Abstract
Mature bone-resorbing osteoclasts (OCs) mediate excessive bone loss seen in several bone disorders, including osteoporosis. Here, we showed that reveromycin A (RM-A), a small natural product with three carboxylic groups in its structure, induced apoptosis specifically in OCs, but not in OC progenitors, nonfunctional osteoclasts, or osteoblasts. RM-A inhibited protein synthesis in OCs by selectively blocking enzymatic activity of isoleucyl-tRNA synthetase. The proapoptotic effect of RM-A was inhibited by neutralization or disruption of the acidic microenvironment, a prominent characteristic of OCs. RM-A was incorporated in OCs but not in nonfunctional osteoclasts and OC progenitors in neutral culture medium. Effects of RM-A on OC apoptosis increased under acidic culture conditions. RM-A not only was incorporated, but also induced apoptosis in OC progenitors in acidic culture medium. RM-A inhibited osteoclastic pit formation, decreased prelabeled 45Ca release in organ cultures, and antagonized increased bone resorption in ovariectomized mice. These results suggested that preventive effects of RM-A on bone resorption in vitro and in vivo were caused by apoptosis through inhibition of isoleucyl-tRNA synthetase in OCs and that specific sensitivity of OCs to RM-A was due to the acidic microenvironment, which increased cell permeability of RM-A by suppressing dissociation of protons from carboxylic acid moieties, making them less polar. This unique mechanism suggested that RM-A might represent a type of therapeutic agent for treating bone disorders associated with increased bone loss.
Keywords: isoleucyl-tRNA synthetase, inhibitor, antiresorptive agent
Mature osteoclasts resorb bone and mediate excessive bone loss seen in several bone disorders, including osteoporosis, arthritis, periodontitis, bone metastasis, and corticosteroid-induced bone loss (1–4). Mature bone-resorbing osteoclasts (OCs) exhibit highly specialized morphological structures, such as actin rings, clear zones, and ruffled borders, which are functional markers of OCs (2–5). These structures permit OCs to establish an isolated acidic microenvironment between themselves and the bone surface. The acidic microenvironment formed by V-ATPase on the ruffled borders of OCs allows dissolution of bone minerals and degradation of the bone matrix by proteases (6, 7). Therefore, these specialized features in OCs represent major potential targets for reducing OC activity and, consequently, could be useful for treatment of bone disorders. In addition, recent studies showed that receptor activator of NF-κB ligand (RANKL), which is an essential factor for OC differentiation and activation, was one of the major targets of anti-resorptive agents (2).
Two types of antiresorptive drugs targeting OCs, i.e., bisphosphonates (BPPs) and calcitonin (CT), are used for clinical treatment of osteoporosis. BPPs, the most effective class of antiresorptive drugs available, inhibit bone resorption by suppressing functions of OCs and by shortening their lifespan (8–10). Because of targeting of BPPs to bone minerals and the ability of OCs to incorporate and then release BPPs accumulated in bone, BPPs decrease overall bone turnover by continuous, rather than transient, inhibition of bone resorption (9–11). As a result, because of this sustained suppression of bone turnover, BPPs decrease beneficial effects of anabolic therapy and therefore cannot be effectively used in combination with the bone anabolic agent parathyroid hormone (PTH) (12, 13). CT inhibits functions of OCs without affecting their survival (14). Although CT transiently inhibits bone resorption without decreasing osteoblastic bone formation, continuous treatment with CT eventually leads to a decrease in its inhibitory effects, because of down-regulation of CT receptors on OCs (15). Therefore, pharmaceutical companies are developing new classes of antiresorptive drugs that specifically target OCs but show different mechanisms of action from those of BPPs and CT.
To develop a class of antiresorptive drugs, we established a screening program to identify small natural molecules targeting OCs. Through this program, we found that reveromycin A (RM-A), which is a small natural product isolated from the genus Streptomyces (16), induced apoptosis specifically in OCs. Here, we showed that RM-A inhibited bone resorption in vitro and in vivo by inducing apoptosis specifically in OCs. These findings suggested that RM-A might be a unique antiresorptive agent for the treatment of bone disorders, including osteoporosis.
Results
RM-A Induces Cell Death Specifically in OCs in Cell Cultures.
