Abstract
The neurosteroid pregnenolone (PREG) and its chemically synthesized analog 3β-methoxypregnenolone (MePREG) bind to microtubule-associated protein 2 (MAP2) and stimulate the polymerization of microtubules. PREG, MePREG, and progesterone (PROG; the physiological immediate metabolite of PREG) significantly enhance neurite outgrowth of nerve growth factor-pretreated PC12 cells. However, PROG, although it binds to MAP2, does not increase the immunostaining of MAP2, contrary to PREG and MePREG. Nocodazole, a microtubule-disrupting agent, induces a major retraction of neurites in control cultures, but pretreatment with PREG/MePREG is protective. Decreasing MAP2 expression by RNA interference does not modify PROG action, but it prevents the stimulatory effects of PREG and MePREG on neurite extension, showing that MAP2 is their specific receptor.
Keywords: RNA interference, PC12, neurites, pregnenolone, neuroprotection
The brain is a target organ for steroid hormones (see ref. 1 for review). Intracellular receptors that are involved in the regulation of specific gene transcription have been identified in neuroendocrine structures, and they account for the many molecular events that are involved in steroid hormone action. However, the characterization of pregnenolone (PREG) in the rat brain (at higher concentrations than in blood) and its persistence after removal of steroidogenic endocrine glands (adrenals and gonads) (2) led to the discovery of a steroid biosynthetic pathway in the nervous system (3). PREG, which retains the carbon skeleton of cholesterol, is the precursor of steroid hormones, but does not bind to any nuclear receptor of steroid hormones (4). PREG sulfate was found to allosterically modulate several neurotransmitter receptors (namely GABA type A, NMDA-type glutamate, and sigma 1 receptors) (5); however, the neuromodulatory effects of neurosteroids could hardly account for their tentatively described neurotrophic and neuroprotective activities. Therefore, it was disappointing not to find receptors proper for the most abundant neurosteroid, PREG.
Rat brain cytosol contains a PREG-binding protein, which is identified as the microtubule associated protein 2 (MAP2), and PREG stimulates MAP2-driven microtubule assembly (6). Electron microscopic observation of PREG-induced microtubules shows their normal conformation, in contrast to microtubules that are assembled in presence of Taxol. Progesterone (PROG), which also binds to MAP2, does not stimulate microtubule polymerization, but counteracts the effect of PREG (as do competitive antagonists).
Microtubules are major structural components of the neuronal cytoskeleton, and they have an essential role in the elaboration of axons and dendrites (7). MAP2 has an important role in neuronal morphogenesis. The specific suppression of MAP2 synthesis prevents the neuronal differentiation of embryonic carcinoma cells exposed to retinoic acid, as shown by the absence of neurites (8).
The PC12 clonal rat pheochromocytoma cell line of neural crest origin, when exposed in culture to nerve growth factor (NGF), ceases to divide and undergoes neuronal differentiation characterized by the elongation of neuritic processes (9). MAP2 is present in PC12 cells during differentiation (10, 11), and there is a direct correlation between the increase of neurite length and the rate of microtubule assembly (12). Here, we examined the neurotrophic and neuroprotective activities of PREG, its 3-methylether derivative 3β-methoxypregnenolone (MePREG) (patent no. WO2004/067010) and PROG. Then, we used RNA interference (RNAi) to definitely demonstrate the involvement of MAP2 in the action of PREG and MePREG, but not of PROG, thus accounting for an uncharacterized mechanism of steroid action.
Results
Kinetics of Microtubule Polymerization in Vitro.
The experiments were carried out with either purified rat brain microtubules (1 mg/ml protein) or PC12 cell cytosol (4 mg/ml protein).
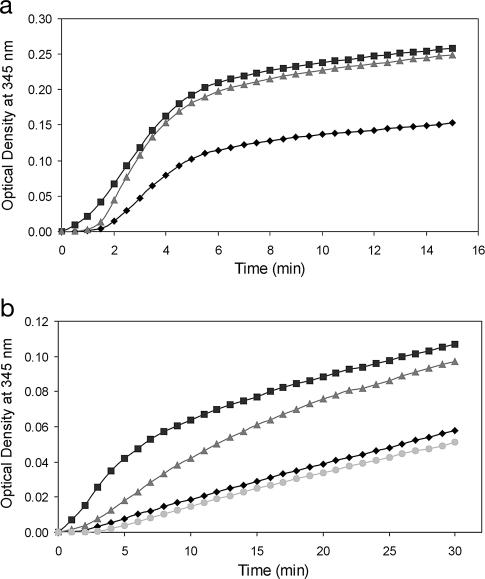
Control microtubules that were purified from rat brain started to polymerize after a time lag of ≈90 s, with a steep initial slope during the first 5 min, followed by a smooth increase up to a variation of OD (ΔOD) of 0.153 after 15 min (Fig. 1a). Rat brain microtubules that were incubated with either PREG or MePREG (40 μM) polymerized faster and reached a ΔOD that was almost the same with PREG and MePREG (0.249 and 0.258, respectively) after 15 min; these ΔODs were 63% or 68% larger than the control level, respectively. Moreover, both steroids increased the initial rate of microtubule polymerization. The effect of PROG was not assayed because it is known not to stimulate the polymerization of microtubules prepared from mammalian brain (6). The purpose of this experiment was to compare the effects of PREG and MePREG.
