Abstract
An insight into a previously unknown step in B12 biosynthesis was unexpectedly obtained through our analysis of a mutant of the symbiotic nitrogen fixing bacterium Sinorhizobium meliloti. This mutant was identified based on its unusually bright fluorescence on plates containing the succinoglycan binding dye calcofluor. The mutant contains a Tn5 insertion in a gene that has not been characterized previously in S. meliloti. The closest known homolog is the bluB gene of Rhodobacter capsulatus, which is implicated in the biosynthesis of B12 (cobalamin). The S. meliloti bluB mutant is unable to grow in minimal media and fails to establish a symbiosis with alfalfa, and these defects can be rescued by the addition of vitamin B12 (cyanocobalamin) or the lower ligand of cobalamin, 5,6-dimethylbenzimidazole (DMB). Biochemical analysis demonstrated that the bluB mutant does not produce cobalamin unless DMB is supplied. Sequence comparison suggests that BluB is a member of the NADH/flavin mononucleotide (FMN)-dependent nitroreductase family, and we propose that it is involved in the conversion of FMN to DMB.
Keywords: cobalamin, symbiosis, bacteria, vitamin B12, biosynthesis
Sinorhizobium meliloti is a Gram-negative α-proteobacterium that exists free-living in soil as well as in symbiosis with specific legume hosts. The symbiosis results in the formation of organs on the plant root known as nodules, in which the bacteria fix atmospheric nitrogen into ammonia in exchange for nutrients provided by the plant (1, 2). The symbiosis is initiated by a series of reciprocal signaling events between the plant and the bacteria. In response to the secretion of flavonoid molecules by the plant, the bacteria induce nodule development in the plant root by producing nod factor. This compound promotes both root hair curling and cortical cell division in the plant root (3, 4). The bacteria invade the plant root hair through tubes known as infection threads (5). The initiation and extension of infection threads is mediated by the bacterial exopolysaccharide succinoglycan (6). The bacteria are released from the infection thread into membrane-bound vesicles within the plant cells of the developing nodule where they differentiate into nitrogen fixing bacteroids (7, 8).
Cobalt has long been known to be essential for both free-living and symbiotic growth of rhizobia (9–13). A major biological function of cobalt is its role as the central atom in B12 (cobalamin) (14). Cobalamin is a cofactor for several enzymatic reactions in animals, protists, and some prokaryotes, yet its biosynthesis is limited to certain prokaryotes (15). Humans have two cobalamin-dependent enzymes: methylmalonyl-CoA mutase, which catalyzes a step in the metabolism of branched chain amino acids and fatty acids, and methionine synthase, which catalyzes the final step in methionine biosynthesis (16). S. meliloti has homologs of these two enzymes, BhbA and MetH, respectively (17, 18). S. meliloti also uses a cobalamin-dependent ribonucleotide reductase, NrdJ, for the synthesis of deoxynucleotides (19, 20). The biosynthesis of methionine and deoxynucleotides occurs only by these cobalamin-dependent pathways in S. meliloti. It is not known whether S. meliloti or humans have other cobalamin-dependent enzymes.
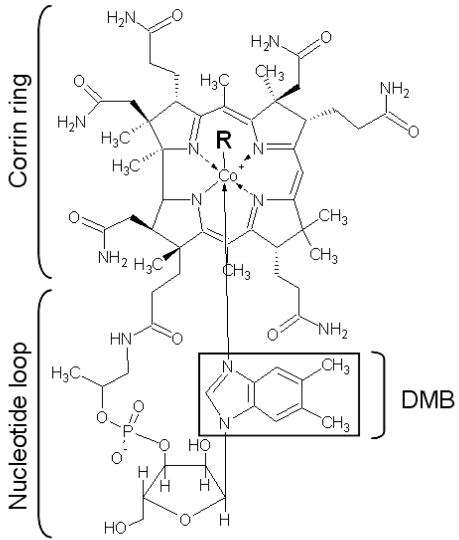
Cobalamin is the largest known nonpolymeric natural compound, as well as one of the most thoroughly studied. The central structural component of cobalamin is a corrin ring (Fig. 1). In the biologically active forms of cobalamin, adenosylcobalamin and methylcobalamin, the upper ligand consists of either 5′-deoxyadenosine or a methyl group, respectively (Fig. 1), and the lower ligand, usually 5,6-dimethylbenzimidazole (DMB), is attached to the corrin ring via the nucleotide loop (15, 21). Cobalamin has been isolated from S. meliloti and shown to contain DMB as the lower ligand (13). Two separate pathways for cobalamin biosynthesis exist in nature, depending on whether the process occurs aerobically or anaerobically, and each pathway requires ≈30 enzymes (21). The S. meliloti genome contains a complete set of genes involved in the aerobic cobalamin biosynthetic pathway.
Fig. 1.
Structure of B12. The corrin ring, the nucleotide loop, and the lower ligand, DMB, are indicated. The upper ligand (R) consists of 5′-deoxyadenosine (adenosylcobalamin), CH3 (methylcobalamin), or CN (cyanocobalamin).