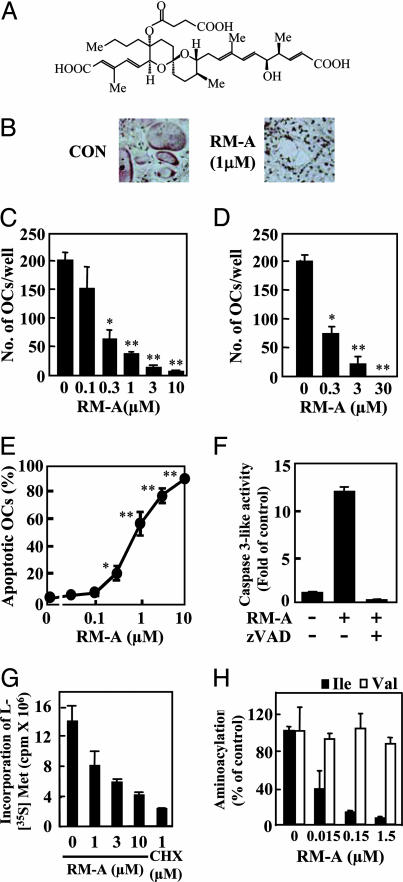
To identify small agents that target OCs, we examined effects of a number of natural products (>3,000 compounds) on OCs. We found that RM-A (Fig. 1A) dose-dependently decreased numbers of OCs remaining on culture plates without affecting osteoblasts (Figs. 1 B and C). RM-A also inhibited survival of purified OCs (Fig. 1D) but did not affect early differentiation of progenitor cells into mononuclear osteoclasts or the fusion of mononuclear osteoclasts into OCs (data not shown). We next determined effects of RM-A on viability of several other cell types using MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide] assays. RM-A inhibited survival of purified OCs with an IC50 value of 0.2 μM. The ED50 of RM-A on bone marrow cells, calvarial osteoblasts, and a number of other human, mouse, and rat cell lines, such as monocytes, macrophages, and immune cell lines including OC progenitor cells (RAW 264 cells), was at least 100-fold higher than that for purified OCs (Table 1, which is published as supporting information on the PNAS web site). This finding indicated that effects of RM-A were highly specific for OCs.
Fig. 1.
RM-A induces apoptosis in OCs through inhibition of protein synthesis. (A) Structure of RM-A. Crude OCs prepared from cocultures of bone marrow cells and osteoblasts in the presence of 1α,25(OH)2D3 were treated with or without RM-A for 24 h. (B) Cells were fixed and stained for TRAP. (C) Remaining TRAP (+) OCs with more than three nuclei were counted. Data are expressed as means ± SD for four cultures. ∗, P < 0.01; ∗∗, P < 0.001 vs. control. (D) Purified OCs were treated with or without RM-A in the presence of RANKL (100 ng/ml) for 24 h. Remaining TRAP (+) OCs with more than three nuclei were counted. Data represent means ± SD for four cultures. ∗, P < 0.01; ∗∗, P < 0.001 vs. control. (E) Purified OCs were treated with or without RM-A in the presence of RANKL (100 ng/ml) for 12 h. Cells were fixed and stained for TRAP, and nuclei were visualized by Hoechst 33258 staining. OCs with normal and condensed nuclei were counted, and percentages of OCs with condensed nuclei compared with those with normal nuclei are shown as apoptotic OCs. Data are expressed as means ± SD for four cultures. ∗, P < 0.05; ∗∗, P < 0.001 vs. control. (F) Purified OCs were cultured for 1 h with or without zVAD-fmk (50 μM) in the presence of RANKL (100 ng/ml) and then treated for 4 h with or without RM-A (1 μM). Cell lysates were used for measurement of caspase 3-like enzyme activity. Data are expressed as means ± SD for three cultures. (G) Protein synthesis was measured by amounts of incorporated l-[35S]methionine into cellular proteins. OCs, formed from a RAW 264 cell culture, were treated for 4 h with appropriate concentrations of RM-A or cycloheximide (CHX). (H) OCs prepared from bone marrow cell cultures in the presence of macrophage colony-stimulating factor (30 ng/ml) and RANKL (300 ng/ml). In vitro aminoacyl-tRNA synthetase assays were carried out in the presence of 0.015, 0.15, and 1.5 μM RM-A. Data are expressed as means ± SD for three cultures.
RM-A Inhibits Incorporation of l-[35S]Methionine into Proteins and Isoleucyl-tRNA Synthetase (IleRS) in OCs.
Recently, IleRS was shown to be a major target of RM-A action in yeasts (17); therefore, we first examined effects of RM-A on total protein synthesis in OCs. RM-A dose-dependently inhibited incorporation of l-[35S]methionine into proteins in OCs (Fig. 1G) (IC50 = 2 μM). Next, we examined effects of RM-A on IleRS activity in vitro. Extracts prepared from OCs were assayed for their ability to catalyze the transfer of [3H]isoleucine into t-RNA in the presence or absence of RM-A. RM-A decreased aminoacylation activity of IleRS in a dose-dependent manner (Fig. 1H) (IC50 = 10 nM). In contrast, aminoacylation activity of ValRS, which, like IleRS, is a type I aminoacyl-tRNA synthetase, was not affected by addition of RM-A (Fig. 1H). These results suggested that inhibition of aminoacylation by RM-A was specific to IleRS.
RM-A Mediated OC Cell Death Resulting from Apoptosis.
