Abstract
Natriuretic peptides (NP) mediate their effects by activating membrane-bound guanylyl cyclase-coupled receptors A (NPR-A) or B (NPR-B). Whereas the pathophysiological role of NPR-A has been widely studied, only limited knowledge on the cardiovascular function of NPR-B is available. In vitro studies suggest antiproliferative and antihypertrophic actions of the NPR-B ligand C-type NP (CNP). Because of the lack of a specific pharmacological inhibitor, these effects could not clearly be attributed to impaired NPR-B signaling. Recently, gene deletion revealed a predominant role of NPR-B in endochondral ossification and development of female reproductive organs. However, morphological abnormalities and premature death of NPR-B-deficient mice preclude detailed cardiovascular phenotyping. In the present study, a dominant-negative mutant (NPR-BΔKC) was used to characterize CNP-dependent NPR-B signaling in vitro and in transgenic rats. Here we demonstrate that reduced CNP- but not atrial NP-dependent cGMP response attenuates antihypertrophic potency of CNP in vitro. In transgenic rats, NPR-BΔKC expression selectively reduced NPR-B but not NPR-A signaling. NPR-BΔKC transgenic rats display progressive, blood pressure-independent cardiac hypertrophy and elevated heart rate. The hypertrophic phenotype is further enhanced in chronic volume overload-induced congestive heart failure. Thus, this study provides evidence linking NPR-B signaling to the control of cardiac growth.
Keywords: C-type natriuretic peptide, knockdown
The family of natriuretic peptides (NP) consists of the structurally homologous but genetically distinct peptide hormones atrial NP (ANP), brain NP (BNP), and C-type NP (CNP). ANP and BNP are predominantly synthesized in atrial and ventricular cardiomyocytes (CMC) and released upon stretch into the circulation (1). CNP is expressed in a wide variety of tissues and acts locally as an autocrine and paracrine hormone (2).
The NP exert their actions by activating guanylyl cyclase-coupled cell surface receptors. ANP and BNP bind specifically to NP receptor (NPR) A, whereas CNP activates NPR-B. ANP, BNP, and CNP display comparable affinity to NPR-C, which lacks intrinsic guanylyl cyclase activity and acts in part as a clearance receptor (3, 4).
NP regulate a variety of physiological processes by intracellular accumulation of their second messenger cGMP (cGMP). The accumulation of cGMP upon ligand stimulation requires a dimerization of receptor molecules including the cytoplasmatic kinase homology domain. This process leads to a tight contact of two guanylyl cyclase domains resulting in conversion of Mg2+-GTP to 3′,5′-cGMP (5, 6).
ANP, BNP, and their receptor, NPR-A, have been established as regulators of blood pressure, water and electrolyte homeostasis, and cellular growth. In vitro studies demonstrated inhibitory effects on proliferation of vascular smooth muscle cells and CMCs as well as extracellular matrix secretion of cardiac fibroblasts (7–9). Genetic models revealed insight into the complementary role of these NP with respect to regulation of myocardial structure. Disruption of the murine NPR-A gene resulted in salt-resistant hypertension, cardiac hypertrophy, and fibrosis (10–12). Gene targeting of ANP led to salt-sensitive hypertension and cardiac hypertrophy, whereas BNP knockout mice exhibited a marked cardiac fibrosis without hypertension or hypertrophy (13–15).
In contrast to ANP and BNP, CNP does not exert systemic effects, but rather acts locally. In vitro studies have shown that CNP mediates antiproliferative and antihypertrophic actions in neonatal CMCs, vascular smooth muscle cells, and cardiac fibroblasts (8, 16, 17). It has not been resolved whether these actions were strictly mediated by NPR-B or whether crossactivation of NPR-A was involved. Targeted disruption of the CNP gene in genetically modified mice resulted in dwarfism due to impaired longitudinal growth of long bones (18). The disruption of the NPR-B gene resulted in impaired endochondral ossification and developmental defects of the female reproductive organs. Blood pressure measurements performed on NPR-B-null mice revealed no differences between knockout and wild-type littermates (19). However, these genetically modified mice died prematurely because of severe skeletal malformation and could not be subjected to further cardiovascular phenotyping.
Thus, the currently available genetic models are not suitable to fully characterize the function of NPR-B in the cardiovascular system. We therefore developed a model that allowed a functional down-regulation of the NPR-B signaling by overexpressing a dominant-negative mutant in transgenic rats. These rats are viable, have a normal lifespan, and are accessible to complex cardiovascular phenotyping, thereby overcoming the limits related to a complete deletion of NPR-B or CNP. Our model presented in this study provides new insights in the physiology of NPR-B and evidence that NPR-B is involved in regulation of CMC growth.
Results
NPR-BΔKC Acts as a Dominant-Negative Molecule in a NPR-B-Specific Manner.
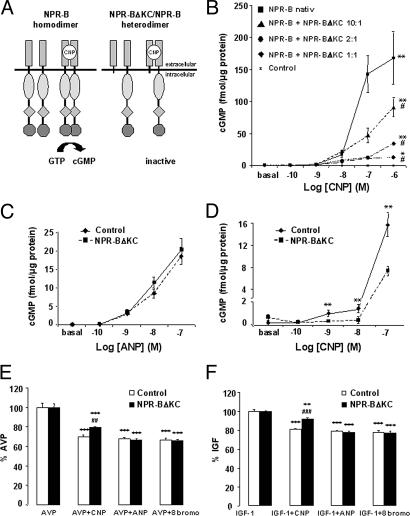
COS-7 cells, which do not express NPR-A or NPR-B, were cotransfected with NPR-B and the NPR-B deletion mutant NPR-BΔKC (Fig. 1A). Intracellular cGMP accumulation upon CNP stimulation was abolished with increasing amounts of NPR-BΔKC, demonstrating a dominant-negative effect of the receptor mutant (Fig. 1B).
