Abstract
Many sensations of pain are evoked by mechanical stimuli, and in inflammatory conditions, sensitivity to such stimuli is commonly increased. Here we used cultured sensory neurons as a model of the peripheral terminal to investigate the effects of inflammatory signaling pathways on mechanosensitive ion channels. Activation of two of these pathways enhanced transduction in a major population of nociceptors. The proinflammatory neurotrophin nerve growth factor caused an up-regulation of mechanically activated currents via a transcriptional mechanism. Activators of PKC, given in vitro and in vivo, also caused an increase in mechanically activated membrane current and behavioral sensitization to mechanical stimulation, respectively. The effect of activating PKC was inhibited by tetanus toxin, suggesting that insertion of new channels into the cell membrane is involved in sensitization. These results reveal previously undescribed mechanisms by which PKC and nerve growth factor synergistically enhance the response of nociceptors to mechanical stimuli, suggesting possible targets for pain treatment.
Keywords: allodynia, dorsal root ganglia, hyperalgesia, mechanosensation, pain
Inflammatory pain conditions are commonly associated with increased sensitivity to mechanical stimuli. In such conditions, normally innocuous mechanical stimuli can become painful (allodynia), and noxious stimuli can evoke enhanced pain responses (hyperalgesia). The mechanisms that underlie these phenomena include both central and peripheral sensitization to sensory input. Centrally, enhanced responsiveness to nociceptor activity and activation of nociceptive pathways by low-threshold mechanoreceptors both can contribute to hypersensitivity (1). Additionally, the excitability of primary nociceptors can be increased by changes in the function of voltage-gated ion channels or by augmentation of the mechanotransduction process (2).
Although the prevalence of peripheral mechanical sensitization has been questioned in cutaneous C fiber nociceptors (3, 4), there are extensive data showing peripheral sensitization to mechanical stimuli of nociceptors innervating a variety of deep tissues, including the meninges (5), joints (6), viscera (7), and injured axons terminating in neuromas (8, 9). Therefore, increased mechanosensitivity could play a major role in the pathophysiology of headache, arthritis, visceral pain, and neuropathic pain.
The development of hyperalgesia to heat stimuli depends on TRPV1 (10, 11), a heat-gated ion channel modulated in multiple ways by inflammatory mediators, such as nerve growth factor (NGF) and bradykinin, at the levels of transcription, translation, and posttranslation (12–14). However, with no unequivocal data regarding the identity of the mechanotransducing ion channels in mammalian sensory neurons, understanding of the molecular mechanisms of mechanical sensitization lags behind that of thermal sensation. Signaling molecules normally present at the peripheral nerve ending in vivo are present on the cell bodies of cultured sensory neurons (15, 16), allowing such cells to be used as a model for the study of transduction processes that normally occur in the sensory terminal. Taking this approach, we have previously characterized mechanotransduction in cultured dorsal root ganglion (DRG) neurons, finding that mechanical stimulation evoked cationic membrane currents and that distinct neuronal subpopulations had distinct mechanosensitive properties (17). In the present study, we investigated whether signaling pathways involved in inflammation modulate the mechanotransducing properties of DRG neurons. It is shown that exposure of a major subclass of nociceptive neurons to NGF and activators of PKC (but not activators of PKA) significantly enhanced mechanosensitivity through distinct mechanisms. In the presumptive TrkA-expressing population of nociceptors, NGF increased the transcription of new mechanosensitive ion channels or a factor that modulates them. Conversely, activation of PKC dramatically increased current amplitude by recruiting channel proteins to the cell surface.
Results
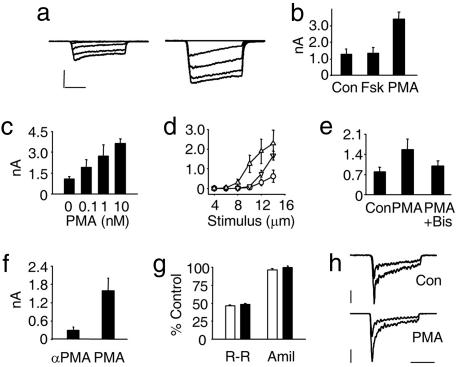
Inflammatory mediators are known to increase nociceptor excitability via activation of PKC- and PKA-dependent signaling pathways (2, 18). To test whether activation of these pathways augmented responses to mechanical stimulation in cultured neurons, cells were incubated overnight in 200 nM forskolin (Fsk, a membrane permeant activator of adenylyl cyclase) or 10 nM phorbol 12-myristate 13-acetate (PMA) (a phorbol ester that activates PKC). Then, using the perforated-patch technique, voltage-clamp recordings of mechanically activated (MA) currents were made. Strikingly, PMA induced an increase of >250% in the peak response to mechanical stimulation (3.42 ± 0.39 nA, n = 7, P = 0.001), whereas currents in Fsk-treated neurons (1.35 ± 0.35 nA, n = 7) were unchanged from control values (1.28 ± 0.32 nA, n = 7) (Fig. 1 a and b). In contrast, voltage-activated sodium currents recorded after overnight PMA exposure (7.35 ± 3.10 nA, n = 30) were not significantly above control values (6.28 ± 2.08 nA, n = 26, P = 0.14). Testing a range of PMA concentrations (0.1–10 nM) showed that its effects were dose-dependent, with a 250% augmentation of MA currents seen at 1 nM (Fig. 1c). It was also found that exposure of neurons to 10 nM PMA for 1 h increased the peak MA current amplitude to levels equivalent to currents in neurons receiving overnight treatment, although it was notable that at lower stimulus intensities, overnight treatment had a greater effect (Fig. 1d). The efficacy of a 1-h treatment in enhancing mechanosensitivity strongly suggested that PKC acted by inducing a posttranslational modification of the transduction apparatus (see below), and all subsequent applications of PMA were for 1 h (unless otherwise stated). To confirm that PMA acted through activation of PKC, neurons were preincubated with the PKC-specific inhibitor bisindolylmaleimide I, and in this condition, PMA (30 nM for 20 min) failed to significantly increase MA current over control values (0.99 ± 0.18 nA, n = 15, vs. 0.80 ± 0.15 nA; n = 13; P = 0.15) (Fig. 1e), whereas PMA alone approximately doubled its amplitude (1.56 ± 0.37 nA; n = 11; P = 0.055 vs. control). In separate experiments (Fig. 1f), neurons treated with the inactive enantiomer of PMA, α-PMA, had small MA currents (0.29 ± 0.11 nA, n = 10) 5-fold lower than in sister cultures treated with PMA (1.59 ± 0.43 nA, n = 8). To assess whether the large MA currents seen after PMA treatment had similar characteristics to those seen in baseline conditions, we tested whether the efficacy of ruthenium red and amiloride in blocking the underlying ion channels remained constant (Fig. 1 g and h). Ruthenium red (5 μM) blocked MA currents by approximately half (residual current: 46 ± 2%, n = 4, vs. 49 ± 1%, n = 5) in each case, whereas amiloride failed to block MA currents in either control or PMA-treated neurons (96 ± 2%, n = 4 vs. 100 ± 2%, n = 5), thus suggesting that the same ion channels are being activated in both states.