Fig. 1.
Effects of steroids on tubulin polymerization in vitro. Assembly of microtubules was monitored at 345 nm at 37°C and recorded every 30 s. The quantity of microtubule formed is proportional to the increase of OD (ΔOD). (a) Microtubule proteins purified from rat brain cytosol (1 mg/ml) were incubated either without steroid (black diamonds) or with 40 μM PREG (gray triangles) or 40 μM MePREG (black squares) for 15 min. Results represent means for six independent observations. (b) PC12 cell cytosol (4 mg/ml) was incubated without steroid (black diamonds) or with PREG (gray triangles), MePREG (black squares), or PROG (gray circles) (40 μM) for 30 min. Results represent means for three independent observations. PREG and MePREG stimulated microtubule polymerization in both conditions, whereas PROG was inactive.
The speed of formation and the amount of microtubules prepared from PC12 cell cytosol, even after prolonged incubation for 30 min, were definitely smaller than those of depolymerized rat brain microtubules and did not reach a plateau level (Fig. 1b). MePREG was more active than PREG (after 30 min, ΔODs were 84% and 67% larger than the control level, respectively). PROG did not stimulate microtubule polymerization.
Effects of Steroids on NGF-Induced PC12 Neurite Outgrowth.
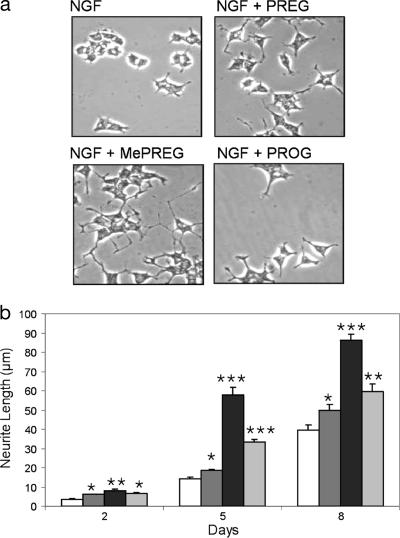
Observation of α-tubulin immunostaining suggested that PC12 cells, cultured in presence of NGF (10 ng/ml) and treated with either PREG or MePREG or PROG (30 μM) for 6 days, had longer neurites than those in control cultures (Fig. 2a).
Fig. 2.
PREG, MePREG, and PROG stimulate neurite outgrowth. (a) Steroid-mediated enhancement of neurite outgrowth in PC12 cells. Cells were treated with NGF (10 ng/ml) without or with PREG, MePREG, or PROG (30 μM) for 6 days and immunostained with anti-α-tubulin Ab. Neurite extension was markedly stimulated by all three steroids. (b) Time course of steroid-stimulated neurite outgrowth in PC12 cells. Cells were treated with NGF (white bar) (10 ng/ml) without or with PREG (dark gray bar), MePREG (black bar), or PROG (light gray bar) (30 μM) and were photographed after 2, 5, and 8 days. Neurite length was quantified from random photographs as described in Materials and Methods. Results represent means ± SEM for three independent observations. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 vs. controls (Newman–Keuls test, ANOVA). MePREG was the most active.
The average neurite length of PC12 cells was measured. Without NGF treatment, neither PREG nor MePREG nor PROG induced neurite outgrowth, whereas every one of these steroids stimulated neurite outgrowth of cells treated with NGF concentrations in the range of 10–100 ng/ml (data not shown). After exposure of PC12 cells to NGF (10 ng/ml) for 5 days, the average neurite length was 14 μm. In the presence of 30 μM PREG, it reached 19 μm; in the presence of 30 μM PROG, it reached 33 μm; and in the presence of 30 μM MePREG, it reached 58 μm (Fig. 2b). Neurite extension was continued at 8 days.
Protection Against Microtubule Depolymerization Induced by Nocodazole.
