Abstract
The human intraparietal sulcus (IPS) is implicated in processing symbolic number information and possibly in nonsymbolic number information. Specific IPS activity for discrete quantities (numerosities) as compared with continuous, analogue quantity has not been demonstrated. Here we use a stimulus-driven paradigm to distinguish automatic estimation of “how many things” from “how much” and “how long.” The discrete analogue response task (DART) uses the perception of hues which can change either abruptly (discrete, numerous stimuli) or smoothly (analogue, nonnumerous stimuli) in space or in time. Subjects decide whether they saw more green or more blue. A conjunction analysis of spatial and temporal conditions revealed that bilateral IPS was significantly more active during the processing of discrete stimuli than during analogue stimuli, as was a parietal-occipital transition zone. We suggest that processing numerosity is a distinct process from processing analogue quantity, whether extended in space or time, and that an intraparietal network connects objects’ segmentation to the estimation of their numerosity.
Keywords: discrete stimuli, magnitude processing, nonsymbolic numerical processing, numerosity, analogue stimuli
Judgments involving symbolic numbers systematically activate the intraparietal sulcus (IPS) area (see ref. 1 for a review). However, two fundamental questions are still unresolved: First, is the IPS engaged in nonsymbolic numerical representation? Second, does the IPS represent the numerosity of sets of discrete objects in the same way as it represents analogue quantities? Of course, a set of discrete objects will have analogue properties that extend in space or in time, such as area, perimeter, and duration, but they are distinct from the abstract property of the numerosity of the set, i.e. how many objects are in it. Our experimental question focuses on whether we can identify a specific neural response tuned to the “numerosity parameter” (1) of the stimulus by using nonsymbolic information.
There is considerable evidence from neuroimaging studies to indicate that the IPS is involved in processing symbolic numerical information with tasks that depend on the perception of Arabic digits or number words, or which require a number–word response (2–7). A few studies have combined digits with nonsymbolic stimuli or nonnumerical dimensions. For example, IPS is activated during target–detection tasks by using Arabic digits compared with letters and colors (8). It is also activated in direct proportion to the difficulty of a comparison task based on the size and the luminance of Arabic digits (9). Left IPS is activated during a magnitude comparison task that included Arabic digits, lengths, and angles (10). A recent study based on the perception of digits and nonnumerical stimuli, i.e., scrambled digits, has suggested that numerosity is represented in the parietal cortex; however, in the context of actions made in response to the numbers, magnitude and action representations might be closely linked (11).
However, the few functional MRI (fMRI) studies investigating specific IPS involvement in nonsymbolic quantity processing have yielded apparently conflicting results (12, 13). For example, a recent study showed fMRI adaptation in the horizontal segment of IPS during passive viewing of discrete numerosities in the form of a fixed sets of dots, from 8 to 32 (12), but, in another study, the IPS showed no difference in activation during the numerical tasks and their control tasks: number and color comparison of two arrays of dots, passive viewing of sets of different shapes varying in numerosity, and number and color comparison of dots in an array with “flashes” of a single dot (13).
How can these discrepant results be explained? One possibility is that the precise activation of the IPS depends on the tasks being performed and contrasted, so that commonalities are difficult to find across different studies. This view is supported by the observation that specialized intraparietal neural populations are differently branched and layered between subjects (14), and that the spatial resolution of fMRI may not be able to separate distinct but intertwined neural populations. Furthermore, single cell recording in monkeys showed that only 10–20% of all IPS neurons respond to displays of different numbers of dots (15). We sought to address this problem by adopting an approach aimed at tapping a core, nonsymbolic, numerosity processing keeping the task constant but exploiting stimulus-driven differences in activation. We reasoned that IPS systems might respond automatically to stimulus properties; that is, equal activation might occur for any number, i.e., salient stimulus, independent of attention or task. Our question was as follows: Is there a specialized system in the IPS that responds automatically to the stimulus property of being numerous as compared with the more general property of being extended? That is, is there a specialized system that responds to “how many” as compared with “how much” by using nonsymbolic stimuli?
