway neutrophil count (expressed in absolute count) follows a logarithmic distribution in CF patients, reflecting the self-amplifying characteristic of CF airway inflammation. Upon NAC treatment, airway neutrophil count was markedly reduced in patients, even more so when the three patients with baseline airway neutrophil count in the normal range were excluded from the analysis (Table 1). Identical results were obtained when neutrophil count was expressed in relative numbers per unit of sputum volume or weight. Data are shown as box plots (see Materials and Methods).
Short-Term High-Dose Oral NAC Treatment Significantly Decreases CF Airway Elastase Activity, the Best Predictor of CF Airway Dysfunction.
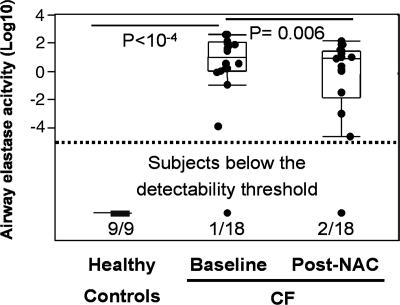
Although increased neutrophil recruitment is a major clinical feature of CF airway inflammation, the subsequent release of effectors by recruited neutrophils plays a crucial role in mediating pathophysiologic effects. Among these effectors, elastase was identified in previous studies as the best predictor of CF pulmonary function (10). Consistent with this knowledge, we found that elastase activity at baseline in sputum was highly correlated with FEV1 and FVC (data not shown). Like sputum neutrophil count, sputum elastase activity was logarithmically distributed and markedly decreased after 4-week treatment with high-dose oral NAC (Fig. 3). However, FEV1 and FVC themselves did not change over the short period of this trial (Table 1).
Fig. 3.
Short-term high-dose oral NAC decreases elastase activity in CF airways. Elastase activity (measured by a specific enzymatic assay; see Materials and Methods) is the best predictor of CF pulmonary function (data not shown). Baseline airway elastase activity was below the measurable range in 9 of 9 healthy controls and only 1 of 18 CF patients included in our study. Upon NAC treatment, 2 of 18 patients were now below the measurable range, and elastase activity was significantly decreased in the patient group as a whole. Data are shown as box plots (see Materials and Methods).
Short-Term High-Dose Oral NAC Treatment Decreases the Overactive Release of Elastase-Rich Granules by CF Airway Neutrophils but Not at the Single-Cell Level.
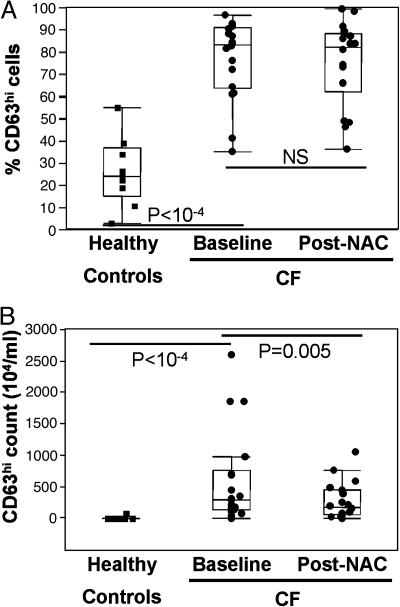
The excessive airway elastase activity in CF airways is generally attributed to passive leakage from postnecrotic neutrophils (29). However, we demonstrate in a related study (R.T., R.B.M., C.K.C., Leonore A. Herzenberg, and Leonard A. Herzenberg, unpublished work) that the release of elastase-rich primary granules is specifically and markedly up-regulated in live nonapoptotic airway neutrophils from CF compared with healthy subjects. This overactive release of primary granules is demonstrated by a significantly increased baseline percentage of airway neutrophils bearing high surface levels of the primary granule marker CD63 (Fig. 4A). This functional defect correlates highly and positively with airway elastase activity, and negatively with the FEV1 and the FVC (data not shown). Short-term treatment with high-dose oral NAC significantly decreased the number of elastase-releasing neutrophils in CF airways (Fig. 4B and Table 1), consistent with its effect on the overall airway neutrophil count (Fig. 1). However, primary granule release measured by FACS on airway neutrophils was not altered by this short-term treatment (Fig. 4A).
