Abstract
We have previously reported the synthesis of four α-cyano-containing ethers based on 2-naphthaldehyde (2-NA) as cytochrome P450 (P450) fluorescent substrates. Activity detection was based on the formation of fluorescent 2-NA following substrate hydrolysis. A major limitation of these substrates was the need to remove NADPH, a required cofactor for P450 oxidation, before measuring 2-NA fluorescence. In this article, we report the synthesis of a new series of novel P450 substrates using 6-dimethylamino-2-naphthaldehyde (6-DMANA), which has a green fluorescent emission that is well separated from the NADPH spectrum. A major advantage of the 6-DMANA substrates is that NADPH removal is not required before fluorescence detection. We used eight α-cyano ether-based substrates to determine the O-dealkylation activity of human, mouse, and rat liver microsomes. In addition, substrate activities were compared with the commercial substrate 7-ethoxyresorufin (7-ER). The catalytic turnover rates of both the 6-DMANA- and 2-NA-based substrates were in some cases threefold faster than the catalytic turnover rate of 7-ER. The 2-NA-based substrates had greater turnover than did the 6-DMANA-based substrates. Murine and rat liver microsomes prepared from animals that had been treated with various P450 inducers were used to examine for isozyme-selective turnover of the substrates. The vastly improved optical properties and synthetic flexibility of the α-cyano ether compounds suggest that they are possibly good general P450 substrates.
Keywords: Cytochrome P450, Fluorescent substrate, Liver microsomes, α-Cyanohydrin ether
Cytochrome P450s (P450s or CYP)1 are heme proteins that exist in variety of forms, with more than 500 different P450 isozymes reported. These enzymes play a significant role in the metabolism of a wide variety of xenobiotics, such as pesticides, food additives, and industrial chemicals, as well as endogenous compounds [1]. Because the P450s are among the most important enzyme families involved in the oxidative metabolism of drugs in mammalian systems [2], analysis of drug metabolism by P450s has become an essential part of the drug development process [3,4]. P450 activity can also be responsible for insect resistance to many pesticides [5-7]. There are a number of different assay systems available for measuring P450 activity, including alkoxyresorufins, alkoxycoumarins, and their modified analogues [8-11].
Our laboratory has reported that α-cyano-containing esterase substrates have very low background fluorescence and are stable under most enzyme assay conditions [2]. The assays using these substrates had increased sensitivity relative to many other standardized assays [12,13]. We have also reported the design and synthesis of a P450 substrate [14] that also possessed the α-cyano group and had very low background fluorescence. P450 activity is detected via decomposition of the O-dealkylation intermediate forming the highly fluorescent 2-naphthaldehyde (2-NA). In addition, these substrates are stable under typical enzyme assay conditions. However, 2-NA was found to have an emission wavelength similar to that of NADPH, which is a required cofactor for P450 oxidation. Therefore, it was necessary to remove excess NADPH from the assay. This process was circumvented by using a new technique for the rapid removal of NADPH, but a major limitation of the resulting assay is that only endpoint measurements, rather than kinetic measurements, were possible.
This study was designed to develop highly sensitive fluorescent substrates of P450 that did not require the removal of NADPH. In this article, we describe the design and synthesis of four fluorescent substrates for P450 that contain an α-cyano group and 6-dimethylamino-2-naphthaldehyde (6-DMANA) as a fluorescent probe. We have tested these substrates as well as the original substrates based on 2-NA to characterize their properties and specificity. For comparison, the P450 activity was also investigated with the most commonly used substrate, 7-ethoxyresorufin (7-ER).
Materials and methods
Reagents
NADPH, glucose-6-phosphate, NADP, glucose-6-phosphate dehydrogenase, dimethyl sulfoxide (DMS O), bovine serum albumin (BSA), and 7-ER were purchased from Sigma (St. Louis, MO, USA). DEAE sepharose fast flow was purchased from Pharmacia (Uppsala, Sweden). Bradford reagent was obtained from Bio-Rad (Hercules, CA, USA). 7-Hydroxy-4-tri-fluoromethylcoumarin was purchased from Molecular Probes (Eugene, OR, USA). All other reagent-grade chemicals were obtained from Aldrich (Milwaukee, WI, USA). Substrates 1–4 have been reported previously [14]. All substrates used in this study were synthesized as racemic mixtures.
Fisher 344 rat liver microsomes and expressed rat CYP1A1 were from Wheelock et al. [15] and Grant et al. [16], respectively. Human microsomes were purchased from BD Gentest (Woburn, MA, USA).
Instrumentation
1H NMR and 13C NMR spectra were obtained on a Mercury 300 spectrometer (Varian, Palo Alto, CA, USA). Chemical shift values are reported in parts per million with CDCl3 as the solvent and TMS as the internal standard. GC/MS values were determined on a Hewlett–Packard model 5890 gas chromatograph equipped with an HP 5973 mass detector (Hewlett–Packard, Arondale, PA, USA) and a 30 m × 0.25-mm i.d. capillary column coated with a 0.25 μm film of 5:95 methylphenyl-substituted dimethylpolysiloxane (DB–5ms, J & W Scientific, Folsom, CA, USA). The LC/MS system consisted of a Micromass Quattro Ultima triple quadrupole tandem mass spectrometer (Micromass, Manchester, UK) equipped with an atmospheric pressure ionization source. Melting points were determined using a Thomas Hoover capillary melting point apparatus (A. H. Thomas, Philadelphia, PA, USA) and are uncorrected. Fluorescent detection was performed with a Fluoromax-2 fluorospectrometer (Instruments SA, Edison, NJ, USA). UV absorption was measured on a Cary 100 Bio spectrophotometer (Varian, Walnut Creek, CA, USA).
Synthesis
All solvents were dried before use. Hexamethylphosphoramide (HMPA) was dried over CaH2 and benzene was dried over sodium and distilled prior to use. Trimethyl orthoformate, triethyl orthoformate, pentyl alcohol, and benzyl alcohol were dried over 4Å molecular sieves. Thin-layer chromatography (TLC) used 0.2-mm glass plates precoated with silica gel 60 F254 (Merck, Darmstadt, Germany), and chemical detection was based on the quenching of fluorescence from UV light at 254nm. Flash chromatographic separations were carried out on 40-μm average particle size Baker silica gel (Fisher, Tustin, CA, USA).