Fig. 1.
NPR-BΔKC acts as NPR-B-specific dominant-negative mutant in vitro. (A) Principle of NPR-B activation and inhibition. (Left) Native receptor. (Right) Dominant-negative mutant. (B) CNP-dependent cGMP response in COS cells cotransfected with NPR-B and various amounts of NPR-BΔKC. ∗, P < 0.05; ∗∗, P < 0.01 vs. control by ANOVA; #, P < 0.05 vs. NPR-B native by ANOVA (n = 6 per group). (C and D) Measurement of cGMP response to ANP (C) or CNP (D) of H9c2 cells. ∗∗, P < 0.01 vs. control (n = 6 per group). (E and F) l-(4,5-3H)leucine incorporation of NPR-BΔKC-transfected H9c2 cells and controls after stimulation with AVP (E) or IGF-1 (F) and treatment with ANP, CNP, or 8-Bromo-cGMP. ∗∗, P < 0.01; ∗∗∗, P < 0.001 vs. single treatment with hypertrophic stimulus; ##, P < 0.01 vs. control AVP plus CNP; ###, P < 0.001 vs. control IGF-1 plus CNP (n = 6 per group).
NPR-BΔKC was stably overexpressed in H9c2 cells, which express NPR-A and NPR-B (20) and respond to ANP or CNP stimulation with accumulation of intracellular cGMP in a concentration-dependent fashion. The cGMP response to ANP was unaffected by NPR-BΔKC expression; however, the CNP-dependent cGMP response was significantly blunted (Fig. 1 C and D), demonstrating NPR-B specificity of NPR-BΔKC in the presence of endogenous NPR-B.
NPR-B Mediates Antihypertrophic Effects of CNP in H9c2 Cells.
H9c2 cells, stably transfected with NPR-BΔKC, were used to study the role of NPR-B signaling on cellular hypertrophy. IGF-1 (10−7 M) and Arg-vasopressin (AVP; 10−7 M) induced protein synthesis in H9c2 cells, accompanied by an increase in cell size (data not shown). ANP (10−7 M) and the membrane-permeable cGMP analogue 8-Bromo-cGMP (10−4 M) were equally potent in blocking IGF-1- and AVP-induced hypertrophy in both NPR-BΔKC-transfected cells and controls (Fig. 1 E and F). In contrast, CNP was significantly less potent in reducing IGF-1- and AVP-induced hypertrophy in NPR-BΔKC-expressing cells, suggesting antihypertrophic effects of NPR-B signaling in vitro.
Generation and Characterization of NPR-BΔKC Transgenic Rats.
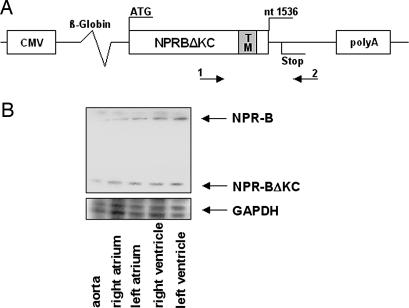
Transgenic rats carrying the dominant-negative mutant NPR-BΔKC were generated to study the function of NPR-B in vivo (Fig. 2A and Fig. 7A, which is published as supporting information on the PNAS web site). NPR-BΔKC transcripts were detected in all tested tissues of transgenic rats by RT-PCR (Fig. 7B). Because this study focused on the role of NPR-B in the cardiovascular system, expression of NPR-B and NPR-BΔKC mRNA was quantified and shown to be equal in aorta, atria, and ventricles of transgenic and wild-type animals (Fig. 2B).
Fig. 2.
Transgenic construct and NPR-BΔKC mRNA expression. (A) Structure of NPR-BΔKC transgenic construct. 1 and 2 indicate primers used to generate the probe for NPR-B/NPR-BΔKC ribonuclease protection assay. Primer 2 matched to NPR-B intracellular domain not present in NPR-BΔKC. CMV, cytomegalovirus promoter for ubiquitous expression of the transgene; β-globin, rabbit β-globin intron for enhanced expression of the transgene; TM, transmembrane domain. (B) Expression of NPR-B and NPR-BΔKC mRNA in the aorta and four heart chambers of transgenic rats shown by ribonuclease protection assay.
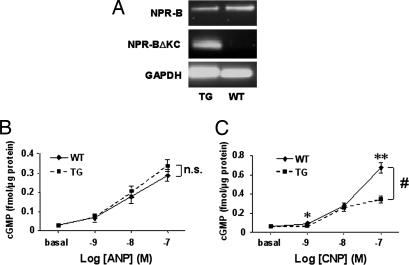
To test whether NPR-BΔKC was able to block the endogenous NPR-B in vivo, we measured the cGMP response to ANP and CNP in primary cells and tissues derived from NPR-BΔKC transgenic rats. Expression of the transgene in cells used for the experiment was confirmed by RT-PCR (Fig. 3A and Fig. 8A, which is published as supporting information on the PNAS web site). Neonatal CMCs isolated from wild-type controls responded to ANP and CNP stimulation with a dose-dependent cGMP increase. The response to ANP stimulation of CMCs from NPR-BΔKC transgenic rats was comparable to wild-type controls, whereas the cGMP response to CNP was attenuated (Fig. 3 B and C). Glomeruli isolated from transgenic rats exhibit reduced ligand-independent cGMP production at baseline. However, glomeruli from both transgenic rats and wild-type controls showed a comparable cGMP response upon stimulation with ANP, whereas the cGMP response to CNP was blunted in glomeruli from transgenic rats (Fig. 8).