The only component of cobalamin whose biosynthesis is not well understood is DMB. Previous work in Propionibacterium shermanii and Salmonella typhimurium has shown that DMB is synthesized from flavin mononucleotide (FMN) aerobically, and that the 1′ carbon of FMN is incorporated into DMB at C2 (22–25). However, the precise reactions and the corresponding enzymes involved in this process have not yet been identified. The condensation of ribosylated DMB with GDP-cobinamide is the final step in the biosynthetic pathway (21).
We report here the characterization of an S. meliloti mutant isolated on the basis of its abnormal succinoglycan production. It contains an insertion in a gene that has sequence similarity to the bluB gene of Rhodobacter capsulatus, which has been implicated in cobalamin production (26). We demonstrate that S. meliloti bluB is required for cobalamin biosynthesis, both in free-living growth and symbiosis with alfalfa. The S. meliloti bluB mutant is specifically defective in DMB biosynthesis, and to our knowledge this is the first report of a gene involved in this process.
Results
Screen for S. meliloti Mutants Altered in Succinoglycan Synthesis.
In an attempt to study the effect of altered succinoglycan production on the ability of S. meliloti to establish a symbiosis with alfalfa, we initiated a genetic screen for mutants that exhibit abnormally high fluorescence on agar plates containing the fluorescent succinoglycan binding dye calcofluor. A calcofluor bright phenotype is indicative of either overproduction of succinoglycan or a change in its structure (27, 28). Approximately 15,000 random Tn5 transposon insertions in wild-type S. meliloti strain Rm1021 were screened for high fluorescence on agar plates containing calcofluor. A total of 150 colonies that exhibited brighter fluorescence than the surrounding colonies were isolated and inoculated onto alfalfa seedlings. Thirty of these mutants exhibited a Fix− phenotype on alfalfa.
One calcofluor bright mutant with a particularly severe symbiotic defect was chosen for further study (Fig. 2A). We sequenced its Tn5 transposon fusion junction and located the transposon in the chromosomal ORF SMc00166. Based on sequence similarity, SMc00166 is predicted to encode a nitroreductase enzyme of the NADH/FMN-dependent oxidoreductase family. The SMc00166 predicted protein sequence has 36% identity to the BluB protein from R. capsulatus (26). The R. capsulatus bluB gene is involved in the biosynthesis of cobalamin and is annotated as a putative cob(II)yrinic acid a,c diamide reductase, which functions in the reduction of the Co(II) center during cobalamin biosynthesis (29). Because of its similarity to the R. capsulatus bluB gene and its mutant phenotype described below, we refer to the S. meliloti SMc00166 gene as bluB. Homologs of bluB exist in numerous bacterial genomes. An alignment of the predicted BluB protein sequences of six representative bacteria is shown in Fig. 6, which is published as supporting information on the PNAS web site.
Fig. 2.
Calcofluor fluorescence of S. meliloti strains. S. meliloti strains were streaked onto LB calcofluor plates (A and B) and LB calcofluor plates containing 50 nM DMB (C) and viewed under UV light. (A) Strains shown are Rm1021 (Left) and the bluB::Tn5 mutant (Right). (B and C) Rm1021 (1), bluB::gus (2), bluB::gus containing the plasmid expressing bluB (3), bhbA::Tn5 (4), and metH::Tn5 (5) are shown.
The bluB Calcofluor Bright Phenotype Is Suppressed by Addition of DMB.
S. meliloti is known to require cobalt for growth in minimal medium, presumably because cobalamin is necessary for the synthesis of methionine and deoxyribonucleotides (30). The predicted function of bluB in cobalamin biosynthesis prompted us to examine the growth of the bluB mutant on LB and M9 minimal agar plates in the presence and absence of cyanocobalamin. Surprisingly, the bluB mutant formed colonies on these plates even in the absence of cyanocobalamin (data not shown). It also grows in rich medium, although more slowly and to a lower final density than Rm1021. In contrast, a cobD mutant, which is unable to synthesize the corrin ring of cobalamin (Fig. 1) (31), did not form colonies on either M9 or LB medium unless cyanocobalamin was supplied (data not shown).
Because the R. capsulatus bluB gene is necessary for the production of a component of cobalamin other than cobinamide (26), we speculated that bluB in S. meliloti could function in the biosynthesis of the lower ligand of cobalamin, DMB. The ability of the bluB mutant, but not the cobD mutant, to grow in LB and to form colonies on M9 agar plates could be due to the presence of trace amounts of DMB in LB and agar. We first tested whether the addition of DMB to LB agar plates would influence the calcofluor fluorescence of the bluB mutant, and indeed, we found this to be the case (Fig. 2C). DMB does not affect the fluorescence of Rm1021 or the bluB mutant expressing bluB from a plasmid (Fig. 2 B and C).
To attribute the calcofluor bright phenotype of the bluB mutant to the decreased activity of a cobalamin-dependent enzyme, we compared the calcofluor fluorescence of bluB to two previously isolated mutants, bhbA::Tn5 and metH::Tn5 (J. Cha, G.R.O.C., and G.C.W., unpublished data). The bhbA strain is significantly brighter than Rm1021 on plates containing calcofluor, but less bright than the bluB strain, and the metH strain is not brighter than wild type (Fig. 2B). The calcofluor phenotypes of the wild-type, bhbA, and metH strains are not affected by DMB (Fig. 2C). The bhbA, metH double mutant appears similar to the bhbA mutant (data not shown). These observations suggest that a reduction in BhbA activity contributes to the calcofluor bright phenotype of the bluB mutant but is not the sole cause of its unusually bright fluorescence.