Several approaches were used to determine whether RM-A caused OC cell death through apoptosis. Hoechst staining revealed that RM-A dose-dependently increased apoptotic OCs showing condensed nuclei, with an IC50 of ≈0.7 μM (Fig. 1E). Next, we determined the effects of RM-A on activation of caspase 3 and release of cytochrome c from mitochondria into the cytosol. RM-A induced activation of caspase 3-like enzyme activity (Fig. 1F). Using Western blot analysis, we confirmed that RM-A induced a decrease in the amount of caspase 3 proform, while increasing that of the active form (Fig. 5A, which is published as supporting information on the PNAS web site). RM-A also induced mitochondrial release of cytochrome c (Fig. 5B). Caspase 3 activation induced by RM-A was inhibited by zVAD-fmk (Fig. 1F), a general caspase inhibitor, whereas cytochrome c release was not inhibited. These findings suggested that RM-A acting on OC mitochondria results in release of cytochrome c, resulting in activation of the caspase 3 and the downstream events of apoptosis.
Apoptotic Effect of RM-A Is Highly Specific for OCs.
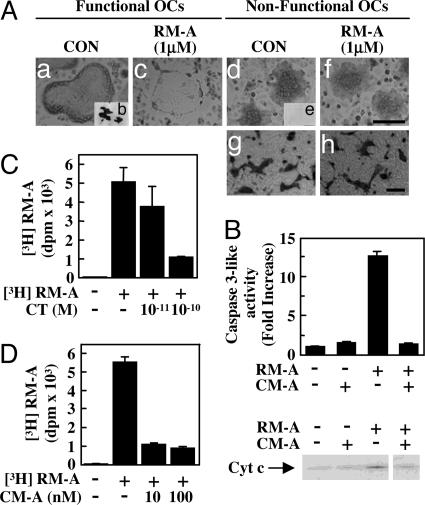
To understand mechanisms of specific effects of RM-A on OCs, we determined whether OCs with an actin ring, which is a functional marker for OCs, were affected by RM-A. RM-A induced apoptosis in OCs with an actin ring, which could form pits on dentine slices (Fig. 2 Aa–Ac). However, RM-A did not induce apoptosis in nonfunctional osteoclasts, which lacked an actin ring and were unable to form pits (Fig. 2 Ad–Af). CT is known to disrupt actin rings in OCs. RM-A did not induce apoptosis in CT-treated nonfunctional osteoclasts (Fig. 2 Ag and Ah). Survival of nonfunctional osteoclasts, which were formed on a collagen gel, was also unaffected by RM-A (data not shown). An acidic microenvironment, another important functional marker of OCs, is formed by targeted secretion of protons by V-ATPase on ruffled borders. We examined whether effects of RM-A were affected by disruption in the acidic microenvironment using concanamycin A (CM-A), a V-ATPase inhibitor that is known to inhibit OC function by disrupting the acidic microenvironment of OCs (18). Acridine orange exhibits an orange fluorescence in acidic pH whereas, at other pHs, it changes to green. Acidic organelles with orange fluorescence in areas surrounding the OC membranes were rarely observed in cultures treated with CM-A (Fig. 6 a and b, which is published as supporting information on the PNAS web site). RM-A did not induce apoptosis in CM-A-treated OCs (Figs. 6 b, d, and f). CM-A also prevented increased caspase 3-like enzyme activity and the release of cytochrome c induced by RM-A (Fig. 2B). These results showed that RM-A selectively induced apoptosis in OCs. To determine whether this specific effect of RM-A depended on RM-A uptake, we examined incorporation of RM-A into OCs using 3H-labeled RM-A. [3H]RM-A was incorporated into OCs, but uptake of [3H]RM-A was decreased in CT-treated OCs (Fig. 2C). CM-A-treated OCs did not incorporate [3H]RM-A (Fig. 2D). These results indicated that selective incorporation of RM-A was involved in the specific effects of RM-A on OCs.
Fig. 2.
RM-A is incorporated into OCs. (A) TRAP (+) mononuclear osteoclasts prepared from cocultures (for 3 days) were cultured for 10 h on culture plates (Aa, Ac, Ad, and Af) and then treated for 8 h with or without RM-A (1 μM) in the presence or absence of RANKL (100 ng/ml) (Aa and Ac) or macrophage colony-stimulating factor (100 ng/ml) (Ad and Af). After culture, cells were fixed and stained for TRAP. TRAP (+) mononuclear osteoclasts were cultured on dentine slices (Ab and Ae) for 24 h. After culture, pits were stained with hematoxylin. (Scale bar: 10 μm.) Crude OCs prepared from cocultures were pretreated with CT (1 nM) and incubated with or without RM-A for 24 h. Cells were fixed and stained for TRAP (Ag and Ah). (Scale bar: 100 μm.) (B) Purified OCs were cultured for 1 h with or without CM-A (100 nM) in the presence of RANKL (100 ng/ml) and treated for 4 h with or without RM-A (1 μM). Cell lysates were used for measurement of caspase 3-like enzyme activity, and cytosolic extracts were used for Western blotting to detect cytosolic cytochrome c. Purified OCs were cultured with CT (C) or CM-A (D) for 1 h and then were treated with or without [3H]RM-A (5 μCi/ml) for 1.5 h. After treatment, cells were washed, and incorporation of [3H]RM-A into OCs was counted by using a liquid scintillation counter. Data are expressed as means ± SD for three cultures.