Fig. 1.
Activation of PKC increases MA current amplitude in neonatal sensory neurons. (a) Examples of voltage-clamp recordings of neurons responding to incrementing mechanical stimuli (2–14 μm) in control conditions (Left) and incubated overnight with PMA (10 nM; Right). (Calibration: vertical, 0.8 nA; horizontal, 100 ms.) (b) Average peak response to 14 μm mechanical stimulus in control conditions, overnight Fsk, and overnight PMA. (c) Overnight PMA increased MA current amplitude in a concentration dependent manner (one-way ANOVA, P < 0.001): control (1.07 ± 0.18 nA; n = 13), 0.1 nM (1.91 ± 0.55 nA; n = 5), 1 nM (2.72 ± 0.82 nA; n = 5; P = 0.04 vs. control), and 10 nM (3.64 ± 0.33 pA; n = 11; P < 0.01 vs. control). (d) MA currents evoked by increasing membrane displacements (from 4 to 14 μm) in control neurons (○, 14-μm step: 0.62 ± 0.30 nA; n = 6), neurons treated overnight with 10 nM PMA (▵, 2.28 ± 0.68 nA; n = 6; P = 0.05 vs. control), and neurons treated for 1 h with 10 nM PMA (▿, 1.67 ± 0.22 nA; n = 6; P = 0.02 vs. control). (e) Average peak response to 14-μm mechanical stimulation in control neurons and in those treated with either PMA alone or PMA plus bisindolylmaleimide I (1 μM, applied for 15 min before PMA). (f) Neurons treated with PMA had significantly larger MA currents than neurons treated with α-PMA, the inactive enantiomer. (g) The efficacy of ruthenium red in blocking MA currents was unchanged before and after PMA treatment, whereas amiloride was ineffective in both conditions. (h) Example traces showing current inhibition by ruthenium red. (Calibration: top vertical, 0.2 nA; bottom vertical, 1 nA; horizontal, 100 ms.)
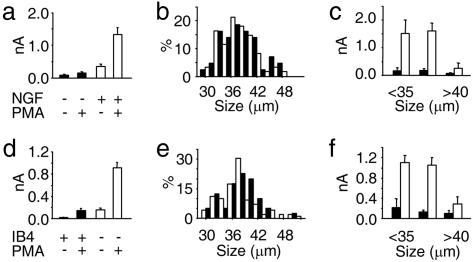
Previously (17, 19) and during the above experiments, we cultured neurons in NGF; to determine whether NGF regulates mechanosensitivity, we cultured adult rat neurons with and without it and then recorded MA currents either in control conditions or after PMA treatment (Fig. 2a). NGF had a striking effect; its omission precluded the expression of large-amplitude MA currents, even after application of PMA. In neurons with neither NGF nor PMA, the mean maximal current size was <100 pA (0.08 ± 0.03 nA, n = 18), and although addition of PMA induced a trend toward current augmentation, it was not significant (0.34 ± 0.08 nA, n = 25, P = 0.2). However, in PMA-treated groups, the presence of NGF in culture increased current amplitude by >9-fold (1.32 ± 0.22 nA, n = 36, vs. 0.14 ± 0.04 nA, n = 25, P < 0.001). To determine that neurons in each group were of the same subpopulation and that NGF had not affected cell viability, we compared their cell size distribution. As is evident in Fig. 2b, we recorded from neurons that had equally distributed somatic diameters, ranging from ≈28 to 50 μm. However, those neurons that responded strongly to NGF had diameters of <40 μm (Fig. 2c), consistent with a nociceptive phenotype and the expression pattern of the high-affinity NGF receptor TrkA in DRG (20).
Fig. 2.
MA currents are differentially regulated by PKC and trophic factors in distinct neuronal subpopulations from adult rats. (a) Up-regulation of MA currents by PMA depends on pretreatment with NGF. Graph shows average, maximal MA currents in control conditions (and after 1-h incubation in 30 nM PMA). (b) Cell diameter frequency distribution of all NGF-deprived (filled columns; n = 43) and NGF-treated (open columns; n = 61) neurons used in a. (c) Average peak MA current of PMA-treated cells seen in a, grouped according to cell size (<35 μm, 35–40 μm, and >40 μm). Open columns, with NGF; filled columns, no NGF. (d) Comparison of MA current amplitude, in control conditions and after PMA treatment, in IB4+ neurons and IB4− neurons. (e) Cell diameter distribution of IB4+ (filled columns; n = 40) and IB4− (empty columns; n = 99) neurons used in d. (f) Average peak MA currents in neurons treated with PMA (from d) grouped according to cell diameter (<35 μm, 35–40 μm, and >40 μm) and IB4 labeling (filled columns, IB4+, empty columns, IB4−).