The S. meliloti bluB Mutant Requires Cobalamin or DMB for Free-Living Growth.
We next compared the growth of the bluB mutant with Rm1021 and the cobD and metH mutants in liquid minimal medium supplemented with different components of cobalamin. To remove residual cobalt or cobalamin from the media and inside the cells, the cells were washed and diluted extensively (see Materials and Methods).
As expected, all strains grew poorly in the absence of added cobalt (Fig. 3A). Growth of Rm1021, but not the other strains, was restored by addition of cobalt (Fig. 3B). Addition of cyanocobalamin to the medium caused increased growth of Rm1021 and the cobD and bluB mutants (Fig. 3C). Cobinamide fully restored the growth of the cobD mutant but only partially stimulated the growth of the bluB mutant (Fig. 3D). Conversely, addition of DMB plus cobalt enhanced the growth of the bluB mutant, but not growth of the cobD mutant (Fig. 3E). Growth of both Rm1021 and the metH mutant was fully restored by the addition of methionine, whereas the growth of the cobD and bluB mutants was only partially enhanced (Fig. 3F). This finding indicates that methionine is the major limiting factor when cobalamin is scarce, but is not the sole basis for the growth defects.
Fig. 3.
Free-living growth. Absorbance at 600 nm was measured for Rm1021 (circles), cobD::gus (squares), bluB::gus (triangles), and metH::Tn5 (diamonds) grown in M9 medium with no supplement (A), CoCl2 (B), cyanocobalamin (C), cobinamide (D), DMB and CoCl2 (E), and methionine (F).
Characterization of the Symbiotic Defect of the bluB Mutant.
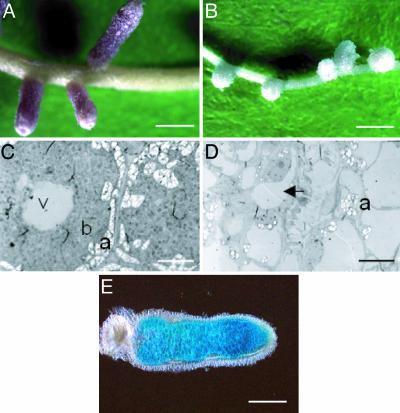
Alfalfa seedlings inoculated with Rm1021 become healthy plants with pink, elongated nodules, whereas the plants inoculated with the bluB mutant are unhealthy and produce small, round, and white nodules (Fig. 4 A and B). Nodules elicited by Rm1021 typically contain many bacteroids that are visible by transmission electron microscopy (Fig. 4C). In contrast, bacteroids are not visible in nodules elicited by the bluB mutant (Fig. 4D). Most bluB mutant derived nodules yield 10- to 1,000-fold fewer bacterial colonies than nodules containing Rm1021 when crushed and plated on LB agar plates (data not shown). However, normal extended infection threads are visible by fluorescence microscopy in plants inoculated with the bluB mutant carrying a plasmid expressing GFP (data not shown) (6). Therefore, the bluB mutant phenotype is distinct from that of the exo mutants, which do not form extended infection threads (6), and mutants such as bacA, which forms defective bacteroids in the nodule (32).
Fig. 4.
Alfalfa nodules elicited by S. meliloti. After 4 weeks of growth, nodules induced by Rm1021 (A) are elongated and pink. In contrast, those elicited by the bluB mutant (B) are small, round, and white. In nodules elicited by Rm1021viewed by transmission electron microscopy (C), cells from the nitrogen fixing zone are packed with bacteroids (b), vacules (v), and small amyloplasts (a). Nodules elicited by the bluB mutant (D) contain plant cells lacking bacteria (for example, arrow) and often have amyloplasts (a) filling portions of the cell. (E) A nodule containing the bluB::gus strain with a plasmid expressing bluB was stained for Gus activity. (Scale bars, 1.5 mm in A and B, 1 μm in C and D, and 0.75 mm in E.)
To confirm that the symbiotic defect of the bluB strain is due to inactivation of bluB, we inoculated alfalfa seedlings with the bluB::gus strain expressing bluB from a plasmid and observed that the plants were indistinguishable from those inoculated with Rm1021. To analyze bluB expression in these plants, the nodules were stained with X-gluc to visualize Gus reporter activity. Gus staining is present in all regions of the nodule, indicating that bluB is expressed throughout the symbiosis (Fig. 4E). bluB is also expressed during free-living growth (data not shown). These results are consistent with the previous observation that other proteins involved in cobalamin biosynthesis are present in both free-living S. meliloti and bacteroids (33).
The S. meliloti bluB Mutant Requires DMB for Symbiosis with Alfalfa.
To relate the symbiotic deficiency of the bluB mutant to its requirement for cobalamin, we inoculated alfalfa seedlings with the bluB mutant on agar plates containing cyanocobalamin, cobinamide, or DMB (Table 1). It was not necessary to add cobalt, because a sufficient amount is present in agar. After 4 weeks of growth, plants were scored for height and number of pink and white nodules. Additionally, acetylene reduction assays were performed on whole plants to measure nitrogenase activity. None of the supplements affected the growth of uninoculated alfalfa or alfalfa inoculated with Rm1021 (Table 1). Plants inoculated with the cobD strain resembled uninoculated plants and produced only a small number of white nodules with any of the supplements provided (data not shown).