All chemical reactions were performed under an inert atmosphere. As shown in Scheme 1, 6-DMANA is readily prepared from the commercially available 6-methoxynaphthaldehyde [17]. The target P450 fluorogenic probes were synthesized according to our previously reported procedure by refluxing the aldehyde with trimethyl orthoformate, triethyl orthoformate, pentyl alcohol, and benzyl alcohol, respectively, to give the requisite diacetals [14]. After removing the excess solvent under reduced pressure, one alkoxy group is replaced with cyanide in high yield following the addition of cyanotrimethylsilane and a catalytic amount of SnCl2. All new compounds were characterized via 1H NMR, GC/MS, and elemental analysis. The purity of all compounds was greater than 97% as determined by LC/MS.
Scheme 1.
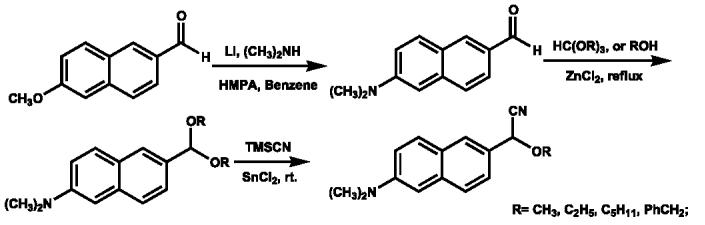
Syntheses of 6-DMANA and α-cyano ether substrates.
6-Dimethylamino-2-naphthaldehyde
Gaseous dimethylamine was introduced into a mixture of 5.7 ml anhydrous hexamethylphosphoramide and 7 ml dry benzene until 1.05 g (23.1 mmol) was dissolved. Hammered Li wire (0.15 g, 21 mmol) was cut into small pieces and added under argon. Shortly thereafter, a deep red color gradually developed in the reaction mixture with some warming. After dissolution of the lithium that occurred in 1–2 h, 1.02 g (5.4 mmol) of 6-methoxy-2-naphthaldehyde was added. The reaction was stirred at room temperature overnight (12–16 h). The reaction was quenched with 2 ml of absolute ethanol and then was poured onto crushed ice, extracted with diethyl ether (3× 20 ml), washed with water (3× 5 ml), and dried over magnesium sulfate. After evaporation of solvent, the solid was recrystallized from 95% ethanol to give a bright yellow crystal (0.91 g) with a total yield of 85%, mp 108–109 °C; TLC Rf 0.60 (hexane:EtOAc = 3.5:1, v/v); 1H NMR (CDCl3): δ 3.07 (s, 6H, N(CH3)2), 6.85–6.86 (m, 1H), 7.13–7.16 (m, 1H), 7.62–7.65 (m, 1H), 7.77–7.83 (m, 2H), 8.11–8.12 (m, 1H), 9.99 (s, 1H, CHO); MS (EI-70 ev): m/z % 199 [M+, 100],170 [(M-CHO)+,10.5], 154 [(M-N(CH3)2)+, 9.5].
2-(2-(Dimethylamino)naphthalen-6-yl)-2-methoxyethanenitrile 5
This was a white plate crystal with a total yield of 83%, mp 99–100 °C; TLC Rf 0.77 (hexane:EtOAc = 3.5:1, v/v); 1H NMR (CDCl3): δ 3.05 (s, 6H, N(CH3)2), 3.50 (s, 3H, OCH3), 5.32 (s, 1H, CHCN), 6.88–7.80 (m, 6H, Ar-H); MS (EI-70 ev): m/z % 240 [M+, 30], 209 [(M-OCH3)+, 100], 193 (12.6), 166 (5.7), 104 (6); ESI–MS: calcd. for C15H16N2O [(M+H)+], 241.1342; found: 241.1345.
2-(2-(Dimethylamino)naphthalen-6-yl)-2-ethoxyethanenitrile 6
This was a bright yellow powder with a total yield of 89%, mp 65–66 °C; TLC Rf 0.56 (hexane:EtOAc = 5:1, v/v). 1H NMR (CDCl3): δ 1.32 (t, J = 6.8 Hz, 3H, CH3), 3.20 (s, 6H, N(CH3)2), 3.67–3.92 (m, 2H, OCH2), 5.42 (s, 1 H, CHCN), 7.60–7.80 (m, 2H), 7.90–8.19 (m, 4 H); MS (EI-70 ev): m/z % 254 [M+, 30], 209 [(M-OCH2CH3)+, 100], 193 [(M-OCH2CH3–CH4)+, 12.4], 166 (5.7), 104 (7.1); anal. calcd. for C16H18N2O: C, 75.56; H, 7.13; N, 11.01; found: C, 75.73; H, 7.38; N, 10.63.
2-(2-(Dimethylamino)naphthalen-6-yl)-2-pentoxyethanenitrile 7
This was a white needle-like crystal with a total yield of 86%, mp 62–63 °C; TLC Rf 0.62 (hexane:EtOAc = 3.5:1, v/v); 1H NMR (CDCl3): δ 0.80–1.00 (m, 5H), 1.23–1.35 (m, 2H), 1.50–1.62 (m, 2H), 3.08 (s, 6H, N(CH3)2), 3.50–3.78 (m, 2H), 5.38 (s, 1H, CHCN), 7.40–7.46 (m, 2H, Ar-H), 7.68–7.84 (m, 4H, Ar-H); MS (EI-70 ev): m/z % 296 [M+, 24.8], 209 [(M-OC5H11)+, 100], 193 [(M-OC5H11-CH4)+ , 10.5], 166 (4.8), 104 (2.4); anal. calcd. for C19H24N2O: C, 76.99; H, 8.16; N, 9.45; found: C, 77.26; H, 8.35; N, 9.50.
2-(2-(Dimethylamino)naphthalen-6-yl)-2-benzyloxyethanenitrile 8
Conversion of the aldehyde to the diacetal is accomplished in only 50% yield. Conversion of the diacetal to the α-cyano ether occurs with yields up to 95%. The target compound is a white needle-like crystal, mp 91–92 °C; TLC Rf 0.73 (hexane:EtOAc = 3.5:1, v/v); 1H NMR (CDCl3): δ 3.10 (s, 6H, N(CH3)2), 4.66–4.84 (m, 2H, OCH2), 5.39 (s, 1H, CHCN), 7.20–7.30 (m, 3H), 7.32–7.46 (m, 5H), 7.66–7.86 (m, 3H); MS (EI-70 ev): m/z % 316 [M+, 23.3], 209 [(M-OCH2Ph)+, 100], 193 [(M-OCH2Ph-CH4)+ ,11.9], 166 (7.6); ESI–MS: calcd. for C21H20N2O [(M+H)+], 317.1655; found: 317.1632.