Next we investigated whether distinct nociceptor populations were differentially sensitive to the effects of PMA and neurotrophins. To distinguish between populations, we labeled neurons with Isolectin B4 (IB4). IB4 binds nonpeptidergic, c-Ret-expressing neurons, that give rise to nociceptive C fibers, but not small-medium sensory neurons that give rise to TrkA-positive, Aδ-fiber, and peptidergic, C fiber nociceptors (20). Consistent with our previous findings in neonatal neurons (17), in adult rat neurons cultured with NGF, we found that IB4+ neurons not exposed to PMA were essentially insensitive to mechanical stimulation (0.02 ± 0.00 nA, n = 11; Fig. 2d). PKC activation led to the appearance of MA currents in some of these cells, although on average, responses were not significantly increased (0.14 ± 0.04 nA, n = 29, P = 0.10; Fig. 2d). Conversely, in IB4− neurons, PMA considerably increased peak MA current amplitude from 0.15 ± 0.04 nA (n = 15) to 0.91 ± 0.10 nA (n = 84, P < 0.001; Fig. 2d). Most IB4+ neurons have small cell bodies (20); however, in this study, we selected size-matched IB4+ and IB4− neurons in the range of 28–52 μm (Fig. 2e). MA current amplitude in PMA-treated cells was independent of cell-size amongst IB4+ neurons; however, those IB4− neurons that responded most to mechanical stimulation after PMA treatment had cell bodies of <40 μm in diameter (Fig. 2f). Together these data suggest that in a subset of IB4− neurons, PKC activation substantially enhances mechanosensitivity and that this effect depends on prior exposure to NGF.
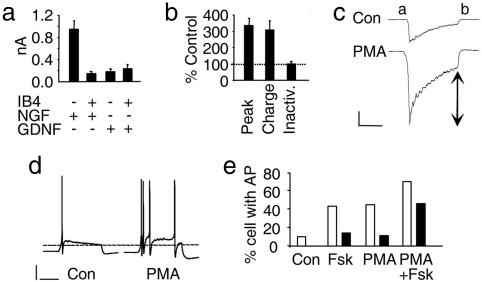
Given that TrkA receptors are localized to IB4− nociceptors, whereas IB4+ neurons express the glial cell line-derived factor (GDNF) receptors c-Ret and GFRα1 (21), we tested whether mechanosensitivity (after treatment with PMA) in the latter group depended on GDNF. However, as is shown in Fig. 3a, when neurons were cultured in the presence of GDNF, IB4+ and IB4− populations had currents that were of similar small amplitude (0.24 ± 0.08 nA and 0.18 ± 0.06 nA, respectively; n = 18) and comparable to those seen in IB4+ neurons cultured in NGF (0.14 ± 0.04 nA, n = 29), whereas IB4− cells in this latter group had much larger MA currents (0.95 ± 0.15 nA, n = 37). To further characterize the IB4− neurons that responded to PMA treatment, we determined their sensitivity to capsaicin and also assessed the kinetics of MA currents expressed in control conditions and after PMA treatment. In a sample of IB4− neurons with a similar size range to those used above, where PMA enhanced MA currents to 337 ± 43% of control values, it was found that capsaicin activated an inward current (>100 pA) in 88% (n = 17) of control neurons and 79% (n = 19) of those treated with PMA, further indicating that these neurons are polymodal nociceptors (data not shown). In this sample, we also assessed the kinetics of MA currents to determine whether up-regulated currents were kinetically similar to control currents. In PMA-treated cells, total charge transfer increased proportionally to peak current (307 ± 57% of control) and current adaptation over the stimulus duration was unchanged (105 ± 5% of control; Fig. 3b). This result is further evidence that channels expressed normally are those up-regulated by PKC activation. Finally, we observed the behavior of these neurons in current-clamp conditions after treatment with PMA and/or Fsk. Interestingly, activation of PKA by Fsk acted synergistically with increasing MA currents through PKC activation. Despite its lack of effect on MA currents, PKA activation alone increased the likelihood of neurons firing at least one action potential from 10% (control, n = 10) to 43% (Fsk, n = 7). Moreover, when Fsk and PMA were applied together, they increased the incidence of repetitive firing from 0% observed in control to 46% (n = 13).
Fig. 3.
Regulation of adult nociceptor mechanosensitivity by neurotrophins and PMA. (a) Average peak currents in IB4-labeled, PMA-treated neurons (30 nM for 1 h). Either NGF or GDNF was applied overnight to culture medium. (b) Kinetics of MA currents are unchanged after PMA treatment. In a population of IB4− neurons, peak current amplitudes and total charge transfer increased at a similar rate after PMA, adaptation was unchanged. (c) Example traces showing current adaptation and the parameters that were used for kinetic analysis: total charge transfer was measured between points a and b; arrows show residual current at the end of the stimulus used to calculate adaptation. (Calibration: vertical, 0.3 nA; horizontal, 100 ms.) (d) Examples of current-clamp recordings made from mechanically stimulated neurons (8-μm membrane deflection): neuron cultured in control conditions (Left) and a neuron incubated for 1 h with 30 nM PMA (Right). (Calibration: vertical, 20 mV; horizontal, 100 ms.) (e) Probability of neurons firing either a single action potential (open columns) of repetitively (filled columns) in response to mechanical stimulation in control conditions (10% and 0%, respectively; n = 10) or incubated for 1 h with 1 μM Fsk (43% and 14%; n = 7), 30 nm PMA (44% and 11%; n = 9), or both (69% and 46%; n = 13).