Table 1.
Symbiotic phenotype of Rm1021 and the bluB mutant in the presence of cyanocobalamin, cobinamide, and DMB
| Plant height, cm | Nodules per plant |
Acetylene reduction (nmol·plant−1·h−1) | ||
|---|---|---|---|---|
| Pink | White | |||
| Uninoculated | ||||
| No addition | 2 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Cyanocobalamin | 2 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Cobinamide | 2 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| DMB | 3 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Wild type | ||||
| No addition | 10 ± 2 | 21 ± 9 | 4 ± 4 | 55 ± 22 |
| Cyanocobalamin | 9 ± 3 | 18 ± 8 | 4 ± 3 | 38 ± 7 |
| Cobinamide | 10 ± 3 | 17 ± 6 | 2 ± 2 | 47 ± 18 |
| DMB | 10 ± 2 | 18 ± 8 | 3 ± 3 | 42 ± 15 |
| bluB::gus | ||||
| No addition | 2 ± 1 | 0 ± 0 | 35 ± 15 | 1 ± 1 |
| Cyanocobalamin | 8 ± 2 | 22 ± 9 | 4 ± 3 | 54 ± 18 |
| Cobinamide | 2 ± 1 | 3 ± 5 | 22 ± 8 | 9 ± 7 |
| DMB | 8 ± 3 | 19 ± 7 | 6 ± 4 | 62 ± 47 |
The plants inoculated with S. meliloti carrying the bluB mutation have a severe Fix− phenotype; their height is comparable to uninoculated plants, no pink nodules are present, and the acetylene reduction activity is 55-fold lower than that of plants inoculated with Rm1021 (Table 1). Addition of cyanocobalamin or DMB completely restores the plant height, nodule numbers, and nitrogenase activity to the wild-type levels. The effects of cyanocobalamin and DMB are concentration dependent, because the mutant phenotype is not rescued by lower concentrations of these compounds (data not shown). Cobinamide did not suppress the Fix− phenotype, although it did allow infected plants to produce a small, variable number of pink nodules and a low level of nitrogenase activity (Table 1). In sum, the effects of these components of cobalamin on the symbiotic deficiency of the bluB mutant are consistent with its free-living growth phenotype.
The bluB Mutant Does Not Produce Cobalamin Unless DMB Is Supplied.
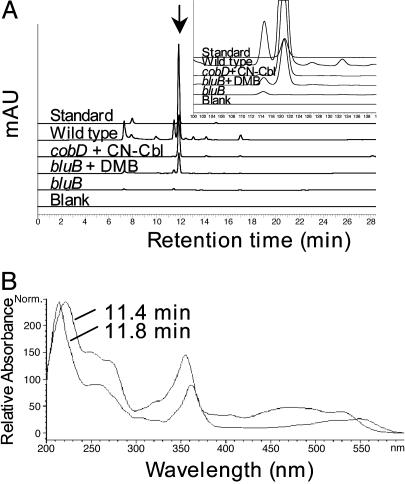
To determine the biochemical defect in cobalamin synthesis in the bluB mutant, we isolated native corrinoids from bacteria grown in liquid culture. The corrinoids were cyanidated, separated by HPLC, and detected at an absorbance of 525 nm (Fig. 5A). A major peak eluting at 11.8 min (arrow) was observed in the extracts of Rm1021 and the cobD strain grown with cyanocobalamin (CN-Cbl). This peak corresponds to cyanocobalamin, as confirmed by comparison of the retention times and DAD UV spectra with the standard. This peak was also present in extracts of the bluB mutant grown with DMB, but absent from extracts when DMB was omitted from the growth medium.
Fig. 5.
HPLC analysis of corrinoids. (A) Corrinoid compounds were extracted from the indicated S. meliloti strains and analyzed by HPLC. Standards contain 0.3 μg of cyanocobalamin and 0.4 μg of 5′-deoxyadenosylcobalamin. (Inset) Enlargement of peaks at 10–14 min. (B) UV DAD of peaks eluting at 11.8 and 11.4 min in the extract of bluB::Tn5 grown in the presence of DMB is shown.
To confirm that the material eluting at 11.8 min was indeed cyanocobalamin in all cases, we analyzed the samples by HPLC-MS. The peaks eluting at 11.8 min for the wild type, cobD, and bluB mutant grown with DMB had mass spectra identical to that of the cyanocobalamin standard (data not shown). The major ion had a mass-to-charge ratio (m/z) of 1355, corresponding to the cyanocobalamin cation. In addition, fragment ions at m/z 998 corresponding to the loss of DMB and at m/z 678 for the doubly charged cyanocobalamin ion [CNCbl+H]2+ were observed. These species were not detected in extracts from the bluB mutant grown in the absence of DMB. Also, consistent with the growth phenotype of the bluB strain, extracts from the bluB mutant grown in the presence of cobinamide also did not contain detectable levels of cyanocobalamin (data not shown).