Fig. 4.
Short-term high-dose oral NAC decreases the overactive release of elastase-rich granules by live CF airway neutrophils, not at the single-cell level, but in aggregate. (A) Live nonapoptotic neutrophils from CF compared with healthy airways show an overactive baseline release of elastase-rich primary granules, reflected by a markedly increased percentage of cells with high surface expression of CD63 (marker specific for primary granules, expressed at the surface upon granule fusion with the plasma membrane). High-dose oral NAC, in this short-term trial, did not inhibit this cellular defect. (B) The physiologically relevant compound index of elastase-rich granule release (percentage CD63hi neutrophils × airway neutrophil count) was significantly decreased by NAC treatment. Data are shown as box plots (see Materials and Methods).
Discussion
In this study, we identify a clinically important cellular link between redox and inflammatory imbalances in CF pathology, and we demonstrate the ability of high-dose oral NAC to ameliorate these imbalances. The redox imbalance, manifested by a deficiency in extracellular GSH, is first observed in the lung fluid, and later in the systemic circulation of CF patients (30, 31). The inflammatory imbalance, by contrast, is localized to airways (1). However, both imbalances have a common cellular link: the neutrophils. Blood neutrophils migrate in high numbers into CF airways and contribute to tissue damage, mucus viscosity, and opportunistic infections, all hallmarks of CF airway disease.
The neutrophilic inflammation of CF airways is a self-amplifying process, with neutrophil-derived effectors such as oxidants, elastase, and IL-8 perpetuating neutrophil exit from the bone marrow, into circulation, and later into CF airways. Here, we observed that neutrophils in the systemic circulation of CF patients with mild to moderately severe airway disease (all in stable clinical condition) were markedly deficient in the antioxidant GSH. Because GSH depletion may impact neutrophil function (17–19), we sought to augment GSH levels in circulating neutrophils by using the well known GSH prodrug NAC (20). In diseases unrelated to CF, oral NAC increased intracellular GSH in blood neutrophils (32–34). Independently, oral NAC decreased neutrophil recruitment to the lungs (27, 28).
In our study, oral NAC was able to do both: GSH in blood neutrophils was significantly augmented and airway neutrophil count and elastase activity were significantly decreased. To this end, we used doses in excess of 1.8 g per day, which had never been used in CF before. The high doses proved to be safe during the 4-week treatment period. Oral NAC treatment likely decreases airway neutrophil count via multiple pathways. First, NAC can increase actin-dependent deformability (35), leading to reduced stasis in narrow lung capillaries (36). Second, NAC can inhibit the NF-κB pathway (37), leading to decreased IL-8 production (as seen in a subset of patients), which can in turn limit neutrophil recruitment.
However, data presented here suggest that high-dose oral NAC, at least in the short-term, does not impact the overactive release of elastase-rich granules by live neutrophils once they have migrated to CF airways. Hence, the observed decrease in elastase activity may be due mostly to a decrease in overall neutrophil count. Additionally, NAC may protect α1-antitrypsin against oxidant-induced inhibition (38), thus allowing this endogenous elastase inhibitor to recover its lost function. Taken together, our findings are consistent with a biphasic model of CF inflammatory airway disease (Fig. 5). In this model, the self-amplifying inflammatory process may be countered early on by the use of high-dose NAC, given orally, as a blocker of the abnormal migration of neutrophils into airways. Other drugs, possibly given as aerosols, may be combined to inhibit the downstream events of active/passive airway neutrophil dysfunction.
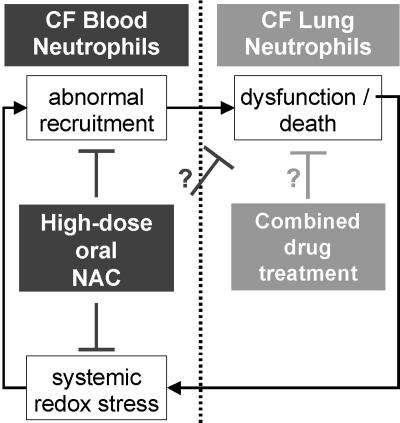
Fig. 5.