Fig. 3.
Reduced CNP-dependent cGMP generation in NPR-BΔKC transgenic rats. (A) Expression of NPR-B and NPR-BΔKC shown by RT-PCR in CMCs. (B and C) ANP-dependent (B) and CNP-dependent (C) cGMP production of CMCs from transgenic and wild-type rats. ∗, P < 0.05; ∗∗, P < 0.01 vs. wild-type control; #, P < 0.05 vs. wild-type control by ANOVA (n = 5–6 per group). TG, transgenic rats; WT, wild-type control.
Aiming to further prove selective inhibition of NPR-BΔKC on NPR-B signaling in an in vivo environment, ANP and CNP were infused i.v., and plasma cGMP concentrations were measured. Application of ANP (0.5 μg/kg per min) resulted in comparable cGMP plasma values in NPR-BΔKC transgenic rats and wild-type controls (wild type, 9.48 ± 0.4 fmol/ml; NPR-BΔKC, 8.8 ± 0.4 fmol/ml). However, plasma concentrations of cGMP after CNP infusion (1.0 μg/ml per min) were lower in NPR-BΔKC transgenic rats (wild type, 9.2 ± 0.9 fmol/ml; NPR-BΔKC, 6.3 ± 0.8 fmol/ml; P < 0.05), suggesting that NPR-BΔKC acts as a dominant-negative mutant specifically interfering with NPR-B signaling in the presented transgenic model.
Blood Pressure Regulation and Increased Heart Rate in NPR-BΔKC Transgenic Rats.
Telemetric blood pressure measurements showed no significant differences in systolic, diastolic, and mean arterial pressure between NPR-BΔKC transgenic rats and wild-type controls (Table 1). However, the heart rate of NPR-BΔKC transgenic rats was found to be modestly elevated. Fourier transformation of systolic blood pressure, pulse interval time series, and cross spectra obtained by telemetry revealed increased sympathetic nerve activity in transgenic rats compared with wild-type controls, as shown by increased low frequency as measure of sympathetic heart rate control, increased low-frequency/high-frequency ratio as a measure of sympathovagal balance, and increased power spectral density of systolic blood pressure calculated in the low-frequency band (Table 1).
Table 1.
Hemodynamic characterization in conscious rats under baseline conditions
| Rats | Mean arterial pressure, mmHg | Systolic blood pressure, mmHg | Diastolic blood pressure, mmHg | Heart rate, bpm | LF-RR, msec2 | HF-RR, msec2 | LF/HF-RR | SBP-LF, mmHg2 |
|---|---|---|---|---|---|---|---|---|
| Wild-type control (n = 10) | 105.0 ± 1.2 | 122.5 ± 1.6 | 89.9 ± 1.0 | 322 ± 4.1 | 0.64 ± 0.15 | 1.55 ± 0.22 | 0.40 ± 0.06 | 2.20 ± 0.19 |
| NPR-BΔKC transgenic (n = 10) | 105.9 ± 0.7 | 125.4 ± 1.6 | 88.0 ± 0.8 | 339 ± 2.4** | 2.17 ± 0.59* | 2.54 ± 0.31* | 0.79 ± 0.14* | 3.47 ± 0.40* |
LF-RR, low-frequency power; HF-RR, high-frequency power; LF/HF, ratio for heart rate variability; SBP-LF, low-frequency power of systolic blood pressure variability.
∗, P < 0.05,
∗∗, P < 0.01 vs. wild-type control.
Normal Renal Function in NPR-BΔKC Transgenic Rats.
Assessment of renal function in metabolic cages revealed no influence of NPR-BΔKC on drinking behavior, natriuresis, diuresis, fractional sodium excretion, and creatinine clearance at baseline or after addition of 1% NaCl in drinking water or 1% NaCl in water plus 4% NaCl in the food (Fig. 9 A–D, which is published as supporting information on the PNAS web site). When the acute renal effect of ANP or CNP infusion was analyzed, both transgenic and wild-type control animals showed comparable dose-dependent diuretic responses to ANP, whereas diuresis did not significantly change after CNP infusion in either group (Fig. 9 E and F). Thus, in contrast to NPR-A, NPR-B signaling does not significantly contribute to regulation of renal function.
Reduced Bone Growth in NPR-BΔKC Transgenic Rats.
As expected and shown for the NPR-B-null mice, x-ray-based measurements revealed a reduced overall length (naso-anal length: wild type, 163.0 ± 1.5 mm; NPR-BΔKC, 150.0 ± 2.0 mm; P < 0.01) and a reduction of the length of several bones (tibia: wild type, 27.5 ± 0.2 mm; NPR-BΔKC, 25.3 ± 0.3 mm; P < 0.001; see Fig. 10, which is published as supporting information on the PNAS web site). No other obvious skeletal malformations were detected.
Cardiac Hypertrophy in NPR-BΔKC Transgenic Rats.
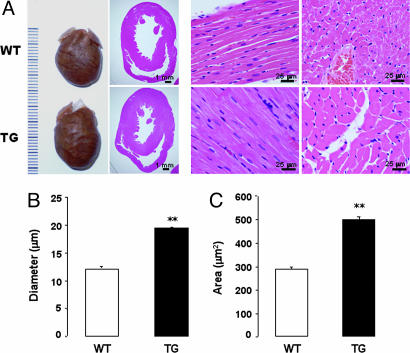
Morphological and histological analysis of hearts from 6-month-old NPR-BΔKC transgenic and wild-type animals revealed a concentric left ventricular hypertrophy along with increased CMC diameter and cross-sectional area in the transgenic rats (Fig. 4). In addition, left ventricular expression of ANP and BNP mRNA (Fig. 11, which is published as supporting information on the PNAS web site), along with ANP and BNP plasma concentrations (ANP: wild type, 71.9 ± 11.6 fmol/ml; NPR-BΔKC, 125.7 ± 21.8 fmol/ml; P < 0.05; BNP: wild type, 15.9 ± 0.9 fmol/ml; NPR-BΔKC, 19.9 ± 0.7 fmol/ml; P < 0.01), were found to be elevated in transgenic rats.