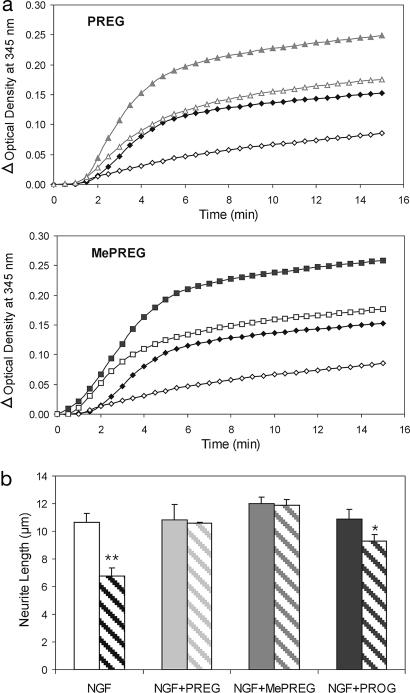
Nocodazole is a microtubule depolymerizing agent. There is one binding site for nocodazole per tubulin monomer, and nocodazole is bound specifically to the β-subunit (13), leading to disruption of microtubule assembly. Several concentrations of nocodazole were tested in the rat brain microtubule polymerization assay; maximal decrease of microtubule assembly (approximately half the control level) was achieved with 10 μM nocodazole (14). Then, microtubule assembly was monitored in the presence of both nocodazole and either 40 μM PREG or 40 μM MePREG (Fig. 3a). Nocodazole decreased the efficiency of both steroids to stimulate microtubule assembly. As a result, the amounts of microtubules formed remained slightly above the level that was achieved in the absence of both steroid and nocodazole. The effect of PROG was not investigated because it did not stimulate microtubule polymerization.
Fig. 3.
Steroids counteract nocodazole effects. (a) Inhibition of microtubule polymerization by nocodazole and partial protection by either PREG or MePREG. Rat microtubule assembly was monitored at 345 nm at 37°C and recorded every 30 s for 15 min without (filled symbols) or with (open symbols) 10 μM nocodazole, and without steroid (black diamonds) or with 40 μM PREG (gray triangles) or 40 μM MePREG (black squares). Results represent means for four independent observations. (b) Neuroprotection. Shortening of neurites by nocodazole and its prevention by steroids is shown. PC12 cells were grown in NGF (10 ng/ml) containing media for 3 days and then incubated with PREG, MePREG, or PROG (20 μM) for 1 h, followed by incubation with either DMSO (filled bars) or 30 μM nocodazole (hatched bars) for 15 min. Neurite length was quantified from random photographs as described in Materials and Methods. Results represent means ± SEM for three independent observations. ∗, P < 0.05; ∗∗, P < 0.01 vs. control (Newman–Keuls test, ANOVA). PREG and MePREG prevented neurite shortening; PROG was less effective.
The effect of nocodazole was tested also in PC12 cells (Fig. 3b). These cells were cultured for 3 days in the presence of NGF (10 ng/ml) to induce neurite outgrowth. Then, they were incubated without steroid (control cultures) or with 20 μM PREG or MePREG for 1 h (a short time of exposure to steroids that had no effect on neurite length). Then, 30 μM nocodazole was added for 15 min. In control cultures, nocodazole induced a prominent retraction of neurites from an average length of 10.6 μm to an average length of 6.75 μm. However, nocodazole was ineffective on PREG- or MePREG-pretreated cells.
MAP2 Immunostaining.
PC12 cells were kept in culture in the presence of NGF for 3 or 6 days without or with PREG, MePREG, or PROG. The MAP2–152 mAb was used. MAP2 is both soluble and bound to microtubules. In the conditions that were used, only the fraction of MAP2 that bound to microtubules was detectable by immunocytochemistry.
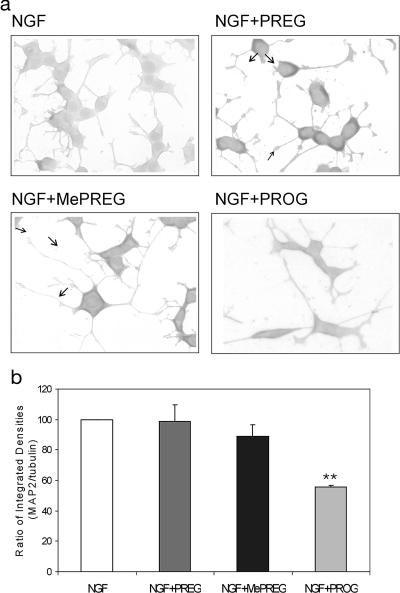
The intensity of MAP2 immunostaining in PC12 cells, grown in presence of NGF (10 ng/ml) for 3 days, was increased both in cell bodies and in neurites after exposure to 20 μM PREG or MePREG, but not to 20 μM PROG (Fig. 4a).
Fig. 4.
Immunostaining of MAP2 and of tubulin. (a) MAP2 immunostaining in PC12 cells. Cells were cultured for 3 days with 10 ng/ml NGF without or with PREG, MePREG, or PROG (20 μM). The anti-MAP2 152 mAb was used. PREG and MePREG increased the brown immunostaining of both cell bodies and neurites (arrows). However, immunostaining after PROG exposure was the same as in cells treated by NGF alone. (b) Densitometric analysis of MAP2 and α-tubulin Western blotting of PC12 cell extracts. Ratios of MAP2/tubulin integrated densities were set at 100% for control cultures. Results represent means ± SEM for four independent observations. ∗∗, P < 0.01 vs. control (Newman–Keuls test, ANOVA). There were no significant differences between PREG or MePREG-treated cells and control cultures, whereas the MAP2/tubulin ratio was decreased by PROG treatment.