Properties of aldehydes and substrates
Fluorescent spectra of aldehydes were measured in 4-ml cuvettes in 0.1 M sodium phosphate buffer (pH 7.8). Solubility of aldehydes and substrates was measured in 0.1 M sodium phosphate buffer (pH 7.8) according to Nellaiah et al. [18]. Absorbance was read at 700 nm. Stability of substrates was checked by TLC and UV spectrum scan. Substrates were spotted on silica gel TLC plates and developed in hexane–ethylacetate (3:1). Developed plates were examined for fluorescence under 254 or 366 nm light. Absorption spectra were recorded from 200 to 500 nm.
Microsome preparation
Male Swiss–Webster mice were purchased from Charles River Breeding Laboratory (Hollister, CA, USA) and were 20–25g on receipt. Mice were housed in HEPA-filtered racks for 7 days before use and were fed and watered ad libitum with a light cycle of 12 h light and 12 h dark. Animal care procedures were approved by the animal use and care committee at the University of California, Davis. β-NaphthoXavone (BNF, 80 mg/kg body weight) in corn oil or phenobarbital (PB, 50 mg/kg body weight) in saline solution was injected intraperitoneally each day for 5 days, and control mice were injected with an equal volume of vehicle alone according to the methods of Chen et al. [19]. On the 6th day, mice were sacrificed with an overdose of pentobarbital. Livers were immediately excised, rinsed in a 0.9% NaCl solution (1% w/v), and frozen at −80°C.
Wistar rats were obtained from the vivarium of the Institute of Cytology and Genetics (Russian Academy of Sciences) and were used for inducer treatment. Rats (4–6 weeks old) were treated with single intraperitoneal injection of 3-methylcholanthene (MC, 75 mg/kg body weight) in corn oil. PB was injected at 24-h intervals for 3 days (80 mg/kg body weight) in saline solution. The animals were sacrificed on the 4th day after the first injection. Control animals received an equal volume of the vehicle only. Livers were promptly removed, perfused with 1.15% KCl solution, and frozen at –80 °C.
Microsomes were isolated using the usual method of differential centrifugation [9], and protein concentrations of the microsomal fractions were determined by the method of either Bradford [20] or Lowry et al. [21] using BSA as the standard.
Time and protein dependency of 6-DMANA and 2-NA
Before determining the enzyme kinetic parameters of the substrates, linearity of the formation of 6-DMANA and 2-NA and its protein dependency were investigated by incubating substrate 2 (final concentration 33μM) in 40mM Tris buffer (or incubating substrate 6 (final concentration 33μM) in 100mM Hepes buffer), 167μM NADPH, containing 18.75, 37.5, 75, 150, or 300 μg rat liver microsomes.
The amount of 6-DMANA produced was detected at different time points. The assay was performed by incubating substrate 6 (final concentration 33 μM) in 100 mM Hepes buffer (pH 7.8), 10 μl NADPH regenerating system (0.5 mM glucose 6-phosphate, 0.1 mM NADP, and 0.14 U/ml glucose-6-phosphate dehydrogenase) [22], 150 μg rat liver microsomes.
Assays for O-dealkylation activity
Substrates based on 6-DMANA
The oxidation of 6-DMANA-based substrates (5–8) was assayed at 37 °C in a final volume of 3 ml of 100 mM Hepes buffer (pH 7.8) with 5 mM MgCl2 and 0.1% BSA. To prepare for the reaction, the substrates (10 μl of 1.5 mM in DMSO, final concentration 50 μM) were incubated with microsomes (200–300 μg) at 37 °C for 5 min, and then the reaction was initiated by the addition of NADPH (final concentration 250 μM). The samples were immediately read by a fluorometer at 531 nm (slit 5 nm) for 5 min with excitation at 410 nm (slit 5 nm), and the real-time increase in fluorescence was recorded. O-deethylation of 7-ER (final concentration 33 μM) was measured at excitation 530 nm (slit 5 nm) and emission 584 nm (slit 5 nm) [23].
Substrates based on 2-NA
Microsomes (200–300 μg) were incubated at 37 °C in a final volume of 3 ml of 40 mM Tris–HCl buffer (pH 7.8), including the substrates (1–4: final concentration 33 μM) in DMSO (DMSO final concentration 0.3%). Reactions were initiated by the addition of 167 μM NADPH. Removal of NADPH was adopted using the method of Zhang et al. [14]. Briefly, after incubation for 30 min, the reactions were added into DEAE sepharose (1.5 ml)-containing tubes and mixed well to remove NADPH. Before use, DEAE ion exchange was washed five times to equilibrate with the reaction buffer. The mixed tubes were centrifuged at 3000 rpm for 5 min. The concentration of 2-NA in the solution was determined by fluorescence with excitation at 344 nm (slit 5 nm) and emission at 460 nm (slit 5 nm).
Determination of Michaelis–Menten parameters for P450 isozyme activities
The kinetics of O-dealkylation of substrates with MC- or PB- induced rat microsomes were determined. Enzyme velocity experiments were carried out over a range of concentrations of the P450 substrates containing 6-DMANA (0.5–33.0 μM) and substrates containing 2-NA (0.05–1.25 μM). Because assays were performed with microsomal preparations versus purified enzymes, kinetic constants are reported as apparent values. The Michaelis constant (Km app) and maximum velocity (Vmax app) with standard errors were estimated by fitting the Michaelis–Menten equation to the data using nonlinear regression analysis (Origin 6.0, OriginLab, Northampton, MA, USA). Initial estimates for nonlinear regression were chosen based on substrate concentration (S) versus reaction velocity (V) plots.