Fig. 4.
Morphological assessment of cardiac hypertrophy in 6-month-old animals. (A) Hearts and hematoxylin and eosin-stained cardiac sections of wild-type (WT) and transgenic (TG) rats. Measurement of CMC diameter (B) and cross-sectional area (C). ∗∗, P < 0.01 vs. wild-type control (n = 5 hearts). Two hundred images per genotype were used for statistical analysis.
The degree of interstitial and perivascular fibrosis, as assessed by Masson's trichrome staining, was not different in transgenic rats from controls (Fig. 12, which is published as supporting information on the PNAS web site). Also, left ventricular mRNA levels of collagen 1 and 3 were not different between the groups (collagen 1: wild type, 0.77 ± 0.02 arbitrary units; NPR-BΔKC, 0.83 ± 0.01 arbitrary units; collagen 3: wild type, 0.74 ± 0.01 arbitrary units; NPR-BΔKC, 0.77 ± 0.01 arbitrary units; Fig. 11).
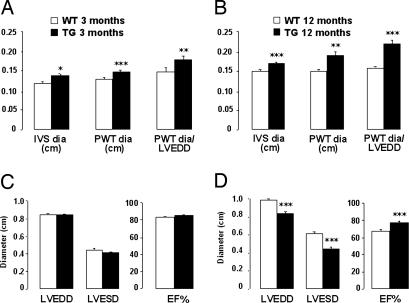
Echocardiographic assessment of cardiac function in NPR-BΔKC transgenic rats and wild-type controls demonstrated early concentric left ventricular hypertrophy but no dilatation at 3 months of age, with increased interventricular septum and posterior wall thickness but unchanged end-diastolic and end-systolic diameters of the left ventricle (Fig. 5 A and C). Left ventricular contractile function was not altered by transgenic expression of NPR-BΔKC, as demonstrated by unchanged EF (Fig. 5C).
Fig. 5.
Echocardiography of 3- and 12-month-old animals. (A and B) Measurements of interventricular septum (IVS dia), posterior wall thickness (PWT dia), and calculated ratio of posterior wall thickness over left ventricular end-diastolic diameter (PWT dia/LVEDD) of 3-month-old rats (A; n = 8 per group) and 12-month-old rats (B; n = 10 per group). ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 vs. wild-type control. TG, transgenic rats; WT, wild-type control. (C and D) Left ventricular end-diastolic diameter (LVEDD), end-systolic diameter (LVESD), and ejection fraction (EF%) of 3-month-old rats (C) and 12-month-old rats (D). ∗∗∗, P < 0.001 vs. wild-type control.
Cardiac hypertrophy was further enhanced in 12-month-old NPR-BΔKC transgenic rats, as demonstrated by increased intraventricular septum and posterior wall thickness and further increased ratio of these parameters, which accounts for hypertrophy (Fig. 5B). Left ventricular end-diastolic and end-systolic diameters were decreased, whereas left ventricular contractility was increased in transgenic rats compared with wild-type controls (Fig. 5D). The relative heart weight of NPR-BΔKC transgenic rats at 12 months of age was significantly increased (wild type, 3.02 ± 0.05 mg/g of body weight; NPR-BΔKC, 3.35 ± 0.13 mg/g of body weight; P < 0.01).
Augmented Cardiac Hypertrophy and Maintained Left Ventricular Function in NPR-BΔKC Transgenic Rats After Chronic Volume Overload.
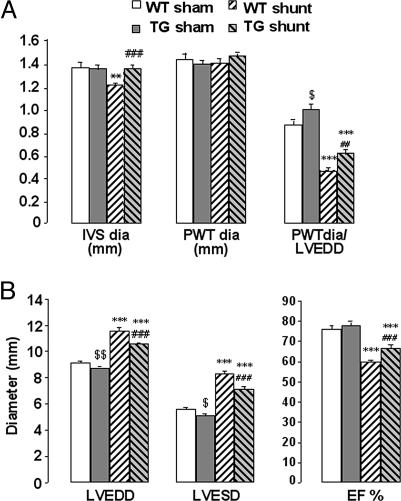
Chronic volume overload led to a significantly pronounced increase in cardiac hypertrophy in NPR-BΔKC transgenic rats compared with wild-type controls measured echocardiographically 6 weeks after induction of an aortocaval shunt (Fig. 6A). In comparison to wild-type controls, left ventricular dilatation was less pronounced and left ventricular ejection fraction was less attenuated in NPR-BΔKC transgenic rats (Fig. 6B).
Fig. 6.
Echocardiography of 14-week-old rats with chronic volume overload 6 weeks after surgery. (A) Measurements of interventricular septum (IVS dia), posterior wall thickness (PWT dia), and calculated ratio of posterior wall thickness over left ventricular end-diastolic diameter (PWT dia/LVEDD). ∗∗, P < 0.01; ∗∗∗, P < 0.001 vs. sham-operated animals; ##, P < 0.01; ###, P < 0.001 vs. wild-type shunt animals; $, P < 0.05 vs. sham-operated wild-type animals (n = 7–10 per group). TG, transgenic rats; WT, wild-type control. (B) Left ventricular end-diastolic diameter (LVEDD), end-systolic diameter (LVESD), and ejection fraction (EF%). ∗∗∗, P < 0.001 vs. sham-operated animals; ###, P < 0.001 vs. wild-type shunt animals; $, P < 0.05; $$, P < 0.01 vs. sham-operated wild-type animals (n = 7–10 per group).