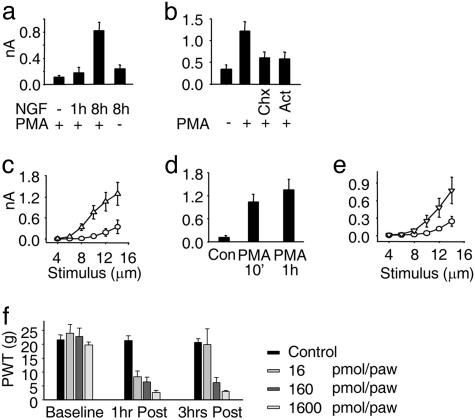
Having characterized the effects of PMA and NGF on the mechanosensitivity of sensory neurons, we sought to determine the mechanisms by which these compounds acted. First, we determined how rapidly NGF modulates mechanosensitivity; IB4- neurons were cultured either without NGF or with it for 1 or 8 h and then treated with PMA before recording MA currents. This experiment showed that the short-term (1 h) application of NGF was ineffective in increasing mechanosensitivity (from 0.11 ± 0.03 nA, n = 30, to 0.17 ± 0.09 nA, n = 20), whereas after 8 h, MA currents were substantially augmented (0.82 ± 0.13 nA, n = 54, P < 0.001 vs. no NGF; Fig. 4a). This observation suggested that NGF acts by increasing the production of new channel proteins whose activity can be increased by activation of a PKC-dependent process. To further test this hypothesis, we incubated neurons for 8 h in NGF, either alone or in the presence of an inhibitor of gene transcription (actinomycin) or an inhibitor of mRNA translation (cyclohexamide). In neurons incubated with NGF alone, PMA treatment induced a 350% increase in MA current amplitude (1.22 ± 0.22 nA, n = 23, vs. 0.34 ± 0.10 nA, n = 9; P < 0.05); conversely, when transcription or translation had been blocked, PMA failed to induce a significant increase in current size (actinomycin: 0.57 ± 0.16 nA, n = 12, P = 0.27; cyclohexamide: 0.60 ± 0.14 nA, n = 21, P = 0.25; Fig. 4b). These data, coupled with the time course of NGF’s action, lead us to conclude that NGF modulates mechanosensitivity by inducing the synthesis of mechanosensitive ion channels (or an auxiliary protein required for their function) via up-regulation of gene transcription. It has been shown recently (22) that, because of the coupling of TrkA receptor to intracellular pathways in DRG neurons, acute regulation of TRPV1 by NGF undergoes maturational changes and NGF is effective only in enhancing TRPV1 function in adult [more than postnatal day (P)12] neurons. In contrast, we observed that if neonatal (P1) neurons were cultured for 9–12 h with or without NGF, a significant enhancement of mechanosensitivity was evident in the NGF-treated group (0.35 ± 0.19 nA, n = 8, vs. 1.29 ± 0.32 nA, n = 9, P < 0.05; Fig. 4c).
Fig. 4.
Mode of action of NGF and time course of cellular and behavioral effects of PMA. (a) MA currents in IB4−, PMA-treated neurons (30 nM for 1 h). Neurons were grown without NGF, or NGF was applied before PMA for either 1 h or 8 h. Mean MA current amplitude recorded after 8 h NGF without PMA was 0.24 ± 0.06 nA (n = 20, P = 0.01 vs. NGF 8 h plus PMA). (b) Cycloheximide (Chx) and actinomycine D (Act) inhibit the effect of NGF on MA currents in IB4− neurons. Cells were treated with NGF for 8 h either alone or with Chx (10 μg/ml) or Act (5 μg/ml, each was added 1 h before NGF), and then 30 nM PMA was added for 1 h. (c) Effect of NGF on short-term (9–12 h) neonatal cultures. Shown are current amplitudes evoked by incrementing mechanical stimuli in IB4− neurons (no PMA added). Currents recorded in neurons without NGF (○) were significantly smaller than those in cells cultured in NGF (▵). (d) Average peak MA currents in IB4− neurons incubated with PMA for 10 min or 1 h. (e) Augmentation of MA currents by PKC activation is maintained after PMA is removed. Shown are current amplitudes evoked by incrementing mechanical stimuli in IB4− neurons. Currents recorded in control conditions (○) were significantly smaller than those in cells treated with PMA for 1 h and then washed for 4 h before recording (▵). (f) PMA caused a dose-dependent decrease in PWT after intraplantar injection. All doses of PMA induced a decrease in PWT 1 h after injection. Three hours after injection, hyperalgesia induced by 160 and 1,600 pmol per paw was maintained, whereas PWTs in rats given the lowest dose returned to near control values (81.0 ± 17.7% of control, P = 0.54) (n = 4 in each group).
Next we examined the mechanism by which PKC activation enhanced MA current amplitude. Using NGF-treated, IB4− neurons only, the time course of PMA actions was studied further. It was found that PMA exposure for either 10 or 60 min increased current levels to comparable levels (1.03 ± 0.20 nA, n = 15, P = 0.01 and 1.36 ± 0.28 nA, n = 11, P = 0.005, respectively, vs. control: 0.12 ± 0.06 nA, n = 6; Fig. 4d), thus suggesting that PMA acted rapidly to modify channel behavior. The same result was obtained when PMA was directly applied to a patched neuron, for as little as 7 min, by using a perfusion pipe positioned next to the cell under study (control: 0.41 ± 0.07 nA, n = 11; vs. PMA treated: 1.23 ± 0.35 nA, n = 8; data not shown). It was further determined that an increase in MA current amplitude was maintained significantly after the cessation of PKC stimulation. As is shown in Fig. 4e, after neurons were exposed to PMA (30 nM) for 1 h, washed, and then incubated in control conditions for 4 h before recording, MA currents remained >300% of control values (0.77 ± 0.23 nA, n = 15, vs. 0.23 ± 0.08 nA, n = 13; P = 0.043).
Peripheral PKC activation has been implicated in the induction of mechanical hyperalgesia (23, 24) and the data presented in this paper suggest a mechanism by which PKC activation can increase the sensitivity of sensory neurons to mechanical stimulation. Therefore, we determined whether intraplantar injections of PMA induced mechanical hypersensitivity with a similar time course and dose dependency as the effects we have observed in vitro. Using automated von Frey testing, it was observed that 1 h after injection, all doses of PMA (16, 160, and 1,600 pmol per paw in 50 μl) caused a severe and dose-dependent reduction in the paw withdrawal threshold (PWT) from a mechanical stimulus [PWT decreased by 64.5 ± 7.7%, P < 0.01; 72.8 ± 6.4%, P < 0.01; and 87.8 ± 3.9% (P < 0.001), respectively; Fig. 4f]. PWTs then were reassessed 3 h after injection, and it was found that although there was a significant recovery in those animals given 16 pmol PMA, mechanical hyperalgesia was entirely sustained at the higher 2 doses (Fig. 4f). Hence, the long-term maintenance of MA current enhancement in vitro mirrors the sustained mechanical hypersensitivity observed in vivo.