In three independent experiments, Western blot analysis showed that, in the conditions that were used (60 μg of protein loaded on each lane), the relative amounts of tubulin and of MAP2, extracted from PC12 cells treated with NGF (10 ng/ml) for 6 days, did not change in the presence of 30 μM PREG or MePREG, as shown by the ratios of MAP2 to tubulin integrated densities, whereas 30 μM PROG led to a decrease of the MAP2/tubulin ratio (Fig. 4b). This observation does not indicate that PROG selectively stimulates tubulin synthesis because the amounts of tubulin were not increased in Western blot analysis. It is more likely that PREG and MePREG increased the fraction of MAP2 linked to tubulin, thus protecting it from proteolysis. Such protection did not occur with PROG, therefore leading to a decrease of MAP2 amount.
PC12 Neurite Outgrowth After MAP2 Suppression by RNAi.
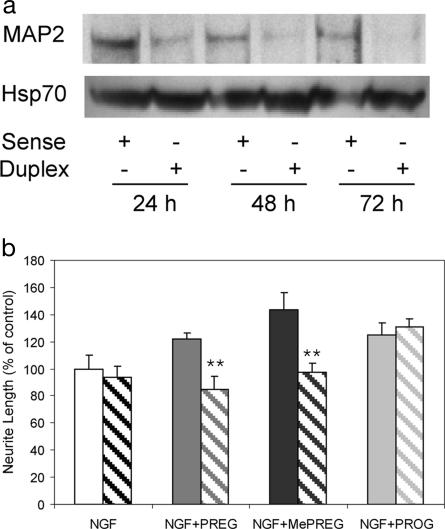
To provide more conclusive evidence for the role of MAP2 in steroid regulated neuritogenesis, the RNAi technology was used. Two 21-bp-long small interfering RNA (siRNA) duplexes specific of MAP2 transcripts were selected and sequentially used. The effectiveness of siRNA was shown by the decrease of MAP2 protein in NGF-treated cells, observed by immunoblotting analysis performed with the mAb AP20 by using Hsp70 as reference standard (Fig. 5a). The prominent decrease of MAP2 expression was accompanied by a complete loss of the stimulatory effects of PREG and MePREG on neurite extension, contrasting with an unchanged effect of PROG (Fig. 5b). Identical results were obtained with both MAP2–siRNAs. Experiments were also made with two times greater amounts of siRNA duplexes, which produced a major decrease of NGF-induced neurite extension (data not shown).
Fig. 5.
Inhibition of MAP2 expression by 21-nt siRNA in PC12 cells. Suppression of PREG/MePREG enhancement of neurite outgrowth is shown. (a) Immunoblotting of MAP2 (AP20 Ab) and of Hsp70: PC12 cells were cultured in the presence of NGF (50 ng/ml) for 11 days and then transfected with MAP2–siRNA duplex or sense oligonucleotide for 24–72 h. We loaded 60 μg of protein in each lane. (b) PC12 Cells were transfected by MAP2-siRNA sense single strand taken as control (filled bars) or by MAP2-siRNA duplex (hatched bars) for 6 h and treated with NGF (50 ng/ml) and either PREG or MePREG or PROG (30 μM) for 48 h. Results represent means ± SEM for three independent observations. ∗∗, P < 0.01 vs. sense control (Newman–Keuls test, ANOVA). The enhancement of neurite outgrowth by PREG and MePREG was completely suppressed by the MAP2–siRNA, whereas the effect of PROG was unchanged.
Discussion
The specific suppression of MAP2 synthesis with antisense oligonucleotides was shown to inhibit minor neurite formation in cultured cerebellar macroneurons (15) and the development of neuronal polarity in cultured hippocampal neurons (16), thus confirming that neuronal MAPs have an important role in neuronal morphogenesis. They promote the assembly and increase the stability of microtubules and form cross-bridge structures between microtubules (bundling) as well as between microtubules and other cytoskeletal elements, thus being involved in the extension of the dendritic tree. Conversely, the outgrowth of cytoplasmic elongations similar to neurites was observed when MAP2 was overexpressed in cultured nonneuronal cells (17).
The PC12 line is a convenient alternative to cultured neurons because it elongates neuritic processes when cultured in the presence of NGF, and the resulting extensions are filled with microtubules (18). MAP2 becomes detectable in PC12 cells after 4 days of exposure to NGF; then, its amount increases 5- to 7-fold when exposure to NGF lasts 12 days, concomitant with intense neurite growth.