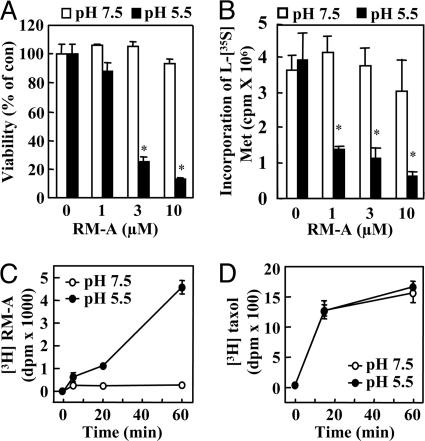
Acidic Culture Medium Promotes RM-A-Induced Apoptosis in OCs and Permits RM-A to Affect Nonfunctional Osteoclasts and OC Progenitor Cells (RAW 264 Cells).
Because RM-A contains three carboxylic groups (Fig. 1A), its specificity for OCs may result from increased cell permeation under acidic conditions. To test this possibility, we determined effects of pH of the medium on RM-A-induced apoptosis in OCs, nonfunctional osteoclasts, and OC progenitor cells (RAW 264 cells). In neutral (pH 7.5) culture medium, RM-A (0.1 μM) did not alter numbers of OCs, but, in acidic (pH 5.5) culture medium, RM-A (0.03 μM) decreased cell numbers by ≈50%. RM-A (1 μM) increased caspase 3-like enzyme activity in nonfunctional osteoclasts under acidic conditions, but not under neutral conditions (Fig. 7A, which is published as supporting information on the PNAS web site). Viability of RAW 264 cells was not affected by RM-A under neutral conditions, but decreased by ≈80% by RM-A (3 μM) under acidic conditions (Fig. 3A). In addition, caspase 3-like enzyme activity in RAW 264 cells was also stimulated by RM-A (3 μM) under acidic conditions, but not at neutral pH (Fig. 7B). Furthermore, inhibitory activity of RM-A on protein synthesis in RAW 264 cells at pH 5.5 medium was greater than that at pH 7.5 medium (Fig. 3B). In contrast, there was no difference in inhibitory effect of cycloheximide on protein synthesis at pH 5.5 and pH 7.5 (data not shown). In addition, to investigate whether an acidic microenvironment was required for RM-A uptake into cells, uptake of [3H]RM-A was measured in RAW 264 cells. [3H]RM-A was incorporated into RAW264 cells in acidic (pH 5.5) culture medium, but uptake of [3H]RM-A did not occur in neutral (pH 7.5) culture medium (Fig. 3C). On the other hand, [3H]taxol was similarly incorporated into cells under acidic and neutral conditions (Fig. 3D). These results supported the conclusion that specificity of RM-A for OCs resulted from the presence of an acidic microenvironment around these cells that promoted cell permeability of RM-A.
Fig. 3.
Acidic culture medium enhances sensitivity to RM-A in OC progenitor cells. (A) RAW 264 cells were seeded and treated for 10 h with RM-A in acidic (pH 5.5) or neutral (pH 7.5) medium and then cultured for 14 h without RM-A. Cell viability was measured by using an MTT assay. Data are expressed as means ± SD for four cultures. ∗, P < 0.01 vs. control. (B) Protein synthesis was measured by amounts of incorporated l-[35S]methionine into cellular proteins. RAW 264 cells were incubated for 4 h with appropriate concentrations of RM-A in acidic (pH 5.5) or neutral (pH 7.5) medium. Data are expressed as means ± SD for four cultures. ∗, P < 0.01 vs. control. RAW 264 cells were treated with [3H]RM-A (2.5 μCi/ml) (C) or [3H]taxol (0.1 μCi/ml) (D) in acidic (pH 5.5) or neutral (pH 7.5) medium. After treatment, cells were washed, and incorporation of drugs into RAW 264 cells was counted by using a liquid scintillation counter. Data are expressed as means ± SD for three cultures.
RM-A Inhibits Bone Resorption in Vitro and in Vivo.
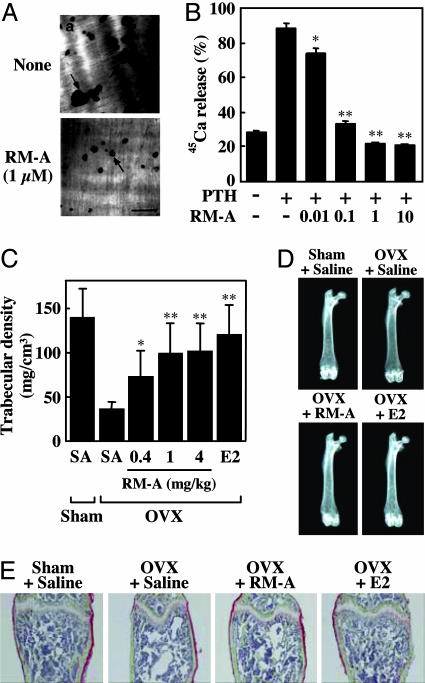
We next examined effects of RM-A on bone resorption in cell and organ cultures. RM-A inhibited pit formation (number and size) by OCs on dentine slices during a 24-h treatment (Fig. 4A). When 45Ca prelabeled fetal rat long bones were treated in the presence of RM-A for 3 days, RM-A (0.01–10 μM) dose-dependently inhibited PTH-stimulated 45Ca release (Fig. 4B). RM-A (0.1 μM) also inhibited IL-1β and -1α, 25(OH)2D3-stimulated 45Ca release by 80%. Effective RM-A concentration for inhibition of bone resorption corresponded to that for inducing cell death in OCs, suggesting that inhibitory effects of RM-A resulted from induction of OC cell death.