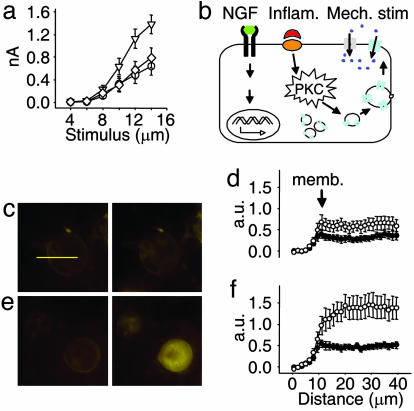
Two aspects of the effect of PKC activation on MA current amplitude suggested the possibility that the underlying mechanism involved the insertion of new functional channels into the neuronal membrane. First, the increase in current amplitude is maintained for a substantial period after the removal of PMA. Second, the apparent NGF-induced increase in ion channel synthesis was alone insufficient to drastically augment MA current amplitude, thus suggesting that newly synthesized channels are not functionally present in the cell membrane. To test this hypothesis, we inhibited vesicular exocytosis in sensory neurons by incubating them in tetanus toxin (TeNT) before application of PMA; TeNT cleaves vesicle-associated protein VAMP-2 to inhibit fusion of vesicles to the cell membrane (25). This treatment inhibited the potentiation of MA currents by PKC activation (Fig. 5a), implying that PMA alone is sufficient to cause increased vesicular turnover. To test this hypothesis further, we used FM1-43 to measure the amount of membrane recycling. Neurons were treated with PMA for 20 min, and the amount of FM1-43 labeling was compared to that of control neurons. Unstimulated neurons showed a very limited time-dependent incorporation of the dye indicative of a low level of membrane recycling (Fig. 5 c and d). Consistent with PKC activation inducing exocytosis, when PMA and FM1-43 were added to neurons together, there was a much higher degree of FM1-43 labeling than in control neurons; this increase was not significant at 5 min (447 ± 64, n = 12, vs. 306 ± 55, n = 11) but after 20 min, labeling was almost 250% of control values (1,437 ± 271, n = 12, vs. 614 ± 151, n = 11; P < 0.02) (Fig. 5 c–f). In separate experiments, PMA was not found to evoke currents in patched DRG neurons (n = 11), negating the possibility that FM1-43 is taken up via an ion channel mediated route. These data thus support the notion that PKC activation by PMA increases mechanosensitivity in a subpopulation of DRG neurons by inducing de novo insertion of mechanosensitive ion channels into the cell membrane via a TeNT-sensitive pathway.
Fig. 5.
PKC activation up-regulates MA currents by a mechanism involving insertion of new channels in the membrane. (a) TeNT inhibits sensitization of MA currents by PMA. Shown are average responses to increasing mechanical stimulation (up to 14 μm) in neonatal neurons. PMA alone up-regulates MA currents (▿; peak: 1.38 ± 0.16 nA, n = 24; P < 0.005 vs. control), but after an 8-h incubation in TeNT (20 nM), PMA had no effect on current amplitude (◇; mean at 14 μm: 0.79 ± 0.18 nA, n = 24; P < 0.05 vs. PMA-treated cells). Peak control current was 0.64 ± 0.16 nA (○, n = 16). (b) Schematic representation of the proposed mechanisms underlying peripheral sensitization to mechanical stimulation. NGF, acting via trkA receptors, increases transcription of MA channels, which are packaged into vesicles. Activation of PKC by proinflammatory signals induces fusion of the vesicles and insertion of new mechanosensitive channels into the cell membrane. (c) Epifluorescence images taken 5 min (Left) and 20 min (Right) after FM1-43, a marker of vesicle recycling, was applied to neurons bathed in control extracellular solution (horizontal bar: 40 μm.) (d) Quantitative analysis of the mean fluorescence (arbitrary units) in 11 neurons whose images were taken as described in c (•, 5 min; ○, 20 min), measured along a 40-μm segment (indicated by yellow line in c). Segments were drawn such that each cell’s profile could be superimposed for comparison. (e) As in c, but FM1-43 was coapplied with 30 nM PMA. (f) Analysis as in d, but neurons (n = 12) were treated with PMA as in e.
Discussion
This study sought to determine whether signaling pathways involved in inflammatory pain conditions directly modulate the mechanotransduction apparatus of sensory neurons. It was found that in a major subclass of nociceptors, two such pathways modulate mechanosensory ion channels. Activation of the PKC signaling cascade increased the mechanosensitivity of DRG neurons by inducing insertion of new transduction channels into the cell membrane, whereas NGF acted at the transcriptional level to increase the availability of these channels or a factor that allows PKC-dependent membrane insertion of the channel (Fig. 5b). NGF levels increase during inflammation (26), and NGF previously has been found to increase transcript levels of TRPV1 (12) and proinflammatory B2 bradykinin receptors (27). PKC-induced insertion of channels was via a VAMP-2 dependent vesicular mechanism, as demonstrated by tetanus toxin sensitivity, whereas the maintenance of enhanced mechanosensitivity hours after the removal of PMA suggests newly inserted channels remain stable in the membrane. Consistent with this interpretation, we found that PKC activation increased the rate of vesicular trafficking as demonstrated by a PMA-induced increase in FM1-43 labeling of neurons. Both regulatory mechanisms that we observed were developmentally maintained, with qualitatively similar effects of PMA and NGF observed in neonatal and adult neurons. PKA activation had no effect on the amplitude of MA currents, but when applied with PMA, had a synergistic effect in increasing action potential firing rates, which could be due to complementary effects on voltage-gated ion channels by PKA, (e.g., NaV1.8, see ref. 28).