Results
Spectral properties of screened aldehydes
A number of aldehydes were screened for their physical properties, including UV spectra, fluorescence, and solubility. Table 1 shows that 6-DMANA, 6-methoxy-2-naphthaldehyde, 9H-fluorene-2-carbaldehyde, anthracene-9-carbaldehyde, naphthene-2-carbaldehyde, and pyrene-1-carbaldehyde have relatively high fluorescent intensities. However, 9H-fluorene-2-carbaldehyde and pyrene-1-carbaldehyde have a very small Stokes' shift, rendering them unsuitable for substrate development. Anthracene-9-carbaldehyde possesses high fluorescent intensity, a large Stokes' shift, and an emission wavelength that is shifted into the green portion of the visible spectrum. Unfortunately, the difference in emission wavelengths between the substrate and the aldehyde is small. In addition, the solubility of anthracene-9-carbadehyde in water is poor [24]. The next aldehyde that was tested as a possible probe for a P450 activity reporter was 6-dimethylamino-2-naphthaldehyde. This aldehyde has a large Stokes' shift, a relatively high fluorescent intensity, a red-shifted wavelength, and a large difference in emission wavelengths between the substrate and the aldehyde. Because of these physical properties, this aldehyde was chosen for further substrate development.
Table 1.
Optical properties of screened aldehydes and common probes
| Aldehyde and common probe | UV absorption |
Fluorescence |
||||
|---|---|---|---|---|---|---|
| λmax (nm) | εmaxa | Ex (nm)b | Emmax (nm)c | Stokes' shift (nm) | Relative intensityd | |
| 2-Naphthaldehyde | 292 | 12.7 | 344 | 444 | 100 | 100 |
| 6-Methoxy-2-naphthaldehyde | 314 | 19.1 | 330 | 460 | 130 | 260 |
| Pyrene-1-carbaldehyde | 397 | 9.8 | 397 | 470 | 73 | 246 |
| 9H-Fluorene-2-carbaldehyde | 318 | 34.6 | 330 | 409 | 79 | 188 |
| 6-Dimethylamino-2-naphthaldehyde | 377 | 18.4 | 396 | 528 | 132 | 173 |
| Anthracene-9-carbaldehyde | 408 | 6.7 | 410 | 519 | 109 | 106 |
| 10-Methyl-anthracene-9-carbaldehyde | 425 | 9.1 | 399 | 531 | 132 | 26 |
| Phenanthrene-9-carbaldehyde | 318 | 13.2 | 334 | 471 | 137 | 25 |
| 10-Chloro-antharacene-9-carbaldehyde | 437 | 6.0 | 410 | 532 | 122 | 22 |
| 3-(4-Nitro-phenyl)-propenal | 308 | 27.0 | 397 | 460 | 63 | 9 |
| 3-(4-Dimethylamino-phenyl)-propenal | 399 | 41.1 | 397 | 494 | 97 | 5 |
| 4-Quinoline-carbaldehyde | 315 | 3.6 | 335 | 430 | 95 | 5 |
| 5-(4-Dimethylamino-phenyl)-penta-2,4-dienal | 409 | 31.1 | 418 | 593 | 175 | 5 |
| 2-Quinoline-carbaldehyde | 301 | 5.7 | 346 | 420 | 74 | 4 |
| 4-Dimethylamino-benzaldehyde | 354 | 33.7 | 330 | 410 | 80 | 3 |
| 3-Quinoline-carbaldehyde | 292 | 10.6 | 345 | 430 | 85 | 2 |
| Resorufin | 571 | 64.55 | 571 | 581 | 10 | 275 |
| 7-Hydroxy-4-trifluoromethyl coumarin | 341 | 11.11 | 356 | 496 | 140 | 276 |
Note. The data were obtained in 0.1 M sodium phosphate buffer (pH 7.8).
Molar absorptivity (103 M−1 cm−1).
Ex: optical excitation wavelength.
Emmax: maximal emission wavelength.
Relative intensity: fluorescent intensity of 2-NA is 100.
Spectral properties of synthesized substrates and the substrates
We first reported an α-cyano ether series of P450 fluorescent substrates that were based on 2-NA with fluorescence in the blue region of the visible spectrum (Table 2). In contrast, the fluorescent spectrum of 6-DMANA in phosphate buffer (pH 7.8) showed excitation and emission maxima at 396 and 528 nm, respectively (Table 1). The emission wavelength of 6-DMANA does not overlap with the emission wavelength of NADPH. The synthesized substrates (5–8) do not display overlapping emission with 6-DMANA. Therefore, substrate background is minimal compared with that of the aldehyde produced in the assay. The fluorescent emission and excitation properties of 6-DMANA were found to be independent of buffer pH and concentration, whereas 7-hydroxy-4-trifluoromethylcoumarin was found to have a high dependence on pH and substrate concentration (Table 3).
Table 2.
Structures of synthesized P450 substrates
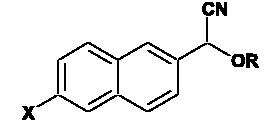 |
| Substrate number | X | R |
|---|---|---|
| 1 | H | CH3 |
| 2 | H | C2H5 |
| 3 | H | C5H11 |
| 4 | H |  |
| 5 | N(CH3)2 | CH3 |
| 6 | N(CH3)2 | C2H5 |
| 7 | N(CH3)2 | C5H11 |
| 8 | N(CH3)2 |  |
Table 3.
Effects of pH and concentration on the fluorescent properties of 6-DMANA and 7-hydroxy-trifluoromethylcoumarin
| 6-DMANA |
7-HFCa |
|||
|---|---|---|---|---|
| Ex (nm) | Emmax (nm) | Ex (nm) | Em (nm) | |
| pHa | ||||
| 6 | 396 | 528 | 340 | 498 |
| 7 | 396 | 528 | 356 | 496 |
| 8 | 396 | 528 | 388 | 531 |
| 9 | 396 | 528 | 367 | 529 |
| Concentrationb(μM) | ||||
| 10 | 396 | 528 | 388 | 531 |
| 1 | 396 | 528 | 391 | 499 |
| 0.1 | 396 | 528 | 395 | 495 |
Note. 7-HFC, 7-hydroxy-4-trifluoromethylcoumarin; Ex, optical excitation wavelength; Emmax, maximal emission wavelength.
Tris–acetate buffer (0.1 M).
Concentration of probes in 0.1 M Hepes buffer (pH 8.0).
Not surprisingly, fluorescent intensity increased with the increasing slit width of the emission (Fig. 1). With an increase of slit width of the emission, the effect of 7-ER on the fluorescent intensity of resorufin was greater than that of substrate 6 on the fluorescent intensity of 6-DMANA (Fig. 1). This observation possibly resulted from greater overlapping between excitation and emission of resorufin (excitation 571 nm, emission 584 nm) compared with that of 6-DMANA (excitation 396 nm, emission 528 nm).