The bluB Mutant Synthesizes a Cobalamin Precursor, GDP–Cobinamide.
The corrinoid extracts of Rm1021 and the bluB mutant show a peak at significant levels eluting at 11.4 min. This peak was absent from extracts of the cobD mutant, suggesting that the compound contains a corrin ring. MS analysis indicated that the major ion in this peak from extracts of Rm1021, the bluB mutant, and the bluB mutant grown with DMB had an m/z of 1440. This mass corresponds to that of GDP-cobinamide, the final intermediate in the cobalamin biosynthetic pathway. Fragment ions detected at m/z 1095, 1078, and 998 were consistent with cleavages between the diphosphate and the corrin ring. Furthermore, the DAD UV spectrum of this compound shows overall similarity to cobalamin, but with an additional peak at 248 nm and a shoulder at 268 nm, characteristic of the presence of guanine (Fig. 5B) (34). These data suggest that cobalamin biosynthesis in the bluB mutant proceeds until the last step, the replacement of GMP with DMB as the lower ligand (21).
Discussion
The bluB mutant of S. meliloti is the first reported DMB auxotroph, and thus addresses a longstanding puzzle in cobalamin biosynthesis: the origin of its lower ligand. Although several possible biosynthetic pathways have been proposed (22, 35), no enzyme has been purified and no mutants blocked in the pathway have been isolated despite deliberate searches in several laboratories. In the absence of direct demonstration of a gene or enzyme, it has been proposed that DMB might be made nonenzymatically by a cascade of uncatalyzed oxidative reactions (36). The failure to find DMB auxotrophs previously prompts an especially close examination of the mutant reported here. A number of factors support our proposal that BluB is a DMB biosynthetic enzyme. First, no cobalamin is detectable in corrinoid extracts of the bluB mutant unless the strain is supplied with DMB. Second, BluB is homologous to enzymes that bind FMN, the generally accepted precursor of DMB during aerobic growth (22–25). Third, consistent with a role as a cobalamin biosynthetic enzyme, the bluB gene is preceded by a putative adenosylcobalamin-binding RNA regulatory motif known as a riboswitch, typically found adjacent to genes involved in cobalamin production (29). Finally, in Streptomyces spp., the bluB gene is fused to cobT, which encodes the enzyme responsible for forming an activated DMB-intermediate, suggesting that these enzymes have related functions (Fig. 6) (37).
Although cobalamin was not detectable in corrinoid extracts of the bluB mutant grown without added DMB, this mutant did show detectable growth during preparation of the extract, albeit much poorer than the wild-type control. Because S. meliloti requires cobalamin for ribonucleotide reductase activity, some form of cobalamin must be available. Trace amounts of DMB in agar and in LB medium may be sufficient to support the observed growth of the bluB mutant. In addition, we cannot rule out the possibility that adenylcobamide (pseudo-B12), the intermediate GDP-cobinamide, or another corrinoid with an alternative lower ligand might partially substitute for cobalamin. Precedent for this possibility exists, because some cobalamin-dependent enzymes can function using B12 with alternative lower ligands (38, 39). The ability of bacteria to compensate for insufficient DMB by using alternate lower ligands or by scavenging DMB from the environment could explain why DMB biosynthetic enzymes have been difficult to identify in the past. Alternatively, because bluB homologs do not exist in S. typhimurium, this organism might synthesize DMB by different enzymes that are more difficult to identify.
We were able to identify a mutant in S. meliloti with a defect in DMB biosynthesis based on its calcofluor bright phenotype. Fortuitously, calcofluor fluorescence apparently is an indicator of an intermediate phenotype in which S. meliloti cells are limiting for cobalamin but nevertheless are able to form colonies. Because bluB mutant colonies are not mucoid, we infer that the succinoglycan is structurally altered rather than overproduced, as is the case for the exoH mutant, whose calcofluor bright phenotype is attributed to its production of an altered succinoglycan lacking the succinyl group (28). The bluB mutant similarly might have a deficiency in succinylation of the polysaccharide because the cobalamin-dependent methylmalonyl CoA mutase enzyme could be a major source of the precursor succinyl CoA. Because a bhbA mutant is significantly brighter than wild type but not as bright as a bluB mutant, decreased ribonucleotide reductase activity might also contribute to the calcofluor bright phenotype of the bluB mutant if stalled DNA replication is related to the succinoglycan synthesis pathway in some way. Alternatively, another as yet unidentified cobalamin-dependent enzyme could contribute to the calcofluor phenotype.
Consistent with a previous report (30), we observed that either cobalt or methionine fully satisfies the growth requirements of wild-type S. meliloti in minimal media. This finding implies that the cobalamin-dependent methionine synthase is the only cobalamin-dependent enzyme that is required for growth. However, we found that, upon significant dilution of the cultures, methionine alone does not completely rescue the growth of the bluB or cobD mutants in minimal medium. This finding indicates that another cobalamin-dependent enzyme, presumably NrdJ, is necessary for growth in minimal media. The complete rescue of growth by methionine in the wild-type strain is likely due to the presence of residual cobalt in the cells at the time of inoculation.