Control of the rate of insertion of ion channels into the cell membrane by vesicular trafficking recently has been the subject of a number of studies. Of particular interest are results concerning members of the TRP channel family, given their general function in sensory systems (29) and the possibility that MA currents are mediated by members of this family (19). For example, TRPC3 is inserted into the cell membrane in a VAMP-2-dependent manner (30), and the surface expression of TRPC5 is regulated in this way by growth factors (31). Moreover, TRPV1, which functions in thermal sensation and hyperalgesia (10, 11), has been shown to associate with two vesicular proteins, whereas in oocytes, activation of PKC increased channel density at the cell surface (14). Hence, ion channel trafficking has an important role in regulating neuronal function in general, and specifically it may play a major role in altering the sensitivity of DRG nerve terminals to physical stimuli. At this stage, it remains unclear which PKC isoform is responsible for mediating membrane insertion of mechanosensitive ion channels, and it will be of interest to determine whether the same one also regulates the trafficking of TRPV1. Interestingly, PKCε null mutants have major deficits in mechanical hyperalgesia induced by epinephrine, whereas a specific inhibitor of PKCε inhibited carageenin-induced mechanical hypersensitivity (32). PKCε is abundant in DRG neurons and translocates to the cell membrane after activation by bradykinin (13).
The effect of NGF was specific to IB4− neurons of <40 μm in diameter, the same population of neurons that were most affected by PKC activation. These data suggest that NGF acts through TrkA receptors located on peptidergic nociceptors and Aδ-fiber nociceptors. A nociceptive phenotype of cultured rat neurons with diameters up to 40 μm is strongly supported by the observation that a substantial number of IB4− neurons in this size range respond to capsaicin, display wide action potentials, and express neuropeptides (33). Furthermore, in a sample of neurons in which we saw PMA augmented mechanosensitivity, the vast majority were capsaicin-sensitive. The other major population of nociceptive neurons are TrkA-negative, IB4+ neurons, which express C-ret in adulthood (20, 21). However, even when neurons were cultured in the presence of GDNF, this population displayed very small MA currents despite the application of PMA. These data raise the possibility that different classes of nociceptors detect mechanical stimuli by using distinct mechanisms. IB4+ neurons may indirectly detect noxious mechanical stimuli via the release of a chemical intermediary released from mechanically damaged cells (34). Dai et al. (35) have produced evidence that activation of P2X3 receptors, which are almost exclusively expressed by IB4+ neurons, play a role in mechanical hyperalgesia after complete Freund’s adjuvant, whereas mechanically stimulated keratinocytes may also signal to DRG neurons by releasing ATP (36).
In inflammatory pain states, increased levels of numerous mediators activate receptors on the sensory terminals of nociceptors to regulate their excitability. Major pathways implicated in this process include those dependent on PKA, PKC, and mitogen-activated protein kinases; targets of these pathways include voltage-activated channels and heat-activated channels (2, 18), and hyperalgesia in a naturalistic setting will involve an interplay between many systems. However, our behavioral data and that of others (24) show that PKC activation is sufficient to induce mechanical hyperalgesia. Furthermore, GPCRs known to be positively coupled to PKC via PLC, such as bradykinin (37) and mGluR5 receptors (23, 38), have been shown to play an important role in mechanical hypersensitivity. Likewise, NGF can induce mechanical hyperalgesia, and it does so with a latency of ≈3 h (39, 40); this delay may imply a central mechanism but our data, suggesting increased expression of a protein required for augmented mechanotransduction, offers an alternative explanation for this delay. It is noted that PMA can activate cellular targets other than PKC, but it is widely held that bisindolylmaleimide I, which binds PKC at a site distinct from the PMA-binding domain, is a selective inhibitor of PKC (41). Also, some phorbol esters are reported to induce ion channel activity, but in our experience, PMA very rarely induces cationic currents in sensory neurons (13, 42). Therefore, PMA effects on MA currents or FM1-43 uptake cannot be accounted for by such a mechanism.
At present, the strongest evidence of a robust peripheral sensitization to mechanical stimuli comes from studies of the nociceptive innervation of joints (6), the viscera (7), the dura (5), and from nerve endings in neuromas (8, 9). In each case, chemical stimulation leads to a decrease in mechanical threshold and increased suprathreshold firing. Most studies have used a combination of inflammatory mediators, meaning the intracellular pathways underlying sensitization are unresolved, although Neugebauer et al. (43) showed bradykinin alone sensitized articular afferents of the knee joint. We recorded from unlabeled neurons from DRG of all spinal levels so were not aware of the peripheral targets of the neurons used. However, given the specificity of PMA- and NGF-induced sensitization to IB4− neurons, it is of interest to note that nociceptive afferents of deep tissues that display pronounced mechanical sensitization are predominately peptidergic neurons. IB4+ neurons are absent from the knee joint (44) and account for only 20% of colonic nociceptors (45), whereas an extensive peptidergic innervation of the dura has been characterized (46). This distribution contrasts with cutaneous nociceptors, most of which bind IB4 (45, 47). The mechanisms underlying peripheral mechanical sensitization are unclear; augmentation of currents carried by voltage-gated sodium channels are likely to contribute (2), and we propose that recruitment of previously uncharacterized mechanosensitive ion channels to the membrane surface will also substantially increase sensitivity.
In conclusion, the molecular understanding of mechanotransduction is still very poor, and very little is known about modulation of this sensory modality in different pain states. Using a functional assay of mechanosensitivity that utilizes cultured neurons as a model of peripheral terminals, we identified two mechanisms potentially involved in nociceptor plasticity after inflammation. These mechanisms may play important roles in visceral pain, arthritis, migraine pain, and neuropathic pain.
Experimental Procedures
Cell Culture.