A biphasic dysregulation model for CF airway inflammatory disease. We propose that inflammatory disease in CF occurs in two distinct steps, which may be targeted by different treatments. The first step, featuring the abnormal recruitment of neutrophils from the systemic circulation to the airways, may be efficiently countered by high-dose oral NAC treatment. The second step in this self-amplifying inflammatory process is the functional dysregulation of neutrophils once they have reached CF airways. We have demonstrated in a related study (R.T., R.B.M., C.K.C., Leonore A. Herzenberg, and Leonard A. Herzenberg, unpublished work) that this dysfunctional regulation features not only an abnormal death and postnecrotic leakage of neutrophil by-products, as generally assumed, but also an upstream overactive release of elastase-rich granules by live nonapoptotic airway neutrophils. High-dose oral NAC in the long term or other drugs may target this defect.
The recognition of neutrophilic inflammation as a major driving force in the progression of CF airway disease has led several groups to propose novel antiinflammatory approaches, including modulators of lipid mediator production (39, 40). So far, none of these approaches has proven safe and efficient in the long term. In contrast, NAC has a proven safety record over long-term use at high doses in several chronic inflammatory diseases and has minimal interaction with other drugs (20). In CF, it could thus be used in combination with other treatments typically prescribed to CF patients, including antibiotics. Although CF pulmonary function was not improved in this short-term trial of high-dose oral NAC, the marked posttreatment decrease in elastase activity, recognized as the best predictor of CF pulmonary function (10), leaves hope that long-term treatment may ultimately improve pulmonary status and outcome by ameliorating the 2–4% per annum decline in lung function usually seen in CF.
Long-term safety, therapeutic effects, and mode of action of high-dose oral NAC treatment remain to be tested. In the meantime, it is important to warn patients against uncontrolled use of the drug, especially because its nutraceutical status prevents proper quality control of most commercial formulations available over the counter. Only in the context of carefully designed and controlled clinical studies may oral NAC eventually prove a useful preventive therapy for both systemic redox imbalance and airway inflammation in CF.
Materials and Methods
Human Subjects.
This study received the approval of the Cystic Fibrosis Foundation and the local Institutional Review Board (Stanford Administrative Panel on Human Subjects in Medical Research, Stanford University, Stanford, CA). All 9 healthy and 18 CF subjects included in this study signed informed consent forms before undergoing clinical procedures, which included venipuncture and sputum induction (see Processing of Samples), as well as spirometry. Spirometry was performed with American Thoracic Society criteria, yielding data on FEV1 as a percent of predicted value, FVC as a percent of predicted value, and other related measurements. Other clinical endpoints included physical examination, complete blood counts, routine blood chemistry panel, and CF quality of life questionnaire.
Selection and Exclusion Criteria for CF Patients.
CF subjects were all followed regularly at the Lucile Packard Children’s Hospital at Stanford University. Selection criteria for inclusion in the phase 1 trial of high-dose oral NAC were as follows: (i) diagnosis of cystic fibrosis by documented sweat chloride >60 milliequivalents per liter by quantitative pilocarpine iontophoresis test and/or genotype with two identifiable mutations consistent with CF, accompanied by one or more clinical features consistent with CF; (ii) clinically stable status; (iii) age of ≥10 years, male or female, of any ethnic background; (iv) weight ≥25 kg; (v) FEV1 >40% predicted (according to Knudson equations based on gender, age, and height); (vi) ability to perform consistent efforts in pulmonary function testing; (vii) ability to produce sputum upon induction; (viii) for female subjects ≥11 years of age or who have reached menarche, a negative urine pregnancy test and when in childbearing age, agreement to use contraception; (ix) signature of written informed consent and Health Insurance Portability and Accountability Act authorization before the performance of any study-related procedure; and (x) ability to comply with all protocol requirements. Exclusion criteria were as follows: (i) age <10 years; (ii) weight <5th percentile for age or evidence of severe malnutrition as body mass index <10%; (iii) severe pulmonary dysfunction (FEV1 <40% predicted); (iv) evidence of clinical CF-related liver disease; (v) evidence of pulmonary exacerbation; (vi) consumption of antioxidants [including NAC, GSH, Immunocal (Immunotac Research, Vandreuil-Dorion, QC, Canada), Nacystelyn (Galephar, Brussels)] in the 4 weeks before recruitment; (vii) daily use of acetaminophen during the 7 preceding days; and (viii) participation in trials for other antiinflammatory or therapeutic investigational drugs simultaneously or <4 weeks before recruitments.