Fig. 4.
RM-A inhibits bone resorption in vitro and in vivo. (A) Crude OCs prepared from cocultures of bone marrow cells and osteoblasts were cultured on dentine slices for 24 h with (Ab) or without (Aa) RM-A (1 μM). At the end of the culture, resorption pits formed by OCs were stained with hematoxylin. Arrows indicate resorption pits. (Scale bar: 100 μm.) (B) 45Ca-labeled fetal rat limb bones were incubated with various concentrations of RM-A (0.01–10 μM) in the presence or absence of PTH (10 nM). Bone-resorbing activity was expressed as the percentage of incorporated 45Ca released into the medium. Data are expressed as means ± SD for four cultures. ∗, P < 0.05; ∗∗, P < 0.001 vs. PTH. RM-A (0.4, 1, and 4 mg/kg body weight twice daily), E2 (0.01 μg/day), or saline was administered s.c. to OVX mice starting 1 day after ovariectomy. After 4 weeks of treatment, mice were killed, and femora were removed for analysis of bone density and structure. (C) Trabecular bone density of distal femora was measured by peripheral quantitative computed tomography. Results were obtained from five mice per group and are expressed as means ± SD. ∗, P < 0.05; ∗∗, P < 0.01 vs. OVX plus saline. (D) Radiographic photographs of femora taken by soft x-ray. (E) Histological appearance of distal femora. Undecalcified bone sections were subjected to Villanueva Goldner staining (green, mineralized bone tissues). OVX + RM-A, OVX plus 4 mg/kg RM-A.
To examine pharmacokinetic profiles of RM-A, RM-A was administered i.v., orally (p.o.), i.p., or s.c. to Sprague–Dawley rats. We found that RM-A was rapidly eliminated from the blood after i.v., i.p., or s.c. administration and was minimally absorbed orally (Table 2, which is published as supporting information on the PNAS web site). Therefore, RM-A was administered twice daily (b.i.d.), i.v. or s.c. in animal experiments. Histomorphometric analysis showed that RM-A significantly decreased numbers of OCs without affecting numbers of osteoblasts in normal Sprague–Dawley rats (Fig. 8, which is published as supporting information on the PNAS web site). It was also shown that bone formation-related parameters declined in RM-A-treated rats compared with saline-treated rats. However, in vitro analysis showed that there were no differences in profiles between mineralization and proliferation in RM-A-treated osteoblasts (data not shown), suggesting that decreased bone formation activity resulted from an indirect action of RM-A, or the coupling mechanism of bone formation and bone resorption. We next examined whether RM-A inhibited bone resorption in ovariectomized (OVX) mice, an in vivo experimental model of postmenopausal osteoporosis. OVX mice were treated with RM-A at 0.4, 1, or 4 mg/kg body weight twice daily for 4 weeks. Femora were then subjected to radiographic analysis and showed a marked loss of mineralized cancellous bone, especially in the distal metaphysis of the femur, in OVX mice (Fig. 4D). Treatment with RM-A or 17β-estradiol (E2) markedly prevented bone loss in the distal metaphysis in OVX mice (Fig. 4D). Villanueva Goldner's staining of distal femora also showed that RM-A as well as E2 effectively prevented OVX-induced loss of cancellous bone (Fig. 4E). As expected, RM-A and E2 caused a significant protective effect on trabecular density (Fig. 4C). Additionally, we did not observe any damage to either the stomach or the kidneys, nor significant change of body weight of the mice that received RM-A (Fig. 9 A and B, which is published as supporting information on the PNAS web site). These results suggested that RM-A inhibited bone resorption in vivo through the induction of OC cell death.
Discussion
RM-A, a small natural molecule isolated from the genus Streptomyces, has been reported to show inhibitory activity on EGF-dependent responses of mouse epidermal cells, antiproliferative activity on human tumor cells, inhibitory activity on protein synthesis in mammalian cells, and antifungal activity (16, 19–22). Recently, IleRS was shown to be a major target of RM-A action in yeasts (17). RM-A was also shown to inhibit antigen receptor-mediated antigen presentation by B lymphoma cells (23). In the present study, we showed that RM-A induced OC cell death and then inhibited bone resorption. RM-A inhibited protein synthesis in OCs by blocking aminoacylation activity of IleRS. It also caused apoptosis through induction of cytochrome c release and caspase 3 activation. Recently, it has been reported that OC survival was highly sensitive to continuous de novo protein synthesis, which is not commonly found in most other cells (24). These facts indicate that induction of apoptosis by RM-A in OCs is caused by inhibition of protein synthesis through blocking activity of IleRS. Identification of proteins whose translations are inhibited by RM-A is still under consideration.