Fig. 1.
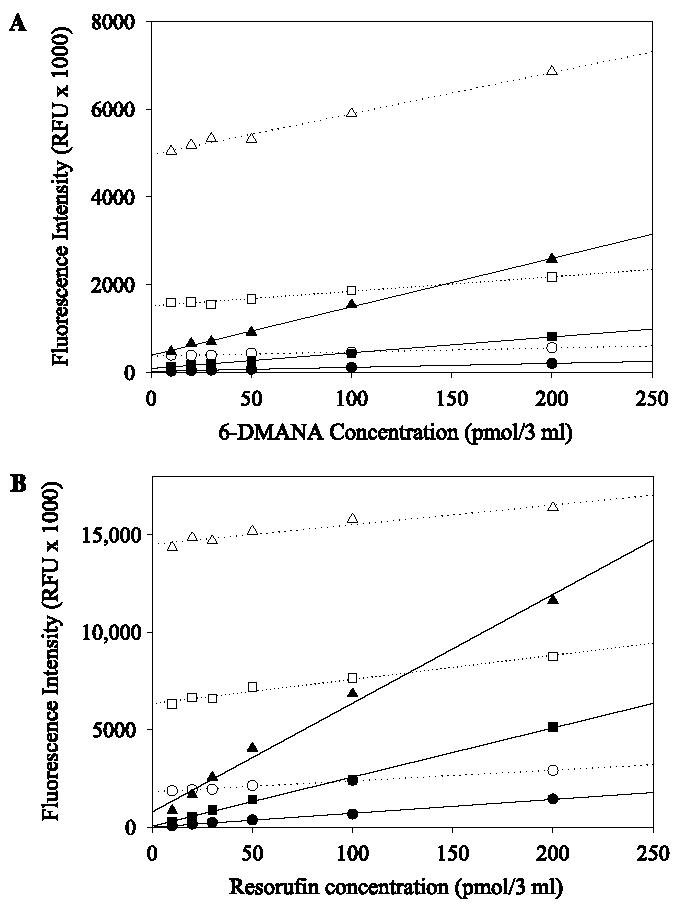
Dependence of fluorescent intensity of 6-DMANA (A) and resorufin (B) on slit width of an emission filter: 5 nm (circle), 10 nm (square), and 20 nm (triangle). The fluorescent intensity was measured both in the presence of probes with their corresponding substrate (33 μM, substrate 6 or 7-ER, open symbols) and without their corresponding substrate (closed symbols).
The solubility of 2-NA was reported to be 1.0–1.5 mM in sodium phosphate buffer [13]. The aqueous solubility of 6-DMANA (0.25–0.5 mM) was less than that of 2-NA. The substrates based on 2-NA were also slightly more soluble (60–80 μM) than those based on 6-DMANA (40–60 μM) in sodium phosphate buffer. The observed order of solubility of 2-NA- and 6-DMANA-based substrates was ethyl (2, 6)>methyl (1, 5) > pentyl (3, 7) > benzyl (4, 8) (data not shown).
To evaluate the stability of these compounds, the ethyl α-cyano ether (6) in DMSO was stored in the dark at room temperature and at −20 °C. Stability was monitored by UV spectra, fluorescence, and TLC. Slight substrate decomposition to the corresponding aldehyde was observed, but the loss was less than 5% at both room temperature (∼23 °C) and −20 °C over 6 months.
Optimization of the assay conditions
Removing NADPH from the enzyme reaction of 2-NA-based substrates
In the case of 2-NA-based substrates, it is important to remove the remaining NADPH at the end of the reaction because its presence increases the background, making it diffcult to measure activity. When DEAE sepharose was used, it gave at least a 95% recovery rate of 2-NA and greater than 96% binding NADPH in 40 mM Tris buffer (data not shown).
Linearity of reaction
Assays with 6-DMANA could not be performed with an excitation at 396 nm due to cofluorescence of the substrate at this wavelength. Background was decreased significantly by using an excitation wavelength of 410 nm. As can be seen in Fig. 2, the formation of fluorescent product was linear (R2 = 0.995) for up to 30 min when a 410-nm excitation wavelength and a 531-nm emission wavelength were used. In addition, formation of 2-NA, 6-DMANA, and resorufin was linear with respect to protein concentration at a substrate concentration of 10 μM (Fig. 3). Based on these results, the turnover rates of substrate 2 and substrate 6 are four and three times faster, respectively, than the turnover rate of 7-ER.
Fig. 2.
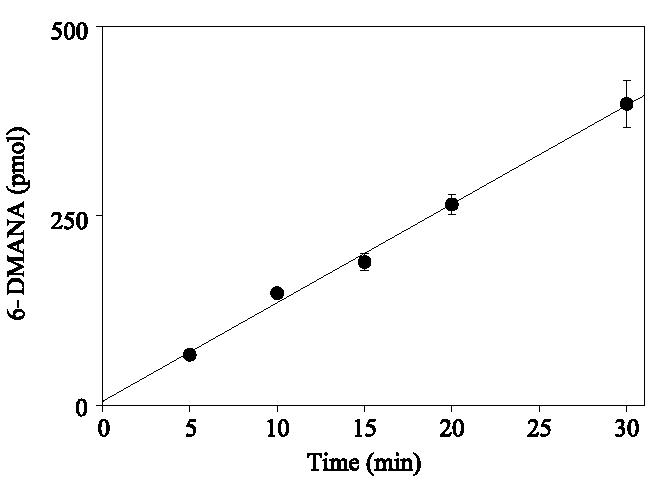
Time-dependent formation of fluorescence expressed as picomoles of 6-DMANA from substrate 6 (r2 = 0.995). Assays were performed at 37 °C in 0.1 M Hepes buffer system (pH 7.8) containing substrate 6 (final concentration 33 μM), NADPH (final concentration 167 μM), and a series concentration of rat liver microsomes (18.75, 37.5, 75, 150, or 300 μg proteins in 3 ml). Data are the means and standard deviations of three replicates.
Fig. 3.