Drug.
NAC used in this study was produced under Good Manufacturing Practice (GMP) conditions and provided by BioAdvantex Pharmaceuticals (Mississauga, ON, Canada).
Processing of Samples.
Blood was obtained by venipuncture. Airway fluid was obtained by sputum induction, as described in ref. 41. We sought to prevent any artifactual activation of neutrophils, which are very sensitive to external stimuli (19, 24). All samples were chilled on melting ice upon collection and kept at 4°C throughout all experimental procedures. Blood samples were not submitted to gradient centrifugation, which activates neutrophils (25, 26). Instead, whole blood was directly processed for staining. Sputum samples were not liquified with DTT at 37°C, as commonly used, because this combination of a potent redox effector and high temperature alters intracellular GSH levels and cell activation (refs. 42 and 43; R.T., unpublished work). Instead, sputum samples were weighed, their volume was measured, and 2 volumes of ice-cold PBS were added. Cells were collected after gentle mechanical dissociation by repeated passage through a sterile 18-gauge needle, followed by filtration through a sterile nylon 40-μm mesh and centrifugation at 400 × g for 10 min. Live airway cells were counted in a dual fluorescence microscope by using the ethidium bromide/acridine orange method and adjusted to 106 cells per ml for staining. The supernatant was further spun at 3,000 × g for 10 min to yield clear sputum fluid for further assays (see Fluid Assays).
Measurement of Whole Blood GSH Levels by HPLC.
Immediately upon collection, 100 μl of whole blood was precipitated with 4% metaphosphoric acid (900 μl). Samples were then vortexed and centrifuged at 3,000 × g for 5 min, and 200 μl of clear supernatant was collected and kept at −80°C until analyzed. Total GSH was determined in thawed supernatants by using an HPLC method with fluorescence detection (44).
Measurement of Intracellular GSH and Elastase-Rich Granule Release in Neutrophils Using Multiparameter Digital FACS.
Whole blood and sputum samples (100 μl) were mixed with the GSH-specific probe monochlorobimane, as detailed in ref. 22. In brief, this method uses the ability of the nonfluorescent probe monochlorobimane to permeate live cells and be conjugated to intracellular GSH by cellular glutathione-S-transferases, thus generating fluorescent GSB adducts (23). Fluorescent adduct formation depends mostly on GSH levels but can also be marginally affected by glutathione-S-transferase activity (which is usually very similar in identical leukocyte subsets from different individuals). Hence, this direct GSH measurement method is considered semiquantitative. Efflux of fluorescent adducts from stained cells was inhibited by addition of probenecid (Sigma) to the RPMI medium 1640 used for staining. After monochlorobimane staining, cells were washed and stained (all reagents from BD Pharmingen) as follows: (i) with the annexin V probe and with antibodies against surface molecules of interest, including CD11b, CD16, CD45, and CD66b to allow for gating of nonapoptotic neutrophils; and (ii) with CD63, to allow for quantitative measurement of elastase-rich granule release. Samples were further washed in probenecid-containing RPMI medium 1640 with 2.5 mM calcium chloride and fixed with 0.5% paraformaldehyde immediately before acquisition on the FACS. Multiparameter digital FACS data were acquired on a Digital Vantage machine (diva software; BD Pharmingen) equipped with three lasers (365, 488, and 598 nm), two scatter detectors (yielding forward scatter and side scatter data), and 12 fluorescent detectors. The FACS machine was calibrated before each session by using a standard set of multicolor fluorescence beads (45–47). Acquisition was controlled by using the diva software (BD Pharmingen). Acquisition speed was kept <2,000 events per second to prevent clumping of cells. Data were exported to the flowjo software (Tree Star, Ashland, OR) for analysis. Live nonapoptotic neutrophils were gated on forward and side scatters, GSB, annexin V, CD66b, and CD45 levels, as detailed elsewhere (R.T., R.B.M., C.K.C., Leonore A. Herzenberg, and Leonard A. Herzenberg, unpublished work), and GSB and CD63 levels were then quantified at the single-cell level.