Cobalamin requires at least 30 genes for its synthesis, and many organisms use cobalamin as a cofactor. However, plants, fungi, and some prokaryotes do not require this cofactor because cobalamin-independent isoforms of most cobalamin-dependent enzymes exist in nature. In fact, many α-proteobacteria closely related to S. meliloti possess cobalamin-independent methionine synthases and ribonucleotide reductases (29). Cobalamin-dependent enzymes likely still exist because they confer some advantage under specific environmental conditions. For S. meliloti, these enzymes may be important for survival within a plant host. During invasion of the plant root hair, S. meliloti encounters an oxidative burst elicited by the plant (40). Cobalamin-independent isoforms of methionine synthase and ribonucleotide reductase in Escherichia coli have been shown to be sensitive to oxidative damage (41, 42). Therefore, cobalamin-dependent enzymes could be critical for invading a eukaryotic host cell. Consistent with this hypothesis, a cobalamin biosynthesis mutant of Brucella melitensis is attenuated in virulence even though this organism possesses a cobalamin-independent ribonucleotide reductase (43). To determine whether cobalamin-dependent enzymes confer a specific advantage in interacting with a eukaryotic host, the role of cobalamin in other symbionts and pathogens should be examined.
Materials and Methods
Bacterial Strains and Growth Conditions.
All S. meliloti strains in this study were derived from the wild-type strain Rm1021 (44). For genetic manipulations and plant inoculations, S. meliloti and E. coli strains were grown under the conditions described (45). For growth experiments, 10–20 colonies of S. meliloti were inoculated into 10 ml of M9 sucrose medium with biotin (46) containing 0.1 mg/ml l-methionine and grown at 30°C with aeration for 18–24 h. Cultures were washed twice in 0.85% NaCl and diluted to an OD600 of 0.01 in M9 sucrose with biotin. Supplements were added at the following concentrations: 50 nM CoCl2, 1 μM cyanocobalamin, 1 μM dicyanocobinamide. 50 nM DMB, and 1 mg/ml l-methionine. Absorbance was measured by using a Beckman Coulter DU530 spectrophotometer at the indicated time points.
Screen for Calcofluor Bright Mutants.
Wild-type S. meliloti strain Rm1021 was mutagenized with transposon Tn5 and plated on LB plates containing 0.02% calcofluor (47). Colonies were screened for bright fluorescence under UV light at a wavelength 254 nm. The Tn5 insertions in strains exhibiting bright fluorescence were reintroduced into Rm1021 by transduction with bacteriophage M12 (48), and those that remained bright were studied further.
Genetic and Molecular Techniques.
The Tn5 insertion in bluB was cloned and sequenced by using a whole genome shotgun approach (49). To construct the bluB::gus strain, the bluB gene and flanking region were amplified by PCR and cloned into the vector pK18mobsacB (50). The GmR cassette from pCRS549 (51) was inserted into an internal AgeI site. The resulting plasmid was introduced into S. meliloti (45), and the bluB::gus GmR allele was inserted into the chromosome by selection on plates containing sucrose (50). The cobD mutant was constructed similarly by cloning cobD into pK18mobsacB and insertion of a GmR cassette, followed by insertion into the chromosome. The plasmid expressing bluB was constructed by cloning the bluB gene and flanking region into plasmid pFAJ1700 (52). All plasmids were introduced into S. meliloti strains by triparental mating (45).
Plant Assays and Microscopy.
Seedlings of Medicago sativa cv. Iroquois were inoculated with S. meliloti on Petri dishes containing Jensen agar as described (6, 47). Jensen agar was supplemented with 5 μM cyanocobalamin, 5 μM dicyanocobinamide, or 50 nM DMB, as indicated. Plant height and nodule numbers (n = 10) were scored after 4 weeks. Nodules were sectioned and examined by electron microscopy (53). Nodules were stained for β-glucuronidase activity with X-gluc and viewed by light microscopy (54). Acetylene reduction assays for whole plants (n = 10) were performed by using a Shimadzu GC-8A gas chromatograph (55).
Cobalamin Extraction and Analysis.
For biochemical analysis of corrinoid compounds, 1-liter cultures of S. meliloti were grown in TY medium (56) supplemented with 1 μM cyanocobalamin or 0.5 μM DMB. Corrinoid compounds were extracted with methanol, and the upper ligand was cyanidated with KCN (37). Extracts were purified by using Sep-Pak cartridges (Waters), concentrated, separated by high performance liquid chromatography (HPLC), and analyzed by mass spectrometry (MS) and UV diode array detection (DAD). Details of the analysis are provided in Supporting Text, which is published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank Stacy Chen, Jisoo Cha, and Per Malkus for assistance with strain construction; Nicki Watson (W. M. Keck Foundation Biological Imaging Facility at the Whitehead Institute, Boston) for help with electron microscopy; Kathryn Jones for assistance in characterizing bluB; and Anke Becker and Karsten Neihaus for helping us determine the Tn5 insertion site in the bluB mutant. We thank Daniel Jarosz and other members of the Walker laboratory for thoughtful discussions and John Campbell for critical reading of the manuscript. This work was supported by National Institutes of Health Grant GM31030 (to G.C.W.), a postdoctoral fellowship from The Jane Coffin Childs Memorial Fund for Medical Research (to M.E.T.), and a postdoctoral fellowship from the Fulbright/Spanish Ministry of Education and Science (to J.L.). G.C.W. is an American Cancer Society Research Professor.