An acidic extracellular microenvironment is the final differentiated characteristic of OCs. It is also a prerequisite feature for OCs to induce bone resorption, because low pH is necessary for degradation of bone tissue (6, 7). OCs generate an acidic microenvironment (pH 4.5 or less) in the attachment zone between cells and on the base of culture dishes (25). RM-A contains three carboxylic groups, and its pKa value (4.2) is close to the acidic pH generated by OCs on culture dishes (17). In the present study, RM-A induced apoptosis only in functional OCs that could generate an acidic microenvironment. RM-A did not induce apoptosis, even in OCs having an actin ring, when V-ATPase-induced acidification was inhibited by CM-A. These observations led us to hypothesize that specificity of RM-A for OCs might result from acidic conditions that increased amounts of nonpolar forms of RM-A, which are more cell permeable. This hypothesis was supported by the following facts: (i) RM-A was incorporated into OCs, but not into nonfunctional osteoclasts; (ii) under acidic (pH 5.5) culture conditions, RM-A was not only incorporated, but also it induced apoptosis in OC progenitor cells and in OCs lacking an actin ring; (iii) under acidic (pH 5.5) culture conditions, RM-A also inhibited protein synthesis in RAW 264 cells; and (iv) growth-inhibiting activity of RM-A on yeasts was shown to be highly dependent on pH of the medium. RM-A (4.5 μM) was shown to inhibit yeast growth at pH 4.5, but not at pH 6.4 (even at a concentration of 100 μg/ml) (26). Therefore, the apoptotic effect of RM-A is highly specific to OCs, and specificity depends upon its carboxylic acid moieties.
RM-A suppressed pit formation by OCs on dentine slices and PTH-stimulated 45Ca release in organ cultures. Furthermore, RM-A dramatically decreased numbers of OCs in the proximal tibiae of rat fed a low-Ca diet (data not shown) and prevented bone loss in the distal metaphysis in OVX mice. BPPs are the most potent therapeutic inhibitors of bone resorption and are widely used for the treatment of osteolytic diseases such as osteoporosis (27). BPPs accumulate in bone minerals, are incorporated into OCs, and then induce disruption of actin rings and apoptosis of OCs. Because of BBP accumulation in bone minerals, inhibitory effects of BPPs on bone resorption are sustained in vivo. Recent studies showed that alendronate, one BPP, impaired the ability of PTH to increase bone mineral density in both women and men with osteoporosis (12, 13). RM-A, which does not accumulate in bone minerals, may be a unique candidate among antiresorptive agents for combination treatment with anabolic drugs.
In summary, RM-A specifically induced apoptosis of OCs through inhibition of protein synthesis and inhibited bone resorption in vitro and in vivo. Specific effects of RM-A on activated OCs may be due to OC-generated acidic conditions that might suppress dissociation of protons from carboxylic acid moieties of RM-A and increase its cell permeation. These findings suggested that RM-A represented a class of antiresorptive drugs whose action and specificity were different from those of BPPs or CT, which could be useful for the treatment of bone diseases such as osteoporosis, rheumatoid arthritis, hypercalcemia, Paget's disease, and tumor metastasis.
Materials and Methods
Animals and Chemicals.
ddY mice and Sprague–Dawley rats were obtained from CLEA Japan (Tokyo). Experimental procedures and housing conditions for animals were approved by the Animal Experiment Committee of RIKEN or the Animal Care Committee of Showa University, and all of the animals were cared for and treated humanely in accordance with the Guidelines for Experiments Using Animals. Recombinant human macrophage colony-stimulating factor (Leukoprol) and recombinant human soluble form of RANKL (sRANKL) were purchased from Welfide (Osaka) and PeproTech (London), respectively. Human PTH was purchased from Peptide Institute (Osaka). 45CaCl2 and [3H]taxol were from Amersham Pharmacia Biotech and Moravek Biochemicals (Brea, CA), respectively. l-[35S]methionine was from Amersham Pharmacia. [3H]isoleucine and [3H]valine were from PerkinElmer Life and Analytical Science. [3H]RM-A was synthesized at BlyChem (Billingham, U.K.), and its specific activity was 14 Ci/mmol (1 Ci = 37 GBq). PD98059 (a specific mitogen-activated protein kinase kinase inhibitor) was from Calbiochem.
Preparation of OCs.
Murine tartrate-resistant acid phosphatase-positive [TRAP (+)] OCs were prepared from a coculture system as described by Takahashi et al. (28). OCs and nonfunctional osteoclasts were prepared by culturing TRAP (+) mononuclear osteoclasts, which were collected from cocultures, in the presence of RANKL and macrophage colony-stimulating factor, respectively, and as described (29). Purified OCs were prepared from crude OCs of cocultures on plastic dishes as described by Jimi et al. (30). In some experiments, OCs were prepared by culturing RAW 264 cells (RIKEN cell bank, Tsukuba, Japan) in the presence of RANKL (50 ng/ml) and PD98059 (20 μM) for 4 days, as described by Hotokezaka et al. (31).