Previous neuroimaging studies investigating nonsymbolic numerosity have manipulated the area of the displayed stimuli, dots or shapes, to control for nonnumerical quantity processing. However, the stimuli have always been presented as discrete objects, hence numerous, in both the experimental and control conditions. In addition, although the importance of investigating whether numerosity is coded for both simultaneously as well as sequentially presented stimuli has been stressed in the past (16, 17), previous studies have not ensured that the numerosity task was independent of type of presentation. Therefore, the challenge for creating a paradigm for numerosity processing was to find a stimulus dimension that can be displayed as either numerous or nonnumerous, either in time (sequentially) or in space (simultaneously). Colors have these following properties: They can be displayed either as discrete, hence numerous, or as analogue, hence extended in time or in space.
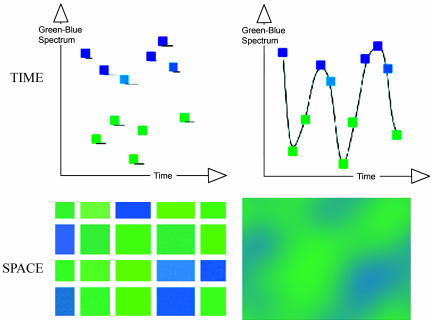
Our experimental paradigm, discrete analogue response task (DART), requires the participant to judge whether there is more blue or more green in the display. The amount of blue and green can be varied continuously or as discrete areas of blue and green. In “analogue” conditions, the relative amount blue and green can be assessed only in terms of analogue quantities (how much blue vs. how much green). However, in the discrete conditions, the assessment can also be made on the basis of numerosity (how many discrete blue rectangles vs. how many discrete green rectangles). In addition, both analogue and discrete displays can be presented in spatial and temporal modalities by using the same stimulus manipulations (see Methods for details and Fig. 1). We can also manipulate the ratio of the amount of blue and green, varying from easy (e.g., 5 green to 15 blue) to more difficult (e.g., 9 green to 11 blue), allowing for investigation of a difficulty effect within the intraparietal region, as suggested by studies of numerical comparison tasks (9, 18, 19). We stress that the logical relationship between numerosity and extent is asymmetrical; that is, discrete stimuli have both numerosity and analogue extent, whereas analogue stimuli have only analogue extent. Thus, DART can reveal brain areas showing an additional effect of numerosity over analogue quantity but not those areas responding to analogue quantity over numerosity.
Fig. 1.
Schematic view of DART stimuli arranged in a 2 × 2 design. The same hues are used in all conditions. (Upper Left) In the discrete temporal condition, a sequence of blue and green squares appears at random intervals between 150 and 400 ms in the same place on the screen. (Upper Right) In the corresponding analogue temporal condition, the same hues are linked by intermediate hue values, so that a single square appears to be smoothly changing hue. In the corresponding discrete spatial condition (Lower Left), the same hues are formed into discrete rectangles separated by gray background, whereas, in the analogue spatial condition (Lower Right), a smoothing function blurs the boundaries between the different hues. Every trial of discrete stimuli is transformed into a trial of analogue stimuli.
Results
Behavioral Data.
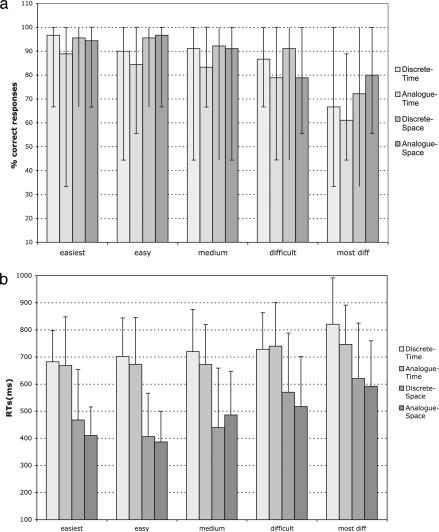
Subjects gave correct responses for 86% of the stimuli (Fig. 2a shows accuracy score for each condition and difficulty level). (For technical reasons, the complete accuracy data from two subjects were not available for analysis, and the behavioral analyses were conducted on ten subjects.) There was no significant difference between correct score (max, nine correct responses in each condition) for discrete stimuli (88%) and for analogue stimuli (83%) [discrete mean = 7.9 (SD = 1.6) vs. analogue mean = 7.5 (SD = 1.6); mean comparison, F = 5.1, P > 0.05)] or for the temporal/spatial presentation [temporal mean = 7.45 (SD = 1.6) vs. spatial mean = 7.5 (SD = 1.6); mean comparison, F = 5.1, P > 0.05]. A further analysis showed that subjects’ accuracy differed over the five difficulty levels (F(1,4) = 35.8, P < 0.001), which refer to the ratio between the two colors: 5:15 (i.e., easiest), 6:14, 7:13, 8:12, and 9:11 (most difficult). In particular, subjects gave fewer correct responses as the difficulty level increased [5:15, mean = 8.45 (SD = 1.2); 6:14, mean = 8.25 (SD = 1.2); 7:15, mean = 8.05 (SD = 1.5); 8:12, mean = 7.55 (SD= 1.4); 9:11, mean = 6.33 (SD = 1.6); and F = 55.9, P < 0.0001].