Fluid Assays.
Elastase activity was measured by a specific spectrophotometric assay that is not sensitive to elastase from Pseudomonas aeruginosa (48). IL-8 levels were measured by standard ELISA (BD Pharmingen). Assays were performed in triplicate.
Statistics.
Statistical analysis was performed by using jmp5 software (SAS Institute, Cary, NC). For baseline comparisons between healthy controls and CF patients, we used the nonparametric Wilcoxon rank-sum test, because unequal numbers in the groups (18 and 9, respectively) precluded rigorous use of parametric tests. For baseline to posttreatment comparisons in CF patients, variables were tested for normality by using the Shapiro–Wilk test and further compared by using the parametric paired t test or nonparametric Wilcoxon signed-rank test, as appropriate. For display purposes, data from individual subjects are presented within box plots, with median line in box delimited by 25th and 75th quantiles ± 1.5 × interquartile range (whiskers). Treatment effects for normally and nonnormally distributed variables are illustrated as percentage changes and as baseline vs. posttreatment 95% confidence intervals, respectively. Correlations between variables were studied by using the nonparametric Spearman test. Significance was set at P < 0.05.
Supplementary Material
Acknowledgments
We thank C. Dunn, Z. Davies, and B. Aram for their invaluable help with portions of the work and D. Parks, J. Tung, and D. Stovel for excellent support and advice. This work was supported by Cystic Fibrosis Foundation Grant CONRAD 04 (to R.T. and C.K.C.) and National Institutes of Health Grant R01 CA85949 (to Leonard A. Herzenberg).
Abbreviations
- CF
cystic fibrosis
- FEV1
functional expiratory volume in 1 s
- FVC
forced vital capacity
- GSB
glutathione-S-bimane
- GSH
glutathione
- NAC
N-acetylcysteine.
Footnotes
Conflict of interest statement: R.T., C.K.C., Leonore A. Herzenberg, R.B.M., and Leonard A. Herzenberg are listed as inventors on a provisional patent application covering NAC as a therapeutic agent for CF. Leonore A. Herzenberg and Leonard A. Herzenberg hold a small amount of equity in BioAdvantex (Mississauga, ON, Canada), which sells European GMP NAC and provided the NAC used in this study.
References
- 1.Davis P. B. Pediatr. Rev. 2001;22:257–264. doi: 10.1542/pir.22-8-257. [DOI] [PubMed] [Google Scholar]
- 2.Cantin A. Am. J. Respir. Crit. Care Med. 1995;151:939–941. doi: 10.1164/ajrccm.151.4.7697269. [DOI] [PubMed] [Google Scholar]
- 3.Khan T. Z., Wagener J. S., Bost T., Martinez J., Accurso F. J., Riches D. W. Am. J. Respir. Crit. Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 4.Tirouvanziam R., Khazaal I., Peault B. Am. J. Physiol. 2002;283:L445–L451. doi: 10.1152/ajplung.00419.2001. [DOI] [PubMed] [Google Scholar]
- 5.Muhlebach M. S., Stewart P. W., Leigh M. W., Noah T. L. Am. J. Respir. Crit. Care Med. 1999;160:186–191. doi: 10.1164/ajrccm.160.1.9808096. [DOI] [PubMed] [Google Scholar]
- 6.Tirouvanziam R., de Bentzmann S., Hubeau C., Hinnrasky J., Jacquot J., Peault B., Puchelle E. Am. J. Respir. Cell Mol. Biol. 2000;23:121–127. doi: 10.1165/ajrcmb.23.2.4214. [DOI] [PubMed] [Google Scholar]
- 7.Vasconcellos C. A., Allen P. G., Wohl M. E., Drazen J. M., Janmey P. A., Stossel T. P. Science. 1994;263:969–971. doi: 10.1126/science.8310295. [DOI] [PubMed] [Google Scholar]