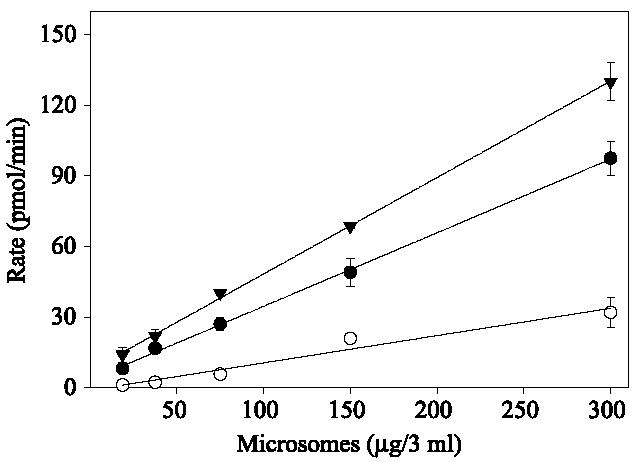
Dependence on the observed reaction rate between amount of microsomes and substrates: substrate 2 (▼), substrate 6 ([unk]), and 7-ER (○). Assays were conducted in a final volume of 3 ml with varying concentrations of rat liver microsomes in 40 mM Tris–HCl at pH 7.8 with a final substrate concentration of 33 μM. Assays with substrate 6 (33 μM) were identical except that 0.1 M Hepes (pH 7.8) buffer was used. Data are the means and standard deviations of three replicates.
O-dealkylation of 6-DMANA substrates by several microsomal preparations
Eight α-cyano ether compounds were tested for measuring P450 activity with rat (Fisher and Wistar), human, expressed rat CYP 1A1, and liver microsomes prepared from mice that had been treated with a variety of P450 inducers (Table 4). In rat (Fisher) liver microsomes, the relative rate of O-dealkylation with 6-DMANA was methyl (5)>ethyl (6)>benzyl (8)>pentyl (7), whereas that with 2-NA-based substrates was methyl (1)>benzyl (4)>pentyl (3)>ethyl (2) [14]. Although the structures of the substrates based on 2-NA and 6-DMANA series are similar, these substrates did exhibit different oxidation profiles. The 2-NA-based substrates had greater turnover than did the 6-DMANA-based substrates in all systems analyzed. The oxidation rate of all substrates using rat (Fisher) liver microsomes was higher than that of 7-ER. In rat (Wistar) liver microsomes, the relative order of the rate using 2-NA-based substrates was methyl (1)>pentyl (3)>ethyl (2)>benzyl (4). In addition, O-dealkylation of pentyl (7) and benzyl (8) ether substrates was not detected with Wistar rat liver microsomes. In the case of human microsomes, the O-dealkylation rates of 2-NA-based substrates 1 to 4 were higher than that of 7-ER, but no O-dealkylation activity could be detected with substrates 5 to 8. In contrast, no activity of expressed CYP 1A1 was detected using 2-NA-based substrates 1 to 4, but activity with 6-DMANA-based substrates was observed. The relative order of O-dealkylation rates using 6-DMANA-based substrates was methyl (5)>ethyl (6)>pentyl (7)>benzyl (8). The order is the same as that of the rat (Fisher) liver microsomes.
Table 4.
Apparent O-dealkylation activity of substrates by microsomes of different species
| Treatment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 7-Ethoxyresorufin | |
|---|---|---|---|---|---|---|---|---|---|---|
| Rat (Fisher) | None | 710 ± 30a | 410 ± 25a | 533 ± 3a | 544 ± 16a | 473 ± 35 | 378 ± 20 | 140 ± 4 | 160 ± 8 | 97 ± 8 |
| Rat (Wistar) | None | 1400 ± 300 | 570 ± 90 | 610 ± 50 | 420 ± 70 | 900 ± 100 | 1100 ± 200 | ND | ND | 70 ± 19 |
| Human | None | 147 ± 19 | 305 ± 21 | 353 ± 19 | 332 ± 15 | ND | ND | ND | ND | 50 ± 5 |
| CYP 1A1b | None | ND | ND | ND | ND | 106 ± 10 | 60 ± 2 | 30 ± 1 | 30 ± 2 | 23 ± 6 |
| Murine | Controlc | 139 ± 4 | 330 ± 27 | 232 ± 32 | 270 ± 27 | ND | ND | ND | 122 ± 2 | 52 ± 4 |
| BNFd | 143 ± 13 | 745 ± 46 | 223 ± 33 | 592 ± 96 | ND | ND | ND | 203 ± 11 | 324 ± 14 | |
| PBe | 113 ± 8 | 636 ± 16 | 126 ± 18 | 172 ± 23 | ND | 134 ± 3 | ND | 244 ± 25 | 66 ± 5 |
Note. Data are the means and standard deviations of three replicates (in pmol/min/mg). ND, not detected (data were below the limit of detection that was considered as corresponding to a signal-to-background ratio of approximately 3).
Rat (Fisher) data from Zhang and coworkers [14].
CYP 1A1 was expressed in insect cells using baculovirus [16].
Control rats were treated by saline or corn oil.
BNF: β-Naphthoflavone (80 mg/kg body weight).
PB: Phenobarbital (50 mg/kg body weight).
To investigate substrate selectivity, microsomes prepared from BNF- and PB-treated mice were used to measure turnover of both the 2-NA- and 6-DMANA-based substrates. BNF and PB are known CYP 1A1 and CYP 2B inducers, respectively [8]. The O-dealkylation rate order of 2-NA-based substrates in the control, BNF, and PB was ethyl (2)>benzyl (4)>pentyl (3)>methyl (1). In BNF-induced microsomes, the ethyl (2) and benzyl (4) ether were twofold higher than in control microsomes. In the case of 6-DMANA substrates, benzyl (8) ether in BNF- and PB-induced microsomes had a two-fold higher debenzylation rate than in control microsomes. In contrast, with the substrates methyl (5), ethyl (6), and pentyl (7) ether, dealkylation rates were not detected in control, BNF-, and PB-induced microsomes except for ethyl (6) ether in PB-induced microsomes. Interestingly, the O-deethylation of ethyl (6) ether was measured only in PB-induced microsomes.
Enzyme kinetics of induced rat microsomes
Kinetic constants for O-dealkylation of the substrates were measured with microsomes prepared from PB- and MC-treated rats as well as untreated rats (Table 5). MC and PB are known CYP 1A and CYP 2B inducers [8]. We observed a significant increase in the P450-mediated oxidation rate of substrates 1 to 4 from microsomes prepared from PB- and MC-treated animals as well as a reduction in the Km app values. When examining the catalytic activity of the microsomes prepared from MC- and PB-treated individuals, the greatest differences were observed with the ethyl derivative (2), with the rates of oxidation increasing 39- and 79-fold, respectively. In addition, the Vmax/Km ratios of the ethyl derivative (2) examined with these microsomes were also highest among the substrates examined (180- and 340-fold, respectively). Unfortunately, these forms of P450 possess overlapping substrate activities toward substrates 1 to 4. P450 activity in the liver of rats treated by PB or MC was increased 1.5- to 2-fold in comparison with controls in the oxidation of methyl (5) or ethyl (6) ether of 6-DMANA. No P450 activity was observed with the pentyl (7) or benzyl (8) ether derivatives.