Fig. 2.
Behavioral results. (a) Subjects’ correct responses for the four tasks and five difficulty levels. Errors bars indicate the range of observed percentages of correct responses (i.e., max = 100%). (b) Subjects’ reaction time for the four tasks and five difficulty levels. Errors bars indicate 1 SD.
Analysis of 12 subjects’ reaction times (RTs) (in ms) revealed the following difference in the temporal/spatial display (F(1,11) = 115.3, P < 0.0001): temporal stimuli (mean = 715.4, SD = 155) evoked slower response than spatial stimuli (mean = 489.7, SD = 187). In addition, subjects’ RTs differ along the difficulty levels (F(1,4) = 9.27, P < 0.0001). In particular, RTs increased linearly as the magnitude distance between the two colors decreased as follows: 5:15 (mean = 562.3, SD = 188); 6:14 (mean = 551.7, SD = 209); 7:13 (mean = 588.4, SD = 203); 8:12 (mean = 651, SD = 195); 9:11 (mean = 718.7, SD = 203) (F = 53.6, P < 0.0001). There was no significant interaction between the degree of difficulty and the stimuli type (analogue vs. discrete) in subjects’ RTs (F(1,4) = 0.72, P = not significant). Fig. 2b shows subjects’ RTs for each condition and each difficulty level.
Neuroimaging Results.
Random effects analysis of neuroimaging data were carried out to evaluate common and differential areas of response during processing discrete vs. analogue stimuli in the temporal and spatial presentations. A mixed effects analysis was carried out treating subject as a random variable. This analysis was implemented by using a two-stage summary statistic approach. In particular, subject-specific contrasts were developed at the first level and then entered into a second level analysis. Thus all inferences are made about the population from which the subjects are (assumed randomly) drawn.
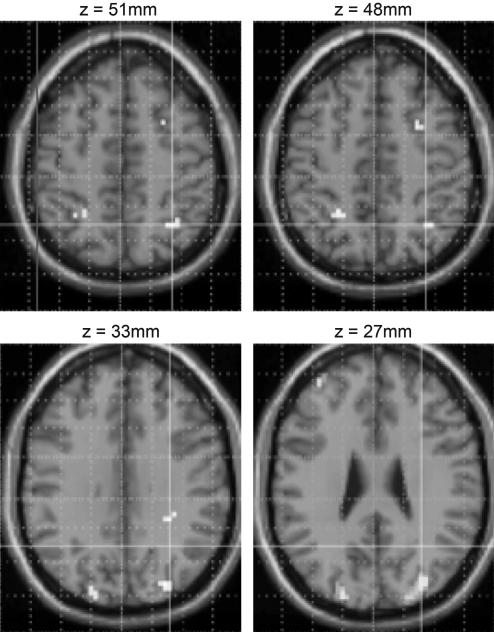
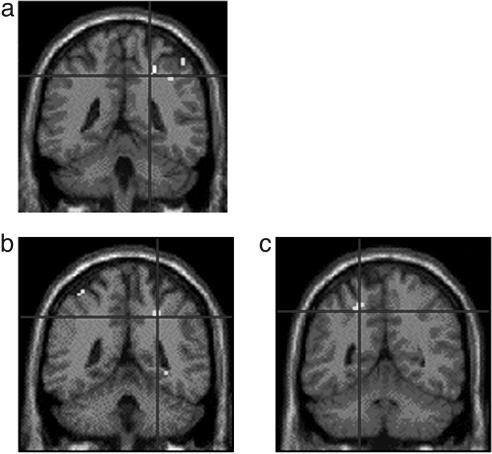
Because of our a priori hypothesis regarding the role of the IPS (see Identification of Activation Loci) on number processing, we looked at bilateral intraparietal areas without correction for multiple comparisons. A conjunction analysis [(discrete temporal > analogue temporal) ⋀ (discrete spatial > analogue spatial)] revealed, as predicted, a bilateral activation along the length of the IPS including the most caudal IPS area, spreading into the transverse occipital sulcus region (Fig. 3). The parametric analyses revealed a linear increase in activation with increasing task difficulty in both spatial and temporal numerosity processing (discrete > analogue) (Table 1). As predicted, the IPS region was activated more as the difference between the two colors decreased (e.g., from 5 greens and 15 blues to 9 blues and 11 greens). In particular, the difficulty of the numerosity estimation in space affects the activation in the bilateral IPS, whereas the difficulty of the numerosity estimation in time affects the activation of right IPS extending into the postcentral sulcus (Fig. 4). No other activations with a level of significant P < 0.05 corrected for multiple comparisons (FamilyWise Error; FWE) have been found during numerosity comparison.