Discussion
In the present article we studied the cardiovascular function of the NPR-B receptor in vitro and in vivo. Employing a genetic model overexpressing a dominant-negative NPR-B mutant in transgenic rats, we demonstrate for the first time that NPR-B signaling is involved in regulation of cardiac hypertrophy.
The dominant-negative properties of NPR-BΔKC were confirmed by a gene dose-dependent reduction of NPR-B signaling by NPR-BΔKC in COS-7 and H9c2 cells as well as in transgenic rats ubiquitously expressing NPR-BΔKC, as shown by attenuated cGMP response after CNP infusion in intact animals or CNP stimulation of adult CMCs and glomeruli ex vivo. These findings mirror data published by Chinkers and Wilson (21) demonstrating a lack of interaction between the extracellular domains of NPR-A and NPR-B. Furthermore, a dominant-negative mutant of NPR-A as well as an alternative splice variant of NPR-B acting in a dominant-negative manner have previously been used to study ANP and CNP physiology, providing evidence for the use of dominant-negative mutants in cell culture and transgenic animal models as a powerful method to study the specific function of NPRs (19, 22–24).
Several other genetically altered models of the NP system provided evidence for antihypertrophic actions of NP (11, 12, 14, 25). Both ANP and CNP were capable of reducing the hypertrophic response to a broad range of hormonal or mechanical stimuli in several cell types, including CMCs, cardiac fibroblasts, and vascular smooth muscle cells (8, 17, 26). This effect was mediated by their second messenger cGMP and could be blocked by an unspecific NPR antagonist. However, because of a paucity of specific pharmacological inhibitors or a suitable genetic model, none of these previous studies could clearly define the role of NPR-B in cardiac hypertrophy.
In accordance with the blunted CNP response in NPR-BΔKC-transfected H9c2 cells, we found a reduced potency of CNP, but not ANP, to block AVP- or IGF-1-induced hypertrophy in H9c2 cells. These data show that the effects of CNP were mediated by NPR-B and cannot be attributed to crossactivation of NPR-A by CNP because of supraphysiological concentrations used in cell culture.
Histological assessment and echocardiography revealed cardiac hypertrophy in NPR-BΔKC transgenic rats, which aggravated with age accompanied by increased cardiac ANP and BNP mRNA expression and increased ANP and BNP plasma concentration. However, there was no evidence for increased interstitial or perivascular fibrosis. Furthermore, chronic volume overload by an infrarenal aortocaval shunt in 8-week-old rats resulted in exaggerated cardiac hypertrophy in NPR-BΔKC transgenic rats 6 weeks after surgery. These findings suggest that NPR-B is implicated in the regulation of CMC growth but not in cardiac fibrosis.
The increased left ventricular contractility observed in 12-month-old transgenic animals and the less affected left ventricular function in the transgenic rats after aortocaval shunt could result from cardiac hypertrophy (27) or from inhibition of NPR-B signaling. Pierkes et al. (28) demonstrated a negative inotropic effect after initial positive inotropic actions of CNP in isolated mouse working hearts, which could contribute to the observed increase in ejection fraction as a consequence of chronic inhibition of CNP signaling in NPR-BΔKC transgenic rats.
Given the possibility of involvement of NPR-B in blood pressure regulation, we aimed to elucidate this function using telemetry in conscious animals. Blood pressure was not different, but the heart rate was moderately elevated in transgenic rats compared with controls. Fourier transformation and power spectral analysis of the data obtained from these telemetric measurements suggest an increased sympathetic nerve activity in transgenic rats. A possible mechanism could be a reduced central nervous CNP action to facilitate vagal heart rate baroreflexes by inhibition of NPR-B (29). Because the increase in heart rate observed in our model is rather small it is unlikely to cause cardiac hypertrophy over a period as short as 3 months, when hypertrophy was first detected.
Our findings do not confirm previous studies that predicted a role of CNP in blood pressure regulation by i.v. administration of supraphysiological CNP concentrations (30) or by exposing isolated vessels to CNP (31, 32), but they are in accordance with the phenotype of NPR-B-null mice, where no blood pressure differences were reported (19). We conclude that normal levels of CNP are most likely not involved in basic blood pressure regulation in vivo and that the observed cardiac hypertrophy is blood pressure-independent.
We found that down-regulation of NPR-B signaling did not affect renal function parameters at baseline or after dietary salt load. Also, in contrast to ANP, CNP infusion did not induce diuresis in wild-type animals. This finding is in accordance with previously published data showing that CNP does not influence renal function in humans and dogs after i.v. administration at low dosages (33, 34). It has been demonstrated that systemic infusion or bolus administration of supraphysiological CNP concentrations in dogs increased aldosterone release and distal nephron sodium reabsorption, suggesting that CNP regulates tubular reabsorption and not glomerular filtration rate (35). Despite conflicting data on acute renal effects of CNP, fluid homeostasis in chronic down-regulation on NPR-B signaling appears to be balanced. Thus, we suggest that NPR-B is not a major regulator of renal function in vivo.
Longitudinal growth of the long bones is a result of endochondral ossification in the cartilaginous growth plate. We report in the present study a growth retardation of long bones in NPR-BΔKC transgenic rats, supporting published data on the role of NPR-B in endochondral ossification (18, 19, 36).