The MAP2 family of MAPs is the only demonstrated target of the neurosteroid PREG, as shown by microtubule binding and microtubule polymerization experiments in vitro (6). The active concentration of PREG, in the range of 10 μm, may be of physiological significance in the context of neurosteroids synthesized in CNS and endowed with paracrine and/or autocrine activities (3, 5). The location of the PREG-binding site on MAP2 isoforms is not known, although preliminary experiments performed with recombinant MAP2C have indicated that this short isoform also binds PREG (E. Plassart-Schiess, V.F.-L., and E.-E.B., unpublished work). Another steroid, dehydroepiandrosterone, has been found to bind recombinant MAP2C (19), although no function was assigned to this binding. However, MAP2 purified from rat brain poorly binds dehydroepiandrosterone, a likely consequence of posttranslational modifications (6).
Here, we show that the microtubules prepared from the extracts of PC12 cells behave as rat brain microtubules as regards the stimulation of polymerization by PREG and MePREG. This effect in vitro is paralleled by the stimulation of neurite extension induced by both steroids in NGF-treated PC12 cells. An additional argument in favor of PC12 microtubules as the target of PREG and MePREG is the protective effect of both steroids against the microtubule retraction induced by nocodazole. However, MAP2 is not the only microtubule associated protein involved in the extension of neurites by PC12 cells. Tau is also involved in the stabilization of microtubules and consequently in neurite morphology (17). Tau antisense oligonucleotides decrease neurite extension of PC12 cells, whereas Tau overexpression renders neurites resistant to nocodazole (20). Attenuation of MAP1B expression by antisense oligonucleotides also inhibits initiation of neurite outgrowth (21).
Therefore, a direct demonstration of MAP2 involvement in the stimulation of neurite extension of PC12 cells was mandatory. The RNAi approach permitted such a demonstration when we used short siRNA duplexes to transfect PC12 cells that were treated with NGF or left untreated, as already shown with neurons (22). After 11 days of culture in presence of NGF (50 ng/ml), sufficient expression of MAP2 was achieved, permitting clear demonstration of high-molecular-weight MAP2 by Western blotting. Potent inhibition of MAP2 expression by transfection of siRNA duplexes occurred. The measurement of neurite extension was performed after only 2 days of culture, a convenient time interval in which to observe the stimulatory effects of steroids. At that time, the average length of neurites was almost unchanged by transfection of both siRNA duplexes, a likely consequence of persisting low levels of MAP2 expression or of compensatory roles of other MAPs (MAP1B and Tau), but the stimulatory effects of PREG and MePREG on neurite extension were completely lost. Therefore, we concluded that the stimulatory activity of both steroids on MAP2-induced microtubule polymerization is involved in the stimulation of neurite extension by PC12 cells, defining an intracellular signaling system.
PROG was reported to reduce apoptosis of PC12 cells after serum and NGF withdrawal (23). The effect of PROG was blocked by a PROG nuclear receptor antagonist. Stimulation of neurite extension by PROG, as described here, did not seem to be mediated by the PROG receptor because the active concentrations were much larger than those that were needed to activate a nuclear receptor. However, other types of PROG receptors have been described (24). Such a PROG effect did not seem to involve MAP2 because the siRNA-induced decrease of MAP2 expression did not impede the stimulatory activity of PROG.
In conclusion, PREG and its synthetic analog MePREG stimulate microtubule polymerization from rat brain and from PC12 cells. In pheochromocytoma cells that are exposed to NGF, both steroids stimulate the extension of neurites. RNAi experiments demonstrate that the inhibition of MAP2 expression results in the suppression of this stimulatory effect. This work describes a mechanism of neurosteroid action and shows that MAP2 is the target of PREG in this effect.
Materials and Methods
Animals.
Male adult Sprague–Dawley rats (body weight, 300 g) were obtained from Janvier (Le Genest-St-Isle, France). Animal care was in accordance with the European Communities Council Directive of November 24, 1986. Animals were killed by decapitation, and their entire brains were used immediately for the preparation of microtubules.
Steroids.
PREG and PROG were purchased from Sigma, and MePREG was either obtained from Steraloids (Newport, RI) or synthesized and guaranteed to be >97% pure by Roowin (Romainville, France)
Abs.
Anti-α-tubulin mAb SC 5286 was purchased from Santa Cruz Biotechnology. mAb 152 (25) was shown to react specifically with high-molecular-weight MAP2. The anti-Hsp70 mAb was purchased from StressGen Biotechnologies (Victoria, Canada), and anti-MAP2 mAb AP20 was purchased from Sigma.
Preparation of Microtubules and Assay of Microtubule Assembly.