Cell Staining.
Staining for TRAP was carried out as described by Takahashi et al. (28). Nuclei in OCs were visualized by Hoechst 33258 staining. Acidified organelles were visualized by acridine orange staining. Stained cells were detected with a fluorescence microscope (Zeiss).
Cell Viability.
Cell viability was measured by using an MTT assay. All cell lines shown in Table 1 were seeded in 96-well plates and incubated overnight. For detailed procedures, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Measurement of Total Protein Synthesis.
Effects of RM-A on protein synthesis in mammalian cells were determined by measuring incorporation of l-[35S]methionine into cellular proteins. For detailed procedures, see Supporting Materials and Methods.
In Vitro Aminoacyl-tRNA Synthetase Assay.
Aminoacyl-tRNA synthetase activities were assayed in a reaction buffer containing cell extract (250 μg of protein) with 1 μM isoleucine, and 10 μCi/ml [3H]isoleucine for the IleRS; and with 1 μM valine, and 10 μCi/ml [3H]valine for valyl-tRNA synthetase. For detailed procedures, see Supporting Materials and Methods.
Caspase Activity Measurement.
RAW 264 cells or OCs were cultured with or without RM-A for 6 h in α-MEM adjusted to pH 7.5 or pH 5.5. Lysate preparation and caspase activity measurement are reported in Supporting Materials and Methods.
Western Blotting.
For detailed procedures, see Supporting Materials and Methods.
Determination of RM-A Uptake.
RAW 264 cells or purified OCs were cultured in the presence of [3H]RM-A or [3H]taxol for appropriate times. After treatment, cells were washed three times with ice-cold PBS and dissolved by using 0.25 M NaOH. Incorporation of drugs into cells was assessed with a liquid scintillation counter.
Bone Resorption Assay in Cell and Organ Cultures.
Crude OCs were placed on 96-well culture plates containing dentine slices (4 mm in diameter) and were cultured for 24 h with or without RM-A (1 μM). Slices were stained with Mayer's hematoxylin (Sigma) to visualize resorption pits. Nineteen-day fetal rat limb bones were prelabeled with 45Ca as described by Raisz (32). For detailed procedures, see Supporting Materials and Methods.
Bone Resorption in OVX Mice.
Eight-week-old female ddY mice were either sham-operated or OVX. One day after surgery, OVX mice were treated with RM-A, E2 (Sigma), or saline (vehicle). RM-A was administered s.c. twice daily for 4 weeks at indicated doses. E2 was dissolved in 20% DMSO in polyethylene glycol-300 and was s.c. administered to OVX mice by using a miniosmotic pump (Alza) at 0.01 μg/day for 4 weeks. After 4 weeks, mice were killed, and femora were removed to analyze bone density and structure. Radiographic photographs of femora were taken by soft x-ray (model SRO-M50; SOFRON, Tokyo). Trabecular bone density of the distal femur was analyzed by using a peripheral quantitative computed tomography (pQCT) system (XCT Research SA, Stratec Medizintechnik GmbH, Birkenfeld, Germany). Femora were dissected and stained by the Villanueva Goldner method.
Statistical Analysis.
Statistical significance of differences between control and experimental groups was determined by using Student's t test. P < 0.05 was considered significant. Differences in 45Ca release in organ cultures were analyzed by using the Newman–Keuls multiple comparison test.
Supplementary Material
Acknowledgments
We thank Drs. N. Takahashi, N. Udagawa, M. Wachi, and T. Kataoka for their helpful discussions and Drs. F. P. Ross, S. L. Teitlebaum, and S. Takeshita for critically reviewing the manuscript. We also thank Drs. K. Wierzba, Y. Minami, and T. Terada (Taiho Pharmaceutical Co., Ltd.) for their technical assistance. This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Abbreviations
- RM-A
reveromycin A
- OC
mature bone-resorbing osteoclast
- BPP
bisphosphonate
- CT
calcitonin
- PTH
parathyroid hormone
- OVX
ovariectomized
- TRAP
tartrate-resistant acid phosphatase
- RANKL
receptor activator of NF-κB ligand
- CM-A
concanamycin A
- E2
17β-estradiol
- IleRS
isoleucyl-tRNA synthetase.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rodan G. A., Martin T. J. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 2.Boyle W. J., Simonet W. S., Lacey D. L. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 3.Chambers T. J. J. Pathol. 2000;192:4–13. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH645>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Teitlelbaum S. L. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 5.Suda T., Nakamura I., Jimi E., Takahashi N. J. Bone Miner. Res. 1997;12:869–879. doi: 10.1359/jbmr.1997.12.6.869. [DOI] [PubMed] [Google Scholar]