In summary, the present study demonstrates that a mutant of NPR-B lacking a substantial part of the cytoplasmatic domain acts as selective inhibitor of the native NPR-B in vitro and in vivo. In contrast to CNP- and NPR-B-deficient mice, which are severely ill, NPR-BΔKC transgenic rats have been proven very valuable for the study of cardiovascular functions of NPR-B. Employing this model, we showed for the first time that NPR-B mediates antihypertrophic effects in CMCs under physiological as well as pathophysiological conditions. Further studies need to address the signaling pathway involved in mediating these effects of CNP/NPR-B.
Methods
Cell Culture and in Vitro Studies.
H9c2 and COS-7 cells were obtained from American Type Culture Collection and were cultivated as described in ref. 20. Rat NPR-B cDNA was a gift from Lincoln Potter (University of Minnesota, Minneapolis). NPR-BΔKC cDNA was cloned into pTarget (Promega) or pcDNA3.1(-) -myc/His (Invitrogen). For titration experiments, COS-7 cells were cotransfected with different ratios of pcDNA3.1/NPR-B and pcDNA3.1/NPR-BΔKC. H9c2 cells were stably transfected with pcDNA3.1/NPR-BΔKC. Transfected cells were serum-starved for 24 h followed by stimulation with 10−7 M AVP or 10−7 M IGF-1 with and without 10−7 M ANP, 10−7 M CNP, or 10−4 M 8-Bromo-cGMP (all from Calbiochem), in the presence of 1 μCi/ml (1 Ci = 37 GBq) l-(4,5-3H)leucine (Amersham Pharmacia). After 24 h, radioactive labeling was measured by liquid scintillation counting.
Generation of Transgenic Rats.
pTarget-NPR-BΔKC was linearized and microinjected into the male pronucleus of rat zygotes as described (37). Founder animals were identified by Southern blotting by using 32P-labeled NPR-BΔKC as a probe and bred to homozygosity. Further genotyping was conducted by PCR (for primers see Table 2, which is published as supporting information on the PNAS web site).
Analysis of mRNA Expression.
After rats were killed, organs were dissected, frozen in liquid nitrogen, and stored at −80°C. Total RNA was extracted by using TRIzol reagent (Invitrogen). Antisense probes for NPR-BΔKC, ANP, BNP, collagen 1 and 3, and GAPDH (for primers see Table 2) were 32P-UTP-labeled by in vitro transcription (Riboprobe combination system Sp6/T7; Promega). RNA expression was measured by RNase protection assay (RPA II kit, Ambion). Protected fragments were separated on a polyacrylamide gel and detected with a Fuji phosphoimager (Fujix BAS 2000, Fuji).
Isolation and Stimulation of Neonatal CMCs and Glomeruli.
Neonatal rat CMCs were obtained from 2- to 3-day-old rats and cultured as described (38). Glomeruli were isolated by differential sieving as published (39). CMC and glomeruli were stimulated at 37°C for 20 min with different concentrations of ANP or CNP in the presence of 0.5 mM 3-isobutyl-1-methylxanthine (Sigma). Samples were kept at −20°C until cGMP measurement.
Analysis of ANP, BNP, and cGMP.
Plasma samples for ANP and BNP were purified on C18 Sep-Pack columns (Waters), and peptide concentrations were determined by using RIA kits (Bachem).
For cGMP analysis, plasma and glomeruli samples were purified on alumina matrix (Sigma). Supernatant from CMC was directly used for the assay. cGMP values were normalized by protein concentration. For experiments with H9c2 and COS-7 cells, intracellular cGMP was analyzed. Determination of cGMP was performed by RIA as described in ref. 40.
Assessment of Renal Function.
Renal excretory function in conscious rats was assessed by using metabolic cages. Diuresis and water intake were monitored under baseline conditions, with 1% NaCl loading in drinking water or 1% NaCl in water plus 4% NaCl in food for 24 h, respectively. The concentrations of sodium and creatinine in urine and plasma were measured by Labor Diagnostik (Leipzig, Germany), and fractional sodium excretion, and creatinine clearance were calculated. The renal effect of acute ANP and CNP infusion was analyzed under isoflurane anesthesia. Briefly, the jugular vein was cannulated. After 20 min of equilibration, ANP (0.5 μg/kg per min) or CNP (1.0 μg/kg per min) were infused over 20 min. Urine was collected over three time periods of 20 min each for calculation of diuresis, and plasma samples were stored at −80°C for analysis of cGMP excretion. After washout, the experiment was repeated with a higher dosage of ANP (1.0 μg/kg per min) or CNP (2.0 μg/kg per min).
Transthoracic Echocardiography, Hemodynamic Evaluation, and Spectral Analysis of Autonomic Nervous System.
Transthoracic two-dimensional guided M-mode echocardiography was performed in 3- and 12-month-old rats under anesthesia with isoflurane, by using an Acuson Sequoia C256 echocardiograph with a 14-MHz probe (Siemens).
For radiotelemetric blood pressure measurements in conscious animals, 10 rats per group were anesthetized with ketamine/xylazine. Pressure transducers were implanted in the abdominal cavity, and the transducer-connected catheter was anchored in the lumen of the abdominal aorta. After recovery for 15 days, data were recorded under baseline conditions. Autonomic nerve activity was assessed by monitoring spontaneous changes in blood pressure and heart rate as described earlier (41).
Histological Analysis.
Hearts from 6-month-old transgenic or control animals were placed in 10% formaldehyde and embedded in paraffin. Longitudinal and transversal sections (5 μm) were stained with hematoxylin and eosin or Masson's trichrome. CMC diameter and cross-sectional area were assessed at ×40 magnification.