Microtubules were prepared from the brains of 50 adult rats by a temperature-dependent in vitro assembly–disassembly procedure (26). Briefly, rat brains were homogenized in a volume of buffer A (0.1 M Mes/1 mM EGTA/0.1 mM EDTA/1 mM MgCl2/1 mM DTT/1 mM PMSF/1 mM GTP, pH 6.4) in a volume (expressed in milliliters) equal to the weight of the pool of brains (expressed in grams), for three 30-s pulses separated by 30-s intervals. After centrifugation of homogenate at 105,000 × g and 4°C for 1 h, 2 M glycerol was added to the supernatant, and the mixture was incubated at 37°C for 30 min. The polymerized preparation obtained was deposited on a cushion of buffer A, supplemented with 1.5 M glycerol. After centrifugation at 105,000 × g and 20°C for 2 h, the final pellets were rinsed with buffer A and stored at −80°C for later use. When required, microtubule pellets were unfrozen and incubated in buffer A at 4°C for 1 h. The solutions of depolymerized microtubules were clarified by centrifugation at 30,000 × g at 4°C for 35 min. Protein concentrations were determined by BC assay (Uptima Interchim, Montluçon, France). The microtubule proteins (1 mg of protein per ml) were incubated with either steroid at concentrations of 40 μM (final ethanol concentration, 1.3%) without or with nocodazole (Sigma) at a concentration of 10 μM (final DMSO concentration, 1%). Control incubations contained the same concentration(s) of solvent(s). Microtubule assembly was monitored by the increase of absorbance at 345 nm vs. time, with an Uvicon spectrophotometer (Kontron, Montigny-le-Bretonneux, France), equipped with an automatic six-sample changer thermostated at 37°C for either 15 or 30 min.
Culture of PC12 Cells.
Cells were plated on plastic dishes coated with poly(l-lysine) and maintained in DMEM supplemented with 10% horse serum and 5% FBS, in an incubator set at 37°C and 5% CO2. Cell culture was performed on 12-well plates (2 × 104 cells per well) for immunocytochemistry and quantification of neurite outgrowth and on six-well plates (3 × 105 cells per well) or 100-mm Petri dishes (1 × 106 cells per dish) for transfection assay and immunoblotting. After ≈6 h, the differentiated neuronal phenotype was induced by adding NGF (10 ng/ml), with or without test agent(s) in fresh DMEM supplemented with 2% horse serum and 1% FBS. All cultures were fed every 2 or 3 days with fresh medium. The steroids were added at concentrations of either 20 or 30 μM (the final concentration of ethanol was 0.1%). To study steroid effects on neurite retraction, cells were treated with NGF for 3 days and then incubated with steroids for 1 h, followed by 30 μM nocodazole for 15 min (dissolved in DMSO at a final concentration of 0.1%). Control cultures contained the same concentration(s) of solvent(s). At different time points during the treatment, cells were either photographed or fixed with 4% paraformaldehyde for later examination.
siRNA Preparation and Transfections.
siRNAs corresponding to MAP2 mRNAs were designed as recommended in ref. 27, with 5′ phosphate, 3′ hydroxyl, and 2-bp overhangs on each strand; they were synthesized by Dharmacon (MWG, Courtaboeuf, France). The following gene-specific sequences were used according to Krichevsky and Kosik (22): siRNA1, 5′-CAGGGCACCUAUUCAGAUAdTdT-3′ (sense) and 5′-UAUCUGAAUAGGUGCCCUGdTdT-3′ (antisense). The following second set of gene-specific sequences was designed according to the recommendations of Tuschl et al. (27): siRNA2, 5′-UUCGCUGAGCCUUUAGACAdTdT-3′ (sense) and 5′-UGUCUAAAGGCUCAGCGAAdTdT-3′ (antisense). Annealing for duplex siRNA formation was performed according to the manufacturer’s instructions.
Cells were maintained in DMEM supplemented with 10% horse serum and 5% FBS. Transfection experiments were initiated 3 or 4 days after plating. Lipofectamine (Invitrogen) diluted in DMEM was applied to the 21-nt duplexes (or to sense oligonucleotide used as control) diluted in OptiMEM, and the formulation was continued for 30 min. Transfections were performed in six-well plates coated with poly(l-lysine) for quantification of neurite outgrowth (3 × 105 cells per well, 7 μl of Lipofectamine, and 120 pmol of 21-bp duplex or sense RNA and DMEM to a final volume of 1 ml per well) and on 100-mm Petri dishes coated with poly(l-lysine) for immunoblot analyses (1.5 × 106 cells per dish, 40 μl of Lipofectamine, and 315 pmol of 21-bp duplex or sense RNA and DMEM to a final volume of 2.5 ml) for ≈6 h. For quantification of neurite outgrowth, 1 ml of DMEM was added to each well, the transfected cells were resuspended, and 0.5 ml of each suspension was seeded in 12-well plates, coated with poly(l-lysine) and containing 0.5 ml of DMEM supplemented with 20% horse serum, 10% FBS, and NGF (final concentration, 50 ng/ml). Steroids (30 μM) were added when indicated. Cells were photographed 48 h after the addition of steroid. For immunoblot analyses, 2.5 ml of DMEM supplemented with 20% horse serum, 10% FBS, and NGF (final concentration, 50 ng/ml) were added to each dish. Proteins were extracted at 24–72 h after transfection.