Abbreviations
- DMB
5,6-dimethylbenzimidazole
- FMN
flavin mononucleotide.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. DQ340866).
References
- 1.Long S. R. Cell. 1989;56:203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- 2.van Rhijn P., Vanderleyden J. Microbiol. Rev. 1995;59:124–142. doi: 10.1128/mr.59.1.124-142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geurts R., Bisseling T. Plant Cell. 2002;14:S239–49. doi: 10.1105/tpc.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long S. R. Plant Physiol. 2001;125:69–72. doi: 10.1104/pp.125.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gage D. J., Margolin W. Curr. Opin. Microbiol. 2000;3:613–617. doi: 10.1016/s1369-5274(00)00149-1. [DOI] [PubMed] [Google Scholar]
- 6.Pellock B. J., Cheng H. P., Walker G. C. J. Bacteriol. 2000;182:4310–4318. doi: 10.1128/jb.182.15.4310-4318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oke V., Long S. R. Curr. Opin. Microbiol. 1999;2:641–646. doi: 10.1016/s1369-5274(99)00035-1. [DOI] [PubMed] [Google Scholar]
- 8.Vasse J., de Billy F., Camut S., Truchet G. J. Bacteriol. 1990;172:4295–4306. doi: 10.1128/jb.172.8.4295-4306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallsworth E. G., Wilson S. B., Greenwood E. A. Nature. 1960;187:79–80. doi: 10.1038/187079a0. [DOI] [PubMed] [Google Scholar]
- 10.Lowe R. H., Evans H. J. J. Bacteriol. 1962;83:210–211. doi: 10.1128/jb.83.1.210-211.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowe R. H., Evans H. J., Ahmed S. Biochem. Biophys. Res. Commun. 1960;3:675–678. doi: 10.1016/0006-291x(60)90085-1. [DOI] [PubMed] [Google Scholar]
- 12.Evans H. J., Kliewer M. Ann. N.Y. Acad. Sci. 1964;112:735–755. doi: 10.1111/j.1749-6632.1964.tb45052.x. [DOI] [PubMed] [Google Scholar]
- 13.Kliewer M., Evans H. J. Arch. Biochem. Biophys. 1962;97:427–429. doi: 10.1016/0003-9861(62)90101-7. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi M., Shimizu S. Eur. J. Biochem. 1999;261:1–9. doi: 10.1046/j.1432-1327.1999.00186.x. [DOI] [PubMed] [Google Scholar]
- 15.Roth J. R., Lawrence J. G., Bobik T. A. Annu. Rev. Microbiol. 1996;50:137–181. doi: 10.1146/annurev.micro.50.1.137. [DOI] [PubMed] [Google Scholar]
- 16.Stabler S. In: Chemistry and Biochemistry of B12. Banerjee R., editor. New York: Wiley; 1999. pp. 343–365. [Google Scholar]
- 17.Charles T. C., Aneja P. Gene. 1999;226:121–127. doi: 10.1016/s0378-1119(98)00555-1. [DOI] [PubMed] [Google Scholar]
- 18.Sato K., Inukai S., Shimizu S. Biochem. Biophys. Res. Commun. 1974;60:723–728. doi: 10.1016/0006-291x(74)90300-3. [DOI] [PubMed] [Google Scholar]
- 19.Cowles J. R., Evans H. J. Arch. Biochem. Biophys. 1968;127:770–778. doi: 10.1016/0003-9861(68)90288-9. [DOI] [PubMed] [Google Scholar]
- 20.Cowles J. R., Evans H. J., Russell S. A. J. Bacteriol. 1969;97:1460–1465. doi: 10.1128/jb.97.3.1460-1465.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren M. J., Raux E., Schubert H. L., Escalante-Semerena J. C. Nat. Prod. Rep. 2002;19:390–412. doi: 10.1039/b108967f. [DOI] [PubMed] [Google Scholar]
- 22.Renz P. FEBS Lett. 1970;6:187–189. doi: 10.1016/0014-5793(70)80053-9. [DOI] [PubMed] [Google Scholar]
- 23.Renz P., Weyhenmeyer R. FEBS Lett. 1972;22:124–126. doi: 10.1016/0014-5793(72)80236-9. [DOI] [PubMed] [Google Scholar]
- 24.Keck B., Munder M., Renz P. Arch. Microbiol. 1998;171:66–68. doi: 10.1007/s002030050679. [DOI] [PubMed] [Google Scholar]
- 25.Lingens B., Schild T. A., Vogler B., Renz P. Eur. J. Biochem. 1992;207:981–985. doi: 10.1111/j.1432-1033.1992.tb17133.x. [DOI] [PubMed] [Google Scholar]
- 26.Pollich M., Klug G. J. Bacteriol. 1995;177:4481–4487. doi: 10.1128/jb.177.15.4481-4487.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doherty D., Leigh J. A., Glazebrook J., Walker G. C. J. Bacteriol. 1988;170:4249–4256. doi: 10.1128/jb.170.9.4249-4256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leigh J. A., Reed J. W., Hanks J. F., Hirsch A. M., Walker G. C. Cell. 1987;51:579–587. doi: 10.1016/0092-8674(87)90127-9. [DOI] [PubMed] [Google Scholar]