- 6.Baron R., Neff L., Louvard D., Courtoy P. J. J. Cell Biol. 1985;101:2210–2222. doi: 10.1083/jcb.101.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blair H. C., Teitelbaum S. L., Ghiselli R., Gluck S. Science. 1989;245:855–857. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- 8.Hughes D. E., Wright K. R., Uy H. L., Sasaki A., Yoneda T., Roodman G. D., Mundy G. R., Boyce B. F. J. Bone Miner. Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 9.Rogers M. J. Curr. Pharm. Des. 2003;9:2643–2658. doi: 10.2174/1381612033453640. [DOI] [PubMed] [Google Scholar]
- 10.Reszka A. A., Rodan G. A. Curr. Rheumatol. Rep. 2003;5:65–74. doi: 10.1007/s11926-003-0085-6. [DOI] [PubMed] [Google Scholar]
- 11.Sato M., Grasser W., Endo N., Akins R., Simmons H., Thompson D. D., Golub E., Rodan G. A. J. Clin. Invest. 1991;88:2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black D. M., Greenspan S. L., Ensrud K. E., Palermo L., McGowan J. A., Lang T. F., Garnero P., Bouxsein M. L., Bilezikian J. P., Rosen C. J. N. Engl. J. Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 13.Finkelstein J. S., Hayes A., Hunzelman J. L., Wyland J. J., Lee H., Neer R. M. N. Engl. J. Med. 2003;349:1216–1226. doi: 10.1056/NEJMoa035725. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki H., Nakamura I., Takahashi N., Ikuhara T., Matsuzaki K., Isogai Y., Hori M., Suda T. Endocrinology. 1996;137:4685–4690. doi: 10.1210/endo.137.11.8895334. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi S., Goldring S., Katz M., Hilsenbeck S., Williams R., Roodman G. D. J. Clin. Invest. 1995;95:167–171. doi: 10.1172/JCI117634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi H., Osada H., Koshino H., Sasaki M., Onose R., Nakakoshi M., Yoshihama M., Isono K. J. Antibiot. 1992;45:1414–1419. doi: 10.7164/antibiotics.45.1414. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto Y., Machida K., Mizunuma M., Emoto Y., Sato N., Miyahara K., Hirata D., Usui T., Takahashi H., Osada H., Miyakawa T. J. Biol. Chem. 2002;277:28810–28814. doi: 10.1074/jbc.M203827200. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki T., Hong M. H., Udagawa N., Moriyama Y. Cell Tissue Res. 1994;278:265–271. doi: 10.1007/BF00414169. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi H., Yamashita Y., Takaoka H., Nakamura J., Yoshihama M., Osada H. Oncol. Res. 1997;9:7–11. [PubMed] [Google Scholar]
- 20.Osada H., Koshino H., Isono K., Takahashi H., Kawanishi G. J. Antibiot. 1991;44:259–261. doi: 10.7164/antibiotics.44.259. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi H., Osada H., Koshino H., Kudo T., Amano S., Shimizu S., Yoshihama M., Isono K. J. Antibiot. 1992;45:1409–1413. doi: 10.7164/antibiotics.45.1409. [DOI] [PubMed] [Google Scholar]
- 22.Koshino H., Takahashi H., Osada H., Isono K. J. Antibiot. 1992;45:1420–1427. doi: 10.7164/antibiotics.45.1420. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka Y., Ishikawa F., Osada H., Imajoh-Ohmi S., Uchida T., Kakiuchi T. J. Antibiot. 2002;55:904–913. doi: 10.7164/antibiotics.55.904. [DOI] [PubMed] [Google Scholar]
- 24.Glantschnig H., Fisher J. E., Wesolowski G., Rodan G. A., Reszka A. A. Cell Death Differ. 2003;10:1165–1177. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- 25.Silver I. A., Murrills R. J., Etherington D. J. Exp. Cell Res. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- 26.Cui Z., Hirata D., Tsuchiya E., Osada H., Miyakawa T. J. Biol. Chem. 1996;271:14712–14716. doi: 10.1074/jbc.271.25.14712. [DOI] [PubMed] [Google Scholar]
- 27.Licata A. A. Ann. Pharmacother. 2005;39:668–677. doi: 10.1345/aph.1E357. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi N., Yamana H., Yoshiki S., Roodman G. D., Mundy G. R., Jones S. J., Boyde A., Suda T. Endocrinology. 1988;122:1373–1382. doi: 10.1210/endo-122-4-1373. [DOI] [PubMed] [Google Scholar]
- 29.Takami M., Woo J. T., Nagai K. Cell Tissue Res. 1999;298:327–334. doi: 10.1007/s004419900092. [DOI] [PubMed] [Google Scholar]
- 30.Jimi E., Ikebe T., Takahashi N., Hirata M., Suda T., Koga T. J. Biol. Chem. 1996;271:4605–4608. doi: 10.1074/jbc.271.9.4605. [DOI] [PubMed] [Google Scholar]
- 31.Hotokezaka H., Sakai E., Kanaoka K., Saito K., Matsuo K., Kitaura H., Yoshida N., Nakayama K. J. Biol. Chem. 2002;277:47366–47372. doi: 10.1074/jbc.M208284200. [DOI] [PubMed] [Google Scholar]
- 32.Raisz L. G. J. Clin. Invest. 1965;44:103–116. doi: 10.1172/JCI105117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.