Aortocaval Shunt.
The infrarenal aortocaval shunt was used as a model of volume overload-induced heart failure in 8-week-old rats as described (40). Sham-operated rats of each genotype were used as controls. Six weeks after surgery, sham- and shunt-operated rats were used for echocardiography and molecular biology studies. All animal studies were carried out in accordance with the local authorities and conforming to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication no. 85-23, revised 1996).
Skeletal Phenotyping.
Thirteen-month-old rats were anesthetized with ketamine/xylazine and underwent soft x-ray examination (Polydoros LX 50 generator; Siemens).
Statistical Analysis.
Differences between two groups were evaluated by using unpaired Student's test, and differences between more than two groups were evaluated by using ANOVA followed by Fisher's probable least-squares difference test using statview software. ANOVA for repeated measurements was applied to evaluate cellular cGMP responses and diuresis after ANP and CNP infusion. The significance level was set at P < 0.05. All data are expressed as mean ± SEM.
Supplementary Material
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Astrid Schiche, Jutta Meisel, Jeannette Mothes, Rita Günzel, Rosemarie Barnow, and Reika Langanki. This project was supported by grants from the Max Delbrück Center for Molecular Medicine Berlin-Buch (to T.H.L., I.P.-L., M.B., J.M., and J.B.) and by a fellowship from the Charité Medical School (to J.B.).
Abbreviations
- NP
natriuretic peptide
- CNP
C-type NP
- ANP
atrial NP
- BNP
brain NP
- NPR
NP receptor
- CMC
cardiomyocyte
- AVP
Arg-vasopressin
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kinnunen P., Vuolteenaho O., Ruskoaho H. Endocrinology. 1993;132:1961–1970. doi: 10.1210/endo.132.5.8477647. [DOI] [PubMed] [Google Scholar]
- 2.Suga S., Nakao K., Itoh H., Komatsu Y., Ogawa Y., Hama N., Imura H. J. Clin. Invest. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suga S., Nakao K., Hosoda K., Mukoyama M., Ogawa Y., Shirakami G., Arai H., Saito Y., Kambayashi Y., Inouye K., et al. Endocrinology. 1992;130:229–239. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- 4.Matsukawa N., Grzesik W. J., Takahashi N., Pandey K. N., Pang S., Yamauchi M., Smithies O. Proc. Natl. Acad. Sci. USA. 1999;96:7403–7408. doi: 10.1073/pnas.96.13.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langenickel T., Buttgereit J., Pagel I., Dietz R., Willenbrock R., Bader M. Hypertension. 2004;43:460–465. doi: 10.1161/01.HYP.0000110907.33263.0b. [DOI] [PubMed] [Google Scholar]
- 6.Labrecque J., Deschenes J., McNicoll N., De L. A. J. Biol. Chem. 2001;276:8064–8072. doi: 10.1074/jbc.M005550200. [DOI] [PubMed] [Google Scholar]
- 7.Itoh H., Pratt R. E., Dzau V. J. J. Clin. Invest. 1990;86:1690–1697. doi: 10.1172/JCI114893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenkranz A. C., Woods R. L., Dusting G. J., Ritchie R. H. Cardiovasc. Res. 2003;57:515–522. doi: 10.1016/s0008-6363(02)00667-3. [DOI] [PubMed] [Google Scholar]
- 9.Redondo J., Bishop J. E., Wilkins M. R. Br. J. Pharmacol. 1998;124:1455–1462. doi: 10.1038/sj.bjp.0701994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez M. J., Wong S. K., Kishimoto I., Dubois S., Mach V., Friesen J., Garbers D. L., Beuve A. Nature. 1995;378:65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 11.Kishimoto I., Rossi K., Garbers D. L. Proc. Natl. Acad. Sci. USA. 2001;98:2703–2706. doi: 10.1073/pnas.051625598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliver P. M., Fox J. E., Kim R., Rockman H. A., Kim H. S., Reddick R. L., Pandey K. N., Milgram S. L., Smithies O., Maeda N. Proc. Natl. Acad. Sci. USA. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melo L. G., Veress A. T., Chong C. K., Pang S. C., Flynn T. G., Sonnenberg H. Am. J. Physiol. 1998;274:R255–R261. doi: 10.1152/ajpregu.1998.274.1.R255. [DOI] [PubMed] [Google Scholar]
- 14.Feng J. A., Perry G., Mori T., Hayashi T., Oparil S., Chen Y. F. Clin. Exp. Pharmacol. Physiol. 2003;30:343–349. doi: 10.1046/j.1440-1681.2003.03836.x. [DOI] [PubMed] [Google Scholar]
- 15.Tamura N., Ogawa Y., Chusho H., Nakamura K., Nakao K., Suda M., Kasahara M., Hashimoto R., Katsuura G., Mukoyama M., et al. Proc. Natl. Acad. Sci. USA. 2000;97:4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arjona A. A., Hsu C. A., Wrenn D. S., Hill N. S. Gen. Pharmacol. 1997;28:387–392. doi: 10.1016/s0306-3623(96)00275-3. [DOI] [PubMed] [Google Scholar]