Table 5.
Apparent kinetic constants for α-cyano ether substrates
| Treatment | 1 |
2 |
3 |
4 |
5 |
6 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vmaxa | Kmb | Vmax | Km | Vmax | Km | Vmax | Km | Vmax | Km | Vmax | Km | |
| Controlc | 1.6 ± 0.1 | 0.59 ± 0.12 | 0.6 ± 0.1 | 0.85 ± 0.21 | 0.7 ± 0.1 | 0.60 ± 0.27 | 0.5 ± 0.1 | 1.62 ± 0.56 | 2.7 ± 0.1 | 19.6 ± 0.3 | 1.8 ± 0.1 | 13.4 ± 0.3 |
| MCd | 31.5 ± 0.9 | 0.29 ± 0.04 | 21.6 ± 1.4 | 0.12 ± 0.03 | 11.9 ± 0.4 | 0.13 ± 0.01 | 5.4 ± 0.2 | 0.19 ± 0.02 | 4.5 ± 0.1 | 20.2 ± 0.2 | 3.4 ± 0.1 | 19.7 ± 0.5 |
| PBe | 22.6 ± 0.8 | 0.20 ± 0.02 | 44.2 ± 3.7 | 0.13 ± 0.04 | 24.0 ± 1.7 | 0.42 ± 0.10 | 13.2 ± 1.1 | 0.25 ± 0.07 | 3.0 ± 0.1 | 18.7 ± 0.3 | 2.5 ± 0.1 | 19.6 ± 0.9 |
Note. Data are from Wistar rat liver microsomes. Activities of substrates 7 and 8 could not be detected. The kinetic data of 7-ER were not determined due to too low activity. Data are the means and standard deviations of three replicates.
Vmax app values are expressed as nanomoles/minute/milligram protein.
Km app values are expressed in micromolars.
Control rats were treated by saline or corn oil.
MC: 3-methylcholanthrene (75 mg/kg body weight).
PB: phenobarbital (80 mg/kg body weight).
Discussion
Recently, our laboratory has reported a series of novel P450 substrates based on 2-NA [14]. The emission wavelength of the substrate is significantly different from that of the aldehyde, which is produced from the dealkylation of the substrate. This difference in fluorescence led to an overall increase in sensitivity of the assay due to the decrease of background of interference. 2-NA has a high fluorescent intensity, a large Stokes' shift, and high solubility in buffer. Because NADPH, which is highly fluorescent at 330 nm, is required as a cofactor for P450 reactions, its presence limits the sensitivity of the assay [9]. In the course of this work, we have developed a convenient protocol for removal of NADPH from incubations by absorption to DEAE sepharose [14], a method that is more efficient and less intensive than published methods involving organic solvent extraction [10] or depletion of the excess NADPH using oxidized glutathione and glutathione reductase [9].
Two aldehydes, 2-NA and 6-DMANA, have been previously reported as chemical reporters for the development of esterase and P450 substrates [12,14]. These aldehydes have a high fluorescent intensity, a high solubility in buffer, and a large Stokes' shift. Therefore, they are good fluorescent reporters. Also, as mentioned previously, in the case of 2-NA, the emission wavelength of the 6-methoxy-2-aldehyde is quite close to that of NADPH, and the assay required an extra step to remove NADPH. Therefore, we screened a series of aldehyde candidates for use as reporter groups in P450 reactions. The selection criteria were as follows: red-shifted emission wavelength, large Stokes' shift, high fluorescent intensity, high aqueous solubility, availability, and low cost. Based on these criteria, 6-DMANA was found to be the most promising candidate.
In this study, four new compounds based on 6-DMANA were synthesized and examined as P450 substrates. The physical properties of 6-DMANA are quite different from those of 2-NA reported previously. First, 6-DMANA has a longer emission wavelength around 530 nm. Common biological materials as well as NADPH have a blue fluorescence emission, whereas 6-DMANA is a green fluorescent compound. Consequently, the background of the assay using 6-DMANA as the reporter group was decreased. Although 6-DMANA-based substrates have lower solubility than do 2-NA-based substrates, 6-DMANA is sufficiently soluble in a buffer system for the enzyme assay. Further work should attempt to develop aldehydes that have increased red shift, improved water solubility, and superior kinetic constants for specific P450 enzymes. The stability of fluorescent intensity or wavelength under various assay conditions is an important factor for enzyme assays. In the case of coumarin, a common reporter for P450 assays, the difference of fluorescent intensity between pHs 7.8 and 9.5 is approximately 50% [10], whereas the fluorescent intensity of 2-NA and 6-DMANA did not change over a pH range of 6 to 9 (data not shown). In addition, the maximal excitation and emission of 7-hydroxy-trifluoromethylcoumarin, an analogue of coumarin, changed depending on pH and reporter concentration. These effects were not observed with 2-NA and 6-DMANA (Table 3). Thus, the pH of the assay with 2-NA and 6-DMANA substrates can be easily changed when optimizing assay conditions.
We measured hydrolysis of all eight substrates using rat, human, and murine liver microsomes. Results showed that O-dealkylation rates of 2-NA-based substrates were higher than the O-dealkylation rate of 6-DMANA. The pattern of the relative rate of O-dealkylation of methyl, ethyl, pentyl, and benzyl ether activities is referred to as the “MEPB profile” [8], which varied among the three species in a species-specific manner. Godden et al. [25] reported that the profile of alkoxyresorufin in rat (Wistar) is ethyl>methyl>benzyl>pentyl. However, this profile was not observed with our substrates. These results demonstrate that each substrate was oxidized by different P450s, unlike alkoxyresorufin. Therefore, these substrates may be useful for development of isozyme-specific P450 assays. In addition, the induction assay results showed the absence of P450 activity in murine microsomes with most of the 6-DMANA-based substrates (5–7). We also found the existence of strong interspecies differences in P450 induction for mice and rats [26]. These results suggest that substrates 1 to4 may be useful in the assessment of CYP 1A and CYP 2B induction. It should be noted that the 6-DMANA derivatives, like other surrogate substrates, have multiple possible routes of metabolism, several of which can yield fluorescent products. The spectral properties of these metabolites are currently being characterized in our laboratory.