- 8.Kirchner K. K., Wagener J. S., Khan T. Z., Copenhaver S. C., Accurso F. J. Am. J. Respir. Crit. Care Med. 1996;154:1426–1429. doi: 10.1164/ajrccm.154.5.8912759. [DOI] [PubMed] [Google Scholar]
- 9.Walker T. S., Tomlin K. L., Worthen G. S., Poch K. R., Lieber J. G., Saavedra M. T., Fessler M. B., Malcolm K. C., Vasil M. L., Nick J. A. Infect. Immun. 2005;73:3693–3701. doi: 10.1128/IAI.73.6.3693-3701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sagel S. D., Sontag M. K., Wagener J. S., Kapsner R. K., Osberg I., Accurso F. J. J. Pediatr. 2002;141:811–817. doi: 10.1067/mpd.2002.129847. [DOI] [PubMed] [Google Scholar]
- 11.Konstan M. W., Davis P. B. Adv. Drug Delivery Rev. 2002;54:1409–1423. doi: 10.1016/s0169-409x(02)00146-1. [DOI] [PubMed] [Google Scholar]
- 12.Keatings V. M., Evans D. J., O’Connor B. J., Barnes P. J. Thorax. 1997;52:372–374. doi: 10.1136/thx.52.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson V. M. Free Radical Biol. Med. 2001;30:1440–1461. doi: 10.1016/s0891-5849(01)00530-5. [DOI] [PubMed] [Google Scholar]
- 14.Cantin A. M. Curr. Opin Pulm. Med. 2004;10:531–536. doi: 10.1097/01.mcp.0000138997.29276.a1. [DOI] [PubMed] [Google Scholar]
- 15.Wood L. G., Fitzgerald D. A., Gibson P. G., Cooper D. M., Collins C. E., Garg M. L. J. Am. Coll. Nutr. 2001;20:157–165. doi: 10.1080/07315724.2001.10719028. [DOI] [PubMed] [Google Scholar]
- 16.Kinnula V. L., Soini Y., Kvist-Makela K., Savolainen E. R., Koistinen P. Antioxid. Redox Signal. 2002;4:27–34. doi: 10.1089/152308602753625825. [DOI] [PubMed] [Google Scholar]
- 17.Seely A. J., Pascual J. L., Christou N. V. Crit. Care. 2003;7:291–307. doi: 10.1186/cc1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santangelo F. Curr. Med. Chem. 2003;10:2599–2610. doi: 10.2174/0929867033456567. [DOI] [PubMed] [Google Scholar]
- 19.Tirouvanziam R. In: Recent Advances in Immunology. Kundu-Raychaudhuri S., editor. Trivandrum, India: Research Signpost; 2005. pp. 1–22. [Google Scholar]
- 20.Kelly G. S. Altern. Med. Rev. 1998;3:114–127. [PubMed] [Google Scholar]
- 21.Duijvestijn Y. C., Brand P. L. Acta Paediatr. 1999;88:38–41. doi: 10.1080/08035259950170574. [DOI] [PubMed] [Google Scholar]
- 22.Anderson M. T., Roederer M., Tjioe I., Herzenberg L. A., Herzenberg L. A. In: Handbook of Experimental Immunology. Blackwell C., editor. Vol. 2. Oxford: Blackwell Scientific; 1996. pp. 54.1–54.9. [Google Scholar]
- 23.Tirouvanziam R., Davidson C. J., Lipsick J. S., Herzenberg L. A. Proc. Natl. Acad. Sci. USA. 2004;101:2912–2917. doi: 10.1073/pnas.0308734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swain S. D., Rohn T. T., Quinn M. T. Antioxid. Redox Signal. 2002;4:69–83. doi: 10.1089/152308602753625870. [DOI] [PubMed] [Google Scholar]
- 25.Venaille T. J., Misso N. L., Phillips M. J., Robinson B. W., Thompson P. J. Scand. J. Clin. Lab. Invest. 1994;54:385–391. doi: 10.3109/00365519409088438. [DOI] [PubMed] [Google Scholar]
- 26.Zahler S., Kowalski C., Brosig A., Kupatt C., Becker B. F., Gerlach E. J. Immunol. Methods. 1997;200:173–179. doi: 10.1016/s0022-1759(96)00206-2. [DOI] [PubMed] [Google Scholar]
- 27.MacNee W., Bridgeman M. M., Marsden M., Drost E., Lannan S., Selby C., Donaldson K. Am. J. Med. 1991;91:60S–66S. doi: 10.1016/0002-9343(91)90285-6. [DOI] [PubMed] [Google Scholar]