Cells were cultured from neonatal (P1 and P2) and adult (P24–P35) Sprague–Dawley rats (17, 19). Briefly, animals were decapitated, and 20–25 DRG were dissected from them. Ganglia were digested by using collagenase type XI and protease type IX and then mechanically triturated before cells were resuspended in culture medium and plated on poly(l-lysine) and laminin. Medium was DMEM with fetal bovine serum, glutamine, and penicillin-streptomycin. NGF (100 ng/ml) (Promega) or 10 ng/ml GDNF (Peprotech) was added as required. Recordings were made 16–30 h after plating. Experimental neurons were compared with control neurons from that day’s cultures to compensate for interculture variability.
Electrophysiology.
Neurons with isolated cell bodies were selected for recording. Electrophysiological recordings were made at a holding potential of −70 mV (voltage-clamp configuration) or at resting potential (current-clamp configuration) by using an Axopatch 200B amplifier (Axon Instruments). Data were acquired at 20 kHz by using pclamp 8 software (Axon Instruments). Experiments were performed in the perforated-patch configuration with a pipette solution containing 90 mM potassium acetate, 50 mM KCl, 1 mM MgCl2, and 10 mM Hepes, pH 7.35 (pH was corrected by using KOH); 200 μg/ml amphotericin B was added immediately before recording. The extracellular solution contained 140 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 4 mM d-glucose, and 10 mM Hepes, pH 7.4. Drugs were prepared on the day of the experiment from stocks kept at −20°C at a concentration at least 1,000-fold the working concentration.
Neurons were mechanically stimulated (17, 19) by using a heat-polished glass pipette (tip diameter ≈5 μm), controlled by a piezo-electric crystal drive (Burleigh Instruments, Fishers, NY). The probe moved at 0.5 μm/msec, and the stimulus was applied for 200 msec. To assess the mechanosensitivity of a neuron, a series of mechanical steps in 2-μm increments up to 14 μm were applied at 15-sec intervals. Current adaptation was measured as the percentage decrease in current amplitude from the peak to the end of the stimulus plateau.
Cell Labeling.
To label neurons with IB4, cells were incubated in 3 μg/ml IB4-Alexa 488 (Molecular Probes) for 10 min and then washed twice in extra cell solution before cell selection. The fluorescent dye FM1-43 (2 μM; Molecular Probes) was added to the dish, with or without PMA, immediately before placing the cells on the stage of a Zeiss Axiovert 200 microscope equipped for epifluorescence. A neuron was randomly selected from the plate in bright-field mode and then fluorescent images of it were taken after 5 and 20 min [Apogee Instruments (Roseville, CA) charge-coupled device camera]. Analysis was performed by using microccd software, a 40-μm line was drawn with the cell’s edge at 10 μm, then for statistics, we compared a single point from midway across each cell after subtraction of background values.
Behavioral Testing.
Mechanical withdrawal thresholds were measured by using an automated von Frey hair applicator, calibrated to apply a pressure ramp of 50 g over 20 sec. At each time point, three stimuli were applied to the animals’ left hind paws, and the mean of these three values was taken. Animals (P28 on day 1) were habituated to the testing room on day 1 and to testing on days 2 and 3. On day 4, a baseline reading was taken 1 h before injection, and then the animals were assessed 1 and 3 h after injection. Injections were given under light anesthesia (2–4% halothane). Drugs were given in a 50-μl volume of standard external solution; controls received a concentration of DMSO (1.62%) equivalent to those animals receiving 1.6 nmol of PMA.
Data Analysis.
Unless otherwise stated, the mean peak amplitude of MA currents in response to a 14-μm mechanical stimulus was used to assess the mechanosensitivity of each group of neurons. Groups were then compared by using the Student t test unless otherwise stated. Data were analyzed by using sigmaplot 8.0 and sigmastat 4.0 software (Systat Software, Point Richmond, CA).
Acknowledgments
We thank Ornella Rossetto (Università di Padova, Padova, Italy) for sharing some reagents and Mark Baccei for comments on the manuscript. This work was supported by grants from the Tronchetti-Provera Foundation (to P.C.) and the Medical Research Council (to L.J.D. and J.N.W.).
Abbreviations
- DRG
dorsal root ganglion
- Fsk
forskolin
- GDNF
glial cell line-derived factor
- IB4
Isolectin B4
- MA
mechanically activated
- NGF
nerve growth factor
- PMA
phorbol 12-myristate 13-acetate
- Pn
postnatal day n
- PWT
paw withdrawal threshold
- TeNT
tetanus toxin.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Woolf C. J., Salter M. W. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 2.Bhave G., Gereau R. W. J. Neurobiol. 2004;61:88–106. doi: 10.1002/neu.20083. [DOI] [PubMed] [Google Scholar]
- 3.Schlegel T., Sauer S. K., Handwerker H. O., Reeh P. W. Neurosci. Lett. 2004;361:163–167. doi: 10.1016/j.neulet.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 4.Andrew D., Greenspan J. D. J. Neurophysiol. 1999;82:2649–2656. doi: 10.1152/jn.1999.82.5.2649. [DOI] [PubMed] [Google Scholar]
- 5.Strassman A. M., Raymond S. A., Burstein R. Nature. 1996;384:560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- 6.Schaible H. G., Schmidt R. F. J. Neurophysiol. 1988;60:2180–2195. doi: 10.1152/jn.1988.60.6.2180. [DOI] [PubMed] [Google Scholar]