Fig. 3.
Conjunction analysis (spatial and temporal presentations). Bilateral areas along the length of the IPS were more activated by numerosity processing (discrete, countable stimuli) than extent processing (analogue, uncountable stimuli). Numerosity processing activates the IPS and the caudal IPS bilaterally.
Table 1.
Conjunction analysis of numerosity (discrete stimuli) vs. extent (analogue stimuli) and parametric analyses of numerosity vs. extent as the difficulty of the estimation increased revealed clusters of activation along bilateral IPS
| Brain region | Coordinates x, y, z | Z score | Cluster size (voxels) | |
|---|---|---|---|---|
| Estimating numerosity: | ||||
| In space and in time | cIPS right | 33, −78, 24 | 5.64 | 102 |
| 24, −90, 21 | 5.44 | |||
| 33, −78, 15 | 4.01 | |||
| cIPS left | −21, −90, 27 | 4.64 | 39 | |
| IPS left | −24, −48, 48 | 3.73 | 12 | |
| −33, −51, 54 | 3.22 | |||
| IPS right | 33, −57, 51 | 4.00 | 6 | |
| Difficulty effect while estimating numerosity: | ||||
| In space | Left IPS/Pocs | −57, −39, 45 | 3.97 | 10 |
| Right IPS/Pocs | 27, −42, 48 | |||
| In time | IPS right | 21, −51, 42 | 3.42 | 6 |
| 24, −42, 42 | 3.30 | |||
P < 0.001. cIPS, caudal IPS; Pocs, postcentral sulcus.
Fig. 4.
Parametric analysis of task difficulty. The activity in the more horizontal segment of the IPS and in the postcentral sulcus increased as the difference between the number of green and blue stimuli decreased. Figures show task difficulty effect activation during numerosity processing in time (a) and in space (b and c).
Discussion
The starting point of the present experiment is the automatic tendency of humans to estimate the numerosity of any set of entities, whether homogeneous or heterogeneous, extended in time or in space (see refs. 20 and 21 for reviews). We distinguished two types of quantity processing in an effort to clarify the nature of the representations involved in relative magnitude judgements: numerosity estimation, based on the perception of countable discrete stimuli, and extent estimation, based on the perception of uncountable analogue entities. Extent processing acted as a control condition for numerosity processing. We used a visual paradigm, the DART that presents matched pairs of discrete and analogue stimuli displayed both simultaneously and sequentially.