- 17.Horio T., Tokudome T., Maki T., Yoshihara F., Suga S., Nishikimi T., Kojima M., Kawano Y., Kangawa K. Endocrinology. 2003;144:2279–2284. doi: 10.1210/en.2003-0128. [DOI] [PubMed] [Google Scholar]
- 18.Komatsu Y., Chusho H., Tamura N., Yasoda A., Miyazawa T., Suda M., Miura M., Ogawa Y., Nakao K. J. Bone Miner. Metab. 2002;20:331–336. doi: 10.1007/s007740200048. [DOI] [PubMed] [Google Scholar]
- 19.Tamura N., Doolittle L. K., Hammer R. E., Shelton J. M., Richardson J. A., Garbers D. L. Proc. Natl. Acad. Sci. USA. 2004;101:17300–17305. doi: 10.1073/pnas.0407894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ha K. C., Chae H. J., Piao C. S., Kim S. H., Kim H. R., Chae S. W. Immunopharmacol. Immunotoxicol. 2005;27:33–51. doi: 10.1081/iph-51292. [DOI] [PubMed] [Google Scholar]
- 21.Chinkers M., Wilson E. M. J. Biol. Chem. 1992;267:18589–18597. [PubMed] [Google Scholar]
- 22.Patel J. B., Valencik M. L., Pritchett A. M., Burnett J. C., Jr., McDonald J. A., Redfield M. M. Am. J. Physiol. 2005;289:H777–H784. doi: 10.1152/ajpheart.00117.2005. [DOI] [PubMed] [Google Scholar]
- 23.Tamura N., Garbers D. L. J. Biol. Chem. 2003;278:48880–48889. doi: 10.1074/jbc.M308680200. [DOI] [PubMed] [Google Scholar]
- 24.Thompson D. K., Garbers D. L. J. Biol. Chem. 1995;270:425–430. doi: 10.1074/jbc.270.1.425. [DOI] [PubMed] [Google Scholar]
- 25.Zahabi A., Picard S., Fortin N., Reudelhuber T. L., Deschepper C. F. J. Biol. Chem. 2003;278:47694–47699. doi: 10.1074/jbc.M309661200. [DOI] [PubMed] [Google Scholar]
- 26.Hutchinson H. G., Trindade P. T., Cunanan D. B., Wu C. F., Pratt R. E. Cardiovasc. Res. 1997;35:158–167. doi: 10.1016/s0008-6363(97)00086-2. [DOI] [PubMed] [Google Scholar]
- 27.Okoshi K., Ribeiro H. B., Okoshi M. P., Matsubara B. B., Goncalves G., Barros R., Cicogna A. C. Jpn. Heart J. 2004;45:647–656. doi: 10.1536/jhj.45.647. [DOI] [PubMed] [Google Scholar]
- 28.Pierkes M., Gambaryan S., Boknik P., Lohmann S. M., Schmitz W., Potthast R., Holtwick R., Kuhn M. Cardiovasc. Res. 2002;53:852–861. doi: 10.1016/s0008-6363(01)00543-0. [DOI] [PubMed] [Google Scholar]
- 29.Thomas C. J., Head G. A., Woods R. L. J. Hypertens. 1999;17:801–806. doi: 10.1097/00004872-199917060-00012. [DOI] [PubMed] [Google Scholar]
- 30.Igaki T., Itoh H., Suga S. I., Hama N., Ogawa Y., Komatsu Y., Yamashita J., Doi K., Chun T. H., Nakao K. Hypertens. Res. 1998;21:7–13. doi: 10.1291/hypres.21.7. [DOI] [PubMed] [Google Scholar]
- 31.Wennberg P. W., Miller V. M., Rabelink T., Burnett J. C., Jr. Am. J. Physiol. 1999;277:H1618–H1621. doi: 10.1152/ajpheart.1999.277.4.H1618. [DOI] [PubMed] [Google Scholar]
- 32.Wei C. M., Aarhus L. L., Miller V. M., Burnett J. C., Jr. Am. J. Physiol. 1993;264:H71–H73. doi: 10.1152/ajpheart.1993.264.1.H71. [DOI] [PubMed] [Google Scholar]
- 33.Barletta G., Lazzeri C., Vecchiarino S., Del B. R., Messeri G., Dello S. A., Mannelli M., La V. G. Hypertension. 1998;31:802–808. doi: 10.1161/01.hyp.31.3.802. [DOI] [PubMed] [Google Scholar]
- 34.Clavell A. L., Stingo A. J., Wei C. M., Heublein D. M., Burnett J. C., Jr. Am. J. Physiol. 1993;264:R290–R295. doi: 10.1152/ajpregu.1993.264.2.R290. [DOI] [PubMed] [Google Scholar]
- 35.Stingo A. J., Clavell A. L., Aarhus L. L., Burnett J. C., Jr. Am. J. Physiol. 1992;262:H308–H312. doi: 10.1152/ajpheart.1992.262.1.H308. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji T., Kunieda T. J. Biol. Chem. 2005;280:14288–14292. doi: 10.1074/jbc.C500024200. [DOI] [PubMed] [Google Scholar]
- 37.Popova E., Krivokharchenko A., Ganten D., Bader M. Therio-genology. 2004;61:1441–1453. doi: 10.1016/j.theriogenology.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Wallukat G., Morwinski R., Kuhn H. J. Biol. Chem. 1994;269:29055–29060. [PubMed] [Google Scholar]
- 39.Misra R. P. Am. J. Clin. Pathol. 1972;58:135–139. doi: 10.1093/ajcp/58.2.135. [DOI] [PubMed] [Google Scholar]
- 40.Langenickel T. H., Pagel I., Buttgereit J., Tenner K., Lindner M., Dietz R., Willenbrock R., Bader M. Am. J. Physiol. 2004;287:H1516–H1521. doi: 10.1152/ajpheart.00947.2003. [DOI] [PubMed] [Google Scholar]
- 41.Gross V., Tank J., Obst M., Plehm R., Blumer K. J., Diedrich A., Jordan J., Luft F. C. Am. J. Physiol. 2005;288:R1134–R1142. doi: 10.1152/ajpregu.00246.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.