- 7.Gebhart G. F. Am. J. Physiol. 2000;278:G834–G838. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- 8.Michaelis M., Vogel C., Blenk K. H., Arnarson A., Janig W. J. Neurosci. 1998;18:7581–7587. doi: 10.1523/JNEUROSCI.18-18-07581.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivera L., Gallar J., Pozo M. A., Belmonte C. J. Physiol. 2000;527:305–313. doi: 10.1111/j.1469-7793.2000.t01-1-00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caterina M. J., Leffler A., Malmberg A. B., Martin W. J., Trafton J., Petersen-Zeitz K. R., Koltzenburg M., Basbaum A. I., Julius D. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 11.Davis J. B., Gray J., Gunthorpe M. J., Hatcher J. P., Davey P. T., Overend P., Harries M. H., Latcham J., Clapham C., Atkinson K., et al. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 12.Nagy I., Santha P., Jancso G., Urban L. Eur. J. Pharmacol. 2004;500:351–369. doi: 10.1016/j.ejphar.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 13.Cesare P., Dekker L. V., Sardini A., Parker P. J., McNaughton P. A. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- 14.Morenilla-Palao C., Planells-Cases R., Garcia-Sanz N., Ferrer-Montiel A. J. Biol. Chem. 2004;279:25665–25672. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- 15.Baccaglini P. I., Hogan P. G. Proc. Natl. Acad. Sci. USA. 1983;80:594–598. doi: 10.1073/pnas.80.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cesare P., McNaughton P. Proc. Natl. Acad. Sci. USA. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drew L. J., Wood J. N., Cesare P. J. Neurosci. 2002;22:RC228. doi: 10.1523/JNEUROSCI.22-12-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Julius D., Basbaum A. I. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 19.Drew L. J., Rohrer D. K., Price M. P., Blaver K. E., Cockayne D. A., Cesare P., Wood J. N. J. Physiol. 2004;556:691–710. doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molliver D. C., Wright D. E., Leitner M. L., Parsadanian A. S., Doster K., Wen D., Yan Q., Snider W. D. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 21.Bennett D. L., Michael G. J., Ramachandran N., Munson J. B., Averill S., Yan Q., McMahon S. B., Priestley J. V. J. Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu W., Galoyan S. M., Petruska J. C., Oxford G. S., Mendell L. M. J. Neurophysiol. 2004;92:3148–3152. doi: 10.1152/jn.00356.2004. [DOI] [PubMed] [Google Scholar]
- 23.Walker K., Reeve A., Bowes M., Winter J., Wotherspoon G., Davis A., Schmid P., Gasparini F., Kuhn R., Urban L. Neuropharmacology. 2001;40:10–19. doi: 10.1016/s0028-3908(00)00114-3. [DOI] [PubMed] [Google Scholar]
- 24.Souza A. L., Moreira F. A., Almeida K. R., Bertollo C. M., Costa K. A., Coelho M. M. Br. J. Pharmacol. 2002;135:239–247. doi: 10.1038/sj.bjp.0704434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossetto O., Seveso M., Caccin P., Schiavo G., Montecucco C. Toxicon. 2001;39:27–41. doi: 10.1016/s0041-0101(00)00163-x. [DOI] [PubMed] [Google Scholar]
- 26.Safieh-Garabedian B., Poole S., Allchorne A., Winter J., Woolf C. J. Br. J. Pharmacol. 1995;115:1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee Y. J., Zachrisson O., Tonge D. A., McNaughton P. A. Mol. Cell. Neurosci. 2002;19:186–200. doi: 10.1006/mcne.2001.1073. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald E. M., Okuse K., Wood J. N., Dolphin A. C., Moss S. J. J. Physiol. 1999;516:433–446. doi: 10.1111/j.1469-7793.1999.0433v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clapham D. E. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 30.Singh B. B., Lockwich T. P., Bandyopadhyay B. C., Liu X., Bollimuntha S., Brazer S. C., Combs C., Das S., Leenders A. G., Sheng Z. H., et al. Mol. Cell. 2004;15:635–646. doi: 10.1016/j.molcel.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Bezzerides V. J., Ramsey I. S., Kotecha S., Greka A., Clapham D. E. Nat. Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 32.Khasar S. G., Lin Y. H., Martin A., Dadgar J., McMahon T., Wang D., Hundle B., Aley K. O., Isenberg W., McCarter G., et al. Neuron. 1999;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petruska J. C., Napaporn J., Johnson R. D., Cooper B. Y. Neuroscience. 2002;115:15–30. doi: 10.1016/s0306-4522(02)00409-8. [DOI] [PubMed] [Google Scholar]
- 34.Burnstock G. J. Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai Y., Fukuoka T., Wang H., Yamanaka H., Obata K., Tokunaga A., Noguchi K. Pain. 2004;108:258–266. doi: 10.1016/j.pain.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 36.Koizumi S., Fujishita K., Inoue K., Shigemoto-Mogami Y., Tsuda M., Inoue K. Biochem. J. 2004;380:329–338. doi: 10.1042/BJ20031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dray A. Can. J. Physiol. Pharmacol. 1997;75:704–712. [PubMed] [Google Scholar]
- 38.Walker K., Bowes M., Panesar M., Davis A., Gentry C., Kesingland A., Gasparini F., Spooren W., Stoehr N., Pagano A., et al. Neuropharmacology. 2001;40:1–9. doi: 10.1016/s0028-3908(00)00113-1. [DOI] [PubMed] [Google Scholar]
- 39.Svensson P., Cairns B. E., Wang K., Arendt-Nielsen L. Pain. 2003;104:241–247. doi: 10.1016/s0304-3959(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 40.Lewin G. R., Rueff A., Mendell L. M. Eur. J. Neurosci. 1994;6:1903–1912. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 41.Brose N., Rosenmund C. J. Cell Sci. 2002;115:4399–4411. doi: 10.1242/jcs.00122. [DOI] [PubMed] [Google Scholar]
- 42.Vellani V., Mapplebeck S., Moriondo A., Davis J. B., McNaughton P. A. J. Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neugebauer V., Schaible H. G., Schmidt R. F. Pflügers Arch. 1989;415:330–335. doi: 10.1007/BF00370884. [DOI] [PubMed] [Google Scholar]
- 44.Ivanavicius S. P., Blake D. R., Chessell I. P., Mapp P. I. Neuroscience. 2004;128:555–560. doi: 10.1016/j.neuroscience.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 45.Robinson D. R., McNaughton P. A., Evans M. L., Hicks G. A. Neurogastroenterol. Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 46.Keller J. T., Marfurt C. F. J. Comp. Neurol. 1991;309:515–534. doi: 10.1002/cne.903090408. [DOI] [PubMed] [Google Scholar]
- 47.Zylka M. J., Rice F. L., Anderson D. J. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]