Measurement of Neurite Outgrowth.
Random field photographs of NGF-treated PC12 cells were analyzed with image (Scion, Frederick, MD). Average neurite length (mean ± SEM) was determined by measuring the longest neurite of at least 200 cells randomly selected in each of three wells.
Immunocytochemistry.
After two washes with PBS for 5 min, the cells were fixed with PFA on the bottom of Petri dishes, then incubated in PBS containing 3% BSA and 0.1% Triton X-100 at room temperature for 45 min. Cells were washed again then incubated at 4°C overnight in the presence of either anti-α-tubulin mAb (1:1,000 dilution) or anti-MAP2 mAb 152 (1:5,000 dilution). After two washes with PBS, rabbit anti-mouse Ig biotin-conjugated IgG, F(ab′)2 fragment (1:500 dilution; Roche Diagnostics) was added at room temperature for 45 min. After three washes, the streptavidin–peroxydase amplification complex (Vectastain kit; AbCys, Paris, France) was added at room temperature for 45 min. The cells were then washed, and the aminoethyl carbazole substrate (Sigma) was deposited on the cells for 15 min. The reaction was stopped with distilled water, and the cells were counterstained with hematoxylin and mounted in Glycergel (DAKO, Serotec).
Total PC12 Cell Extract and Cytosol Enriched in Microtubule Proteins.
PC12 cells (cultured to confluence on 100-mm dishes) were rinsed, removed by gentle trituration in PBS, and centrifuged at 500 × g for 4 min, and the cell pellets were kept at −80°C. For electrophoreses and immunoblot analyses, the frozen pellets were boiled for 5 min in sample buffer, containing 2% SDS, 5% 2-mercaptoethanol, 62.5 mM Tris·HCl (pH 6.8), and 10% glycerol (total cell extracts). To assay microtubule polymerization, the pellets were homogenized in a Dounce glass homogenizer with buffer A containing protease inhibitor mixture Complete (Roche Diagnostics), left at 4°C for 1 h to induce microtubule depolymerization, and centrifuged at 100,000 × g at 4°C for 45 min. The supernatants that were enriched in microtubule proteins (4 mg/ml) were assayed immediately or stored at −80°C until analysis. Protein concentrations were determined with the BC assay.
Western Blot Analysis.
Total cell extracts (60 μg of protein) were separated on 10% SDS/PAGE gels or on 3–8% Tris·acetate gels (NOVEX, San Diego; Invitrogen) and then transferred to nitrocellulose membranes. The membranes were blocked by incubation in 5% skim milk in TBS buffer (0.1% Tween 20/10 mM Tris/50 mM NaCl, pH 8.8) at room temperature for 1 h. All washes were performed in TBS buffer. The membranes were then incubated at 4°C overnight with all of the following mAbs (dilutions given in parentheses): anti-tubulin (1:500), anti-MAP2 152 (1:1,000), anti-MAP2 AP20 (1:200), and anti-Hsp70 (1:1,000). Incubation with the secondary goat anti-mouse Ig peroxydase-conjugated IgG F(ab′)2 fragment (1:10,000) (Perbio Science, Brebières, France) was performed in TBS buffer containing 5% skim milk for 45 min at room temperature. The signal was detected by the enhanced chemiluminescence system (Amersham Pharmacia), according to the manufacturer’s instructions, with Kodak X-Omat film and analyzed for quantification with image.
Statistical Analyses.
One-way ANOVA was applied to determine significant differences between control and treated conditions. The level of significance was set at P < 0.05. The Newman–Keuls post hoc test was used.
Acknowledgments
We thank Dr. K. Rajkowski (Institut National de la Santé et de la Recherche Médicale, Unité 788, Bicêtre) for critical reading of the manuscript. This work was supported by the Institute for the Study of Aging (New York), The Harold and Leila Y. Mathers Charitable Foundation (New York), and Association pour la Recherche sur le Cancer (Villejuif, France) Contract 3232.