- 28.Villa P., Saccani A., Sica A., Ghezzi P. J. Infect. Dis. 2002;185:1115–1120. doi: 10.1086/340042. [DOI] [PubMed] [Google Scholar]
- 29.Vandivier R. W., Fadok V. A., Hoffmann P. R., Bratton D. L., Penvari C., Brown K. K., Brain J. D., Accurso F. J., Henson P. M. J. Clin. Invest. 2002;109:661–670. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hull J., Vervaart P., Grimwood K., Phelan P. Thorax. 1997;52:557–560. doi: 10.1136/thx.52.6.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roum J. H., Buhl R., Mcelvaney N. G., Borok Z., Crystal R. G. J. Appl. Physiol. 1993;75:2419–2424. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- 32.Laurent T., Markert M., Feihl F., Schaller M. D., Perret C. Chest. 1996;109:163–166. doi: 10.1378/chest.109.1.163. [DOI] [PubMed] [Google Scholar]
- 33.Behr J., Degenkolb B., Krombach F., Vogelmeier C. Eur. Respir. J. 2002;19:906–911. doi: 10.1183/09031936.02.00204902. [DOI] [PubMed] [Google Scholar]
- 34.Martensson J., Gustafsson J., Larsson A. J. Inherited Metab. Dis. 1989;12:120–130. doi: 10.1007/BF01800713. [DOI] [PubMed] [Google Scholar]
- 35.Drost E. M., Selby C., Lannan S., Lowe G. D., MacNee W. Am. J. Respir. Cell Mol. Biol. 1992;6:287–295. doi: 10.1165/ajrcmb/6.3.287. [DOI] [PubMed] [Google Scholar]
- 36.Doerschuk C. M. Microcirculation. 2001;8:71–88. [PubMed] [Google Scholar]
- 37.Arrigo A. P. Free Radical Biol. Med. 1999;27:936–944. doi: 10.1016/s0891-5849(99)00175-6. [DOI] [PubMed] [Google Scholar]
- 38.Gressier B., Lebegue S., Gosset P., Brunet C., Luyckx M., Dine T., Cazin M., Cazin J. C., Wallaert B. Fundam. Clin. Pharmacol. 1994;8:518–524. doi: 10.1111/j.1472-8206.1994.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 39.Schmitt-Grohe S., Zielen S. Paediatr. Drugs. 2005;7:353–363. doi: 10.2165/00148581-200507060-00004. [DOI] [PubMed] [Google Scholar]
- 40.Karp C. L., Flick L. M., Park K. W., Softic S., Greer T. M., Keledjian R., Yang R., Uddin J., Guggino W. B., Atabani S. F., et al. Nat. Immunol. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 41.Henig N. R., Tonelli M. R., Pier M. V., Burns J. L., Aitken M. L. Thorax. 2001;56:306–311. doi: 10.1136/thorax.56.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai Y., Carty K., Gibson P., Henry R. Pediatr. Pulmonol. 1996;22:402–407. doi: 10.1002/(SICI)1099-0496(199612)22:6<402::AID-PPUL9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 43.Woolhouse I. S., Bayley D. L., Stockley R. A. Thorax. 2002;57:667–671. doi: 10.1136/thorax.57.8.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abukhalaf I. K., Silvestrov N. A., Menter J. M., von Deutsch D. A., Bayorh M. A., Socci R. R., Ganafa A. A. J. Pharm. Biomed. Anal. 2002;28:637–643. doi: 10.1016/s0731-7085(01)00658-6. [DOI] [PubMed] [Google Scholar]
- 45.Perfetto S. P., Chattopadhyay P. K., Roederer M. Nat. Rev. Immunol. 2004;4:648–655. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- 46.Tung J. W., Parks D. R., Moore W. A., Herzenberg L. A., Herzenberg L. A. Clin. Immunol. 2004;110:277–283. doi: 10.1016/j.clim.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 47.Baumgarth N., Roederer M. J. Immunol. Methods. 2000;243:77–97. doi: 10.1016/s0022-1759(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 48.Pelletier A., Dimicoli J. L., Boudier C., Bieth J. G. Am. J. Respir. Cell Mol. Biol. 1989;1:37–39. doi: 10.1165/ajrcmb/1.1.37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.