We showed that specific estimation of numerosity activates bilaterally the IPS by using nonsymbolic stimuli alone, in both temporal and spatial modes of display. The greatest challenge in mapping parietal cortex is that many of the functions that it probably subserves, such as shifting and maintaining attention, directing eye movements, using working memory (23), in addition to number-processing tasks, are a vital component in many cognitive tasks (22). Given the architectonic complexity of the human intraparietal neurons (14), the limitation of fMRI techniques (16, 24), and contrasting findings relative to IPS involvement with nonsymbolic number information (12, 13), it is possible that the precise activation of this area depends on the tasks being performed and contrasted. We were able to exclude condition-dependent task difficulty as a factor in the IPS activation, because the task was held as far as possible constant in the paradigm by using the neutral question “more green or more blue?” for all four tasks. Overall, the difficulty of task for the extent stimuli and the numerosity stimuli were equivalent as measured by response accuracy. In addition, we are able to exclude the possibility that the IPS activity is because of the obvious visual differences between temporal and spatial presentations by making a “cognitive conjunction” (25) and considering only the higher-order feature of the stimuli, namely, being countable or not countable, whether extended in time or in space.
However, it can be argued that subjects might be using a nonnumerical intermediate perceptual representation as a basis for magnitude comparison. We are able to rule out this hypothesis because blood oxygenation level-dependent activity in the horizontal segment of the IPS (hIPS) and postcentral sulcus increased as the difficulty of the estimation task increased during the discrete stimuli processing (i.e., numerosity) compared with the difficulty of the estimation task during analogue stimuli processing (i.e., extent). This finding indicates that the anterior region of IPS is engaged by the numerosity properties alone, and that its activity is modulated according to the changes in the numerical ratio of the stimuli. This finding replicates previous studies with numerical comparison tasks where the distance effect (i.e., it is easier and quicker to select the larger of two numbers when they are numerically dissimilar than when they are similar) (26) revealed activation in bilateral hIPS (1, 9).
We report activation along the length of the IPS including its most caudal extent spreading into the transverse occipital sulcus region. This visual region has not been fully characterized in other studies (24). However, human neuroimaging has identified this area during object matching and grasping as well as during discriminations of object size and orientation (24, 27). Interestingly, single cell recordings in the monkey parietal lobe indicated that most of the neurons that respond to numerosity displays are located in the ventral area of the IPS (16). As noted above, we have excluded specific low level visual aspects of the discrete stimuli by use of the conjunction, and so we deduce that we have identified a high-level visual feature to which this area is sensitive. It is important to note that in the parametric analyses, we could find no evidence that this caudal region was sensitive to manipulating the relative proportions of the blue and green stimuli. This result supports the contention that this region is concerned with segmenting the scene into discrete objects but not with estimating numerosity. Because we were not able to record eye movements in the scanner during the four conditions, we cannot quantify or characterize saccade differences contributing to this increased activation during discrete stimuli processing vs. analogue stimuli processing. We can speculate that this region serves as a “location map” that, in some computational models of numerosity extraction, serves as a first step of object segmentation before numerosity estimation (28). The concept of a countable entity can be therefore defined as a thing that firstly must be segmented from the background and only then can it be counted or numerically manipulated. It seems therefore that a wide range of properties of the world is processed along the length of the IPS, beginning with segmenting the visual scene into objects and proceeding to representing their number. The concepts of object and of countability are clearly related, and our findings therefore suggest that regions responsible for the representation and processing of numerosities may be built from the perception of “objecthood.”
How can the specificity of a numerosity system be justified as one of the functions that the parietal cortex subserves? It has been argued by Walsh (29) that the inferior parietal cortex (IPC) is the site engaged by a common magnitude system, which encompasses estimation of time, space, and quantity. No distinction is made between numerosity and extent, that is, countable and uncountable quantity. The hypothesis of a general magnitude system is based on the view that the linking function of the parietal cortex is to extract the information about the external world for representing the coordinates of action. Thus, according to Walsh, the competence of the IPC, rather than computing “where” in space, is computing “how far, how fast, how much, how long, and how many with respect to action” (ref. 29, p. 486). The present study provides a complementary evidence for a subregion of the magnitude system located along the IPS, which is more active during discrete quantity estimation, both in time and in space, than during analogue quantity estimation. These findings support the view of a functional parcellation of a magnitude system that, in turn, may reflect the architectonic differentiation of the cortex lining the IPS (15, 30). Future investigations are needed to clarify differentiations within IPS neural populations.