Abbreviations
- NGF
nerve growth factor
- ΔOD
variation of OD
- MAP
microtubule-associated protein
- MePREG
3β-methoxypregnenolone
- PREG
pregnenolone
- PROG
progesterone
- RNAi
RNA interference
- siRNA
small interfering RNA
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.McEwen B. S., Coirini H., Westlind-Danielsson A., Frankfurt M., Gould E., Schumacher M., Woolley C. J. Steroid Biochem. Mol. Biol. 1991;39:223–232. doi: 10.1016/0960-0760(91)90067-f. [DOI] [PubMed] [Google Scholar]
- 2.Corpechot C., Synguelakis M., Talha S., Axelson M., Sjovall J., Vihko R., Baulieu E. E., Robel P. Brain Res. 1983;270:119–125. doi: 10.1016/0006-8993(83)90797-7. [DOI] [PubMed] [Google Scholar]
- 3.Baulieu E. E. Recent Prog. Horm. Res. 1997;52:1–32. [PubMed] [Google Scholar]
- 4.Evans R. M. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robel P., Schumacher M., Baulieu E. E. In: Neurosteroids: From Definition and Biochemistry to Physiopathologic Function. Baulieu E. E., Robel P., Schumacher M., editors. Totowa, NJ: Humana; 1999. pp. 1–25. [Google Scholar]
- 6.Murakami K., Fellous A., Baulieu E. E., Robel P. Proc. Natl. Acad. Sci. USA. 2000;97:3579–3584. doi: 10.1073/pnas.97.7.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada K. M., Spooner B. S., Wessells N. K. Proc. Natl. Acad. Sci. USA. 1970;66:1206–1212. doi: 10.1073/pnas.66.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinsmore J. H., Solomon F. Cell. 1991;64:817–826. doi: 10.1016/0092-8674(91)90510-6. [DOI] [PubMed] [Google Scholar]
- 9.Greene L. A., Tischler A. S. Proc. Natl. Acad. Sci. USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black M. M., Aletta J. M., Greene L. A. J. Cell Biol. 1986;103:545–557. doi: 10.1083/jcb.103.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer I., Richter-Landsberg C., Safaei R. Exp. Cell Res. 1991;194:195–201. doi: 10.1016/0014-4827(91)90354-w. [DOI] [PubMed] [Google Scholar]
- 12.Richter-Landsberg C., Landreth G. E., Shooter E. M. Dev. Neurosci. 1983–1984;6:32–44. doi: 10.1159/000112330. [DOI] [PubMed] [Google Scholar]
- 13.Black M. M., Greene L. A. J. Cell Biol. 1982;95:379–386. doi: 10.1083/jcb.95.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Head J., Lee L. L., Field D. J., Lee J. C. J. Biol. Chem. 1985;260:11060–11066. [PubMed] [Google Scholar]
- 15.Caceres A., Mautino J., Kosik K. S. Neuron. 1992;9:607–618. doi: 10.1016/0896-6273(92)90025-9. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Billault C., Engelke M., Jimenez-Mateos E. M., Wandosell F., Caceres A., Avila J. J. Neurosci. Res. 2002;67:713–719. doi: 10.1002/jnr.10161. [DOI] [PubMed] [Google Scholar]
- 17.Kaech S., Ludin B., Matus A. Neuron. 1996;17:1189–1199. doi: 10.1016/s0896-6273(00)80249-4. [DOI] [PubMed] [Google Scholar]
- 18.Luckenbill-Edds L., Van Horn C., Greene L. A. J. Neurocytol. 1979;8:493–511. doi: 10.1007/BF01214805. [DOI] [PubMed] [Google Scholar]
- 19.Laurine E., Lafitte D., Gregoire C., Seree E., Loret E., Douillard S., Michel B., Briand C., Verdier J. M. J. Biol. Chem. 2003;278:29979–29986. doi: 10.1074/jbc.M303242200. [DOI] [PubMed] [Google Scholar]
- 20.Esmaeli-Azad B., McCarty J. H., Feinstein S. C. J. Cell Sci. 1994;107:869–879. doi: 10.1242/jcs.107.4.869. [DOI] [PubMed] [Google Scholar]
- 21.Brugg B., Reddy D., Matus A. Neuroscience. 1993;52:489–496. doi: 10.1016/0306-4522(93)90401-z. [DOI] [PubMed] [Google Scholar]
- 22.Krichevsky A. M., Kosik K. S. Proc. Natl. Acad. Sci. USA. 2002;99:11926–11929. doi: 10.1073/pnas.182272699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLusky N. J., Chalmers-Redman R., Kay G., Ju W., Nethrapalli I. S., Tatton W. G. Neuroscience. 2003;118:741–754. doi: 10.1016/s0306-4522(02)00940-5. [DOI] [PubMed] [Google Scholar]
- 24.Boonyaratanakornkit V., Edwards D. P. Essays Biochem. 2004;40:105–120. doi: 10.1042/bse0400105. [DOI] [PubMed] [Google Scholar]
- 25.Kalil J., Fellous A., Fellous M. In: Culture de Cellules Animales, Méthodologies, Applications. Adolphe M., Barlovatz-Meimon G., editors. Paris: Institut National de la Santé et de la Recherche Médicale; 1988. pp. 101–130. [Google Scholar]
- 26.Fellous A., Francon J., Lennon A. M., Nunez J. Eur. J. Biochem. 1977;78:167–174. doi: 10.1111/j.1432-1033.1977.tb11726.x. [DOI] [PubMed] [Google Scholar]
- 27.Elbashir S. M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Nature. 2001;24:428–429. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]