- 29.Rodionov D. A., Vitreschak A. G., Mironov A. A., Gelfand M. S. J. Biol. Chem. 2003;278:41148–41159. doi: 10.1074/jbc.M305837200. [DOI] [PubMed] [Google Scholar]
- 30.Watson R. J., Heys R., Martin T., Savard M. Appl. Environ. Microbiol. 2001;67:3767–3770. doi: 10.1128/AEM.67.8.3767-3770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grabau C., Roth J. R. J. Bacteriol. 1992;174:2138–2144. doi: 10.1128/jb.174.7.2138-2144.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glazebrook J., Ichige A., Walker G. C. Genes Dev. 1993;7:1485–1497. doi: 10.1101/gad.7.8.1485. [DOI] [PubMed] [Google Scholar]
- 33.Djordjevic M. A. Proteomics. 2004;4:1859–1872. doi: 10.1002/pmic.200300802. [DOI] [PubMed] [Google Scholar]
- 34.Parish J. H. Principles and Practice of Experiments with Nucleic Acids. London: Longman; 1972. [Google Scholar]
- 35.Renz P. In: Chemistry and Biochemistry of B12. Banerjee R., editor. New York: Wiley; 1999. pp. 557–575. [Google Scholar]
- 36.Maggio-Hall L. A., Dorrestein P. C., Escalante-Semerena J. C., Begley T. P. Org. Lett. 2003;5:2211–2213. doi: 10.1021/ol034530m. [DOI] [PubMed] [Google Scholar]
- 37.Maggio-Hall L. A., Escalante-Semerena J. C. Microbiology. 2003;149:983–990. doi: 10.1099/mic.0.26040-0. [DOI] [PubMed] [Google Scholar]
- 38.Chowdhury S., Banerjee R. Biochemistry. 1999;38:15287–15294. doi: 10.1021/bi9914762. [DOI] [PubMed] [Google Scholar]
- 39.Stupperich E., Steiner I., Eisinger H. J. J. Bacteriol. 1987;169:3076–3081. doi: 10.1128/jb.169.7.3076-3081.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos R., Herouart D., Sigaud S., Touati D., Puppo A. Mol. Plant–Microbe. Interact. 2001;14:86–89. doi: 10.1094/MPMI.2001.14.1.86. [DOI] [PubMed] [Google Scholar]
- 41.Hondorp E. R., Matthews R. G. PLoS Biol. 2004;2:e336. doi: 10.1371/journal.pbio.0020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roy B., Lepoivre M., Henry Y., Fontecave M. Biochemistry. 1995;34:5411–5418. doi: 10.1021/bi00016a012. [DOI] [PubMed] [Google Scholar]
- 43.Lestrate P., Dricot A., Delrue R. M., Lambert C., Martinelli V., De Bolle X., Letesson J. J., Tibor A. Infect Immun. 2003;71:7053–7060. doi: 10.1128/IAI.71.12.7053-7060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. J. Bacteriol. 1982;149:114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glazebrook J., Walker G. C. Methods Enzymol. 1991;204:398–418. doi: 10.1016/0076-6879(91)04021-f. [DOI] [PubMed] [Google Scholar]
- 46.Maniatis T., Fritsch E. F., Sambrook J. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1982. [Google Scholar]
- 47.Leigh J. A., Signer E. R., Walker G. C. Proc. Natl. Acad. Sci. USA. 1985;82:6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finan T. M., Hartweig E., LeMieux K., Bergman K., Walker G. C., Signer E. R. J. Bacteriol. 1984;159:120–124. doi: 10.1128/jb.159.1.120-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell G. R., Sharypova L. A., Scheidle H., Jones K. M., Niehaus K., Becker A., Walker G. C. J. Bacteriol. 2003;185:3853–3862. doi: 10.1128/JB.185.13.3853-3862.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schafer A., Tauch A., Jager W., Kalinowski J., Thierbach G., Puhler A. Gene. 1994;145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 51.Reeve W. G., Tiwari R. P., Worsley P. S., Dilworth M. J., Glenn A. R., Howieson J. G. Microbiology. 1999;145:1307–1316. doi: 10.1099/13500872-145-6-1307. [DOI] [PubMed] [Google Scholar]
- 52.Dombrecht B., Vanderleyden J., Michiels J. Mol. Plant–Microbe. Interact. 2001;14:426–430. doi: 10.1094/MPMI.2001.14.3.426. [DOI] [PubMed] [Google Scholar]
- 53.Hirsch A. M., Bang M., Ausubel F. M. J. Bacteriol. 1983;155:367–380. doi: 10.1128/jb.155.1.367-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swanson J. A., Mulligan J. T., Long S. R. Genetics. 1993;134:435–444. doi: 10.1093/genetics/134.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oke V., Long S. R. Mol. Microbiol. 1999;32:837–849. doi: 10.1046/j.1365-2958.1999.01402.x. [DOI] [PubMed] [Google Scholar]
- 56.Rosado M., Gage D. J. FEMS Microbiol. Lett. 2003;226:15–22. doi: 10.1016/S0378-1097(03)00603-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.