Interestingly, Walsh (29) points out that it would be maladaptive for the infant brain not to use a common metric for estimating time, space, and quantity because experience teaches the infant that there is a covariance of time, space, and quantity in the physical world. However, we further speculate that this covariance may represent a confound for correct numerosity judgements. Numerosity abilities in infants have been investigated by using control tasks based on perception of continuous extent (31). However, these tasks suffer the same limitations of past neuroimaging studies: The stimuli for the control condition are presented as discrete objects, hence numerous. Further investigations are needed to determine whether a differentiation between two different systems, numerosity and extent, develops with experience. We propose that an exact representation of numerosity seems to better capture our intuitive understanding of numerosity, because it maps directly onto lower level perceptual processes (e.g., object identification) and enumeration procedures (e.g., subitizing, counting).
In conclusion, the present study made a clear distinction between discrete and analogue quantities and compared them directly by using nonsymbolic stimuli. We suggest that the fundamental ability to compare magnitudes evolved from the capacity to extract information about the discreteness of a scene. An object can be defined as an entity that can be segmented from a background and can also be thought of as a countable entity. Our findings reveal a network along the length of the IPS that connects object segmentation to the estimation of numerosity during a magnitude comparison task.
Methods
Subjects.
Twelve neurologically healthy subjects (4 females and 8 males; mean age, 24.0 years; range, 18.0–34.1 years), all but one right-handed, gave written informed consent according to procedures approved by the National Hospital and Institute of Neurology Ethics Committee (London).
Experimental Design.
The DART visual stimuli were presented both sequentially (in time), as discrete entities (DT) or analogue stimuli (AT), and simultaneously (in space), as discrete entities (DS) or analogue stimuli (AS) (see Fig. 1). The stimuli were presented in nine blocks of four conditions (DT, AT, DS, AS) for each of the five difficulty levels (in a randomized order).
Stimuli Presentation.
Each stimulus was presented on a screen situated outside the scanner and projected onto a mirror 30 cm above subjects, subtending a visual angle of ≈10°. The stimuli were created with matlab (MathWorks, Natick, MA). The stimulation consisted of a rectangle filled with blue and green hues. The background was gray. The stimuli of all four conditions were shown at the same location, within the rectangle. The four conditions differed as function of the type of presentation (in time and in space) and of the type of transition between each hue (abrupt or continuous). Twenty hues in total were presented for each condition. The difficulty of the “more blue or more green?” judgment was controlled by varying the ratios of the amounts of the two hues: 5:15 (easier), 6:14, 7:13, 8:12, 9:11 (more difficult). Within each block, the higher ratio was counterbalanced between the two colors. The speed and the randomization of the presentation were designed to prevent explicit counting of the two sets of hues, as confirmed by subjects’ report at the end of the experiment.
Stimuli Presentation in Time (Sequential Presentation).
In the discrete condition, the interstimulus interval of each hue varied randomly from 150 to 400 ms, so that subjects perceived the visual signal as a sequence of twenty blue and green squares appearing at irregular intervals (Fig. 1 Upper Left). In the analogue condition, the transition between the 20 different hues included a fixed number of intermediate steps so that subjects perceived a continuous light changing from blues to greens (Fig. 1 Upper Right). The intermediate steps between the 20 blue and green squares were created by a matlab program to assure the perception of a smooth transition between each pair of squares.
The average duration of the display of both discrete and analogue stimuli in the temporal presentation was of 9 s plus a 3-s window within which the subject had to respond.
Stimuli Presentation in Space (Simultaneous Presentation).
In the discrete spatial condition, the hues were presented as a rectangular grid composed of blue and green rectangles (5 × 4) of irregular size separated by gray background (Fig. 1 Lower Left). In the analogue spatial condition, the hues were presented as a single large rectangle composed of blurred blue-to-green areas that correspond to blurred versions of the discrete spatial configuration (Fig. 1 Lower Right). The average duration of the display of both discrete and analogue stimuli in the spatial presentation was of 0.6 s plus a 3-s window for forced choice response.
Instructions.
The subjects were instructed to watch the sequences and to decide at the end whether they saw more blue or more green. They answered by pressing one of two keys (left or right press for each response varied and counterbalanced across subjects). Scanning was preceded by a practice session with examples of the four conditions and a key press.
Image Acquisition.
Imaging was performed by using a 2-Tesla scanner (Siemens VISION, Erlagen, Germany). Structural images were acquired with a T1–weighted sequence (repetition time (TR) = 9.5 s, echo time (TE) = 4 ms, inversion time (TI) = 600 ms, nominal voxel size 1 × 1 × 1.5 mm, 108 axial slices). Functional images were acquired with a gradient echoplanar T2* sequence by using blood oxygenation level-dependent contrasts. The image matrix size was 64 × 64. Each functional image was composed of 32 transverse slices (2.5-mm thickness, 1.3-mm gap, nominal voxel size 3 × 3 × 2.5 mm, TE = 40 ms) covering the whole brain. A total of 518 functional volumes were acquired continuously with an effective repetition time of 2.432 s.
Data Analysis.
The data were analyzed with SPM02 (Wellcome Department of Cognitive Neurology; http://fil.ion.ucl.ac.uk/spm). The first five volumes of images were discarded to allow for T1 equilibration. The remaining 513 image volumes were realigned to the first image, sinc-interpolated over time to correct for phase advance during volume acquisition, and normalized to the Montreal Neurological Institute (Montreal) reference brain. The data were spatially smoothed with a Gaussian kernel (8 mm, full width at half maximum) and a high-pass temporal filter with a frequency cut-off at 200 s was applied. Individual events were modeled first by a canonical synthetic hemodynamic response function, its temporal and dispersion derivatives. We used a general linear model to generate parameter estimates for event-related activity at each voxel for each subject in response to the presentation of each if the four stimulus types and the button presses. Movement parameters derived from realignment corrections were also entered as covariates of no interest.
Identification of Activation Loci.
Because of our a priori hypothesis regarding the role of the IPS region on the basis of previous studies of number processing (1–13), activations along the IPS, i.e., the sulcus, which is ≈7 cm long and ≈2 cm deep, located between the transverse occipital sulcus near the parieto-occipital sulcus and the postcentral sulcus (32) are reported as significant at P < 0.001 uncorrected for multiple comparisons. We accepted a level of significance of P < 0.05 corrected for multiple comparisons [FamilyWise Error (FWE)] for all other brain regions.
Given the difference between the duration of the spatial (≈0.6 s) and the temporal (≈9 s) presentations, two subtraction analyses were initially performed separately for each modality (numerosity processing vs. extent processing), followed by a conjunction analysis for identification of areas that were activated more by numerosity processing than by extent processing in both the temporal and spatial presentations. Finally, two parametric analyses tested whether the previously identified areas (numerosity processing vs. analogue processing in both the temporal and spatial presentations) exhibited a linear increase in activation with increasing task difficulty.
Acknowledgments
This work was supported by European Union Research Training Network Grant HPRN-CT-2000-00076 (Neuromath) (to F.C. and B.B.) and Medical Research Council Cooperative Group Grant G9900106 (to D.E.G.).
Abbreviations
- DART
discrete analogue response task
- fMRI
functional MRI
- IPS
intraparietal sulcus.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Dehaene S., Piazza M., Pinel P., Cohen L. Cognit. Neuropsychol. 2003;20:487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- 2.Dehaene S., Spelke E., Pinel P., Stanescu R., Tsivkin S. Science. 1999;284:970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- 3.Pesenti M., Thioux M., Seron X., De Volder A. J. Cognit. Neurosci. 2000;12:461–479. doi: 10.1162/089892900562273. [DOI] [PubMed] [Google Scholar]
- 4.Stanescu-Cosson R., Pinel P., van de Moortele P.-F., Le Bihan D., Cohen L., Dehaene S. Brain. 2000;123:2240–2255. doi: 10.1093/brain/123.11.2240. [DOI] [PubMed] [Google Scholar]
- 5.Pinel P., Dehaene S., Riviere D., Le Bihan D. NeuroImage. 2001;14:1013–1026. doi: 10.1006/nimg.2001.0913. [DOI] [PubMed] [Google Scholar]
- 6.Piazza M., Mechelli A., Butterworth B., Price C. J. NeuroImage. 2002;15:435–446. doi: 10.1006/nimg.2001.0980. [DOI] [PubMed] [Google Scholar]
- 7.Simon O., Cohen L., Mangin J. F., Le Bihan D., Dehaene S. Neuron. 2002;33:475–487. doi: 10.1016/s0896-6273(02)00575-5. [DOI] [PubMed] [Google Scholar]
- 8.Eger E., Sterzer P., Russ M. O., Giraud A. L., Kleinschmidt A. Neuron. 2003;37:719–725. doi: 10.1016/s0896-6273(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 9.Pinel P., Piazza M., Le Bihan D., Dehaene S. Neuron. 2004;41:983–993. doi: 10.1016/s0896-6273(04)00107-2. [DOI] [PubMed] [Google Scholar]
- 10.Fias W., Lammertyn J., Reynvoet B., Dupont P., Orban G. A. J. Cognit. Neurosci. 2003;15:1–11. doi: 10.1162/089892903321107819. [DOI] [PubMed] [Google Scholar]
- 11.Gobel S. M., Johansen-Berg H., Behrens T., Rushworth M. F. S. J. Cognit. Neurosci. 2004;16:1536–1551. doi: 10.1162/0898929042568442. [DOI] [PubMed] [Google Scholar]
- 12.Piazza M., Izard V., Pinel P., Le Bihan D., Dehaene S. Neuron. 2004;44:547–555. doi: 10.1016/j.neuron.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Shuman M., Kanwisher N. Neuron. 2004;44:557–569. doi: 10.1016/j.neuron.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Zilles K., Eickhoff S., Palomero-Gallagher N. Adv. Neurol. 2003;93:1–21. [PubMed] [Google Scholar]
- 15.Nieder A., Miller E. K. Neuron. 2003;37:149–157. doi: 10.1016/s0896-6273(02)01144-3. [DOI] [PubMed] [Google Scholar]
- 16.Nieder A. Neuron. 2004;44:407–409. doi: 10.1016/j.neuron.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Carey S. Science. 1998;282:641–642. doi: 10.1126/science.282.5389.641. [DOI] [PubMed] [Google Scholar]
- 18.Pinel P., Le Clec’H G., van de Moortele P.-F., Naccache L., Le Bihan D., Dehaene S. NeuroReport. 1999;10:1473–1479. doi: 10.1097/00001756-199905140-00015. [DOI] [PubMed] [Google Scholar]
- 19.Kiefer M., Dehaene S. Math. Cogn. 1997;3:1–30. [Google Scholar]
- 20.Dehaene S. The Number Sense: How the Mind Creates Mathematics. New York: Oxford Univ. Press; 1997. [Google Scholar]
- 21.Butterworth B. The Mathematical Brain. New York: Macmillan; 1999. [Google Scholar]
- 22.Culham J. C., Kanwisher N. G. Curr. Opin. Neurobiol. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- 23.Corbetta M., Akbudak E., Conturo T. E., Snyder A. Z., Ollinger J. M., Drury H. A., Linenweber M. R., Petersen S. E., Raichle M. E., Van Essen D. C., et al. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 24.Kleinschmidt A. Neuron. 2004;41:842–844. doi: 10.1016/s0896-6273(04)00154-0. [DOI] [PubMed] [Google Scholar]
- 25.Price C. J., Friston K. J. NeuroImage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- 26.Moyer R., Landauer T. Nature. 1967;215:1519–1520. doi: 10.1038/2151519a0. [DOI] [PubMed] [Google Scholar]
- 27.Faillenot I., Toni I., Decety J., Gregoire M. C., Jeannerod M. Cereb. Cortex. 1997;7:77–85. doi: 10.1093/cercor/7.1.77. [DOI] [PubMed] [Google Scholar]
- 28.Dehaene S., Changeux J. P. J. Cognit. Neurosci. 1993;5:390–407. doi: 10.1162/jocn.1993.5.4.390. [DOI] [PubMed] [Google Scholar]
- 29.Walsh V. Trends Cogn. Sci. 2003;7:483–488. doi: 10.1016/j.tics.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Zilles K., Palomero-Gallagher N. NeuroImage. 2001;14:S8–S20. doi: 10.1006/nimg.2001.0823. [DOI] [PubMed] [Google Scholar]
- 31.Feigenson L., Carey S., Spelke E. Cognit. Psychol. 2002;44:33–66. doi: 10.1006/cogp.2001.0760. [DOI] [PubMed] [Google Scholar]
- 32.Ono M., Kubik S., Abernathey C. D. Atlas of the Cerebral Sulci. Stuttgart: Thieme; 1990. [Google Scholar]