Using 7-ER, MC pretreatment of rats (Wistar) caused a 145-fold increase in the Vmax app value [25]. Treating rats (Fisher F-344/N) with MC resulted in a 6-fold increase in the rates of microsomal O-deethylation with 3-cyano-7-ethoxycoumarin as the substrate, whereas PB treatment caused a 21-fold increase [11]. With substrate 2, the O-deethylation of MC- and PB-induced rat microsomes increased 39- and 79-fold, respectively. Using induced rat microsomes, the increase of Vmax app value with substrate 2 was higher than that with 3-cyano-7-ethoxycoumarin but was less than that with 7-ER. Substrates that have high Vmax/Km ratios are optimal for measuring enzymatic activity [27]. Therefore, substrate 2 is the best substrate for measuring O-dealkylation activity in rat liver microsomes among the substrates we developed.
Most existing commercial substrates for P450 are phenyl ethers that contain a phenol derivative as the leaving group. In contrast, the α-cyano ethers reported here employ a non-aromatic leaving group. Thus, when examined with a variety of recombinant P450s, they can be expected to show a new range of specificities. The fundamental structure introduces new possibilities of synthetic variations in the oxidized group, the reporter, and the chiral α-cyano carbon. Possibly the greatest strength of the aromatic aldehyde reporters is their optical properties. The very large Stokes' shift of 6-DMANA and the very low background fluorescence of substrate 6 compared with resorufin and 7-ER translate into assays that are far more sensitive. For example, large slit width or large bandwidth filters can be used to vastly improve sensitivity, and less expensive filters and lamps can be used. These optical properties facilitate development of higher density format.
In conclusion, we have found a useful reporter, 6-DMANA, for P450 activity detection. The substrates synthesized from 6-DMANA overcome the high background problem due to NADPH. Consequently, the enzymatic reaction does not need an extra step for measuring the activity, and the assay is more convenient for measuring enzyme activity. In addition, results from enzymatic assays showed that the turnover rates of α-cyano ether compounds were in some cases three- to fourfold higher than the turnover rate of 7-ER. The concept of using prefluorescent cyanohydrins should be generally applicable to many enzyme and chemical reporter systems.
Acknowledgments
We thank Shuzio G. Kamita for kindly providing expressed CYP 1A1. We also thank David F. Grant, Jeanette E. Stok, Michael D. Denison, and Anthony Cornel for their valuable critique of the manuscript. Paul Jones was supported by an NIH postdoctoral training grant (T32 DK07355-22) and an NIH/NHLBI Ruth L. Kirchstein training grant (F32 HL078096). Craig Wheelock was supported by an NIH postdoctoral training grant (T32 DK07355-22) and a UC Toxic Substances Research & Teaching Program graduate fellowship. This project was funded in part by grants from the NIH/NIAID (U01 AI058267), the USDA Competitive Research Grants Program (2003-35302-13499), the National Institute of Environmental Health Sciences (NIEHS) (R37 ES02710), the NIEHS Superfund Basic Research Program (P42 ES04699), and the NIEHS Center (P30 ES05707).
Footnotes
Abbreviations used: P450 or CYP, cytochrome P450; 2-NA, 2-naphthaldehyde; 6-DMANA, 6-dimethylamino-2-naphthaldehyde; 7-ER, 7-ethoxyresorufin; DMSO, dimethyl sulfoxide; BSA, bovine serum albumin; HMPA, hexamethylphosphoramide; TLC, thin-layer chromatography; BNF, β-naphthoflavone; PB, phenobarbital; MC, 3-methylcholanthene.
References
- 1.Estabrook RW. A passion for P450s (remembrances of the early history of research on cytochrome P450) Drug Metab. Dispos. 2003;31:1461–1473. doi: 10.1124/dmd.31.12.1461. [DOI] [PubMed] [Google Scholar]
- 2.Chauret N, Tremblay N, Lackman RL, Gauthier JY, Silva JM, Marois J, Yergey JA, Nicoll-Griffith DA. Description of a 96-well plate assay to measure cytochrome P4503A inhibition in human liver microsomes using a selective fluorescent probe. Anal. Biochem. 1999;276:215–226. doi: 10.1006/abio.1999.4348. [DOI] [PubMed] [Google Scholar]
- 3.Stresser DM, Turner SD, Blanchard AP, Miller VP, Crespi CL. Cytochrome P450 fluorometric substrates: Identification of isoform-selective probes for rat CYP2D2 and human CYP3A4. Drug Metab. Dispos. 2002;30:845–852. doi: 10.1124/dmd.30.7.845. [DOI] [PubMed] [Google Scholar]
- 4.Venhorst J, Onderwater RCA, Meerman JHN, Commandeur JNM, Vermeulen NPE. Influence of N-substitution of 7-methoxy-4-(aminomethyl)-coumarin on cytochrome P450 metabolism and selectivity. Drug Metab. Dispos. 2000;28:1524–1532. [PubMed] [Google Scholar]
- 5.Feyereisen R. Insect P450 enzymes. Annu. Rev. Entomol. 1999;44:507–533. doi: 10.1146/annurev.ento.44.1.507. [DOI] [PubMed] [Google Scholar]
- 6.Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 7.Mayer RT, Jermyn JW, Burke MD, Prough RA. Methoxyresorufin as a substrate for fluorometric assay of insect microsomal O-dealkylases. Pestic. Biochem. Phys. 1977;7:349–354. [Google Scholar]
- 8.Weaver RJ, Thompson S, Smith G, Dickins M, Elcombe CR, Mayer RT, Burke MD. A comparative study of constitutive and induced alkoxyresorufin O-dealkylation and individual cyto-chrome-P450 forms in cynomolgus monkey (Macaca fascicularis), human, mouse, rat, and hamster liver microsomes. Biochem. Pharmacol. 1994;47:763–773. doi: 10.1016/0006-2952(94)90475-8. [DOI] [PubMed] [Google Scholar]
- 9.Deluca JG, Dysart GR, Rasnick D, Bradley MO. A direct, highly sensitive assay for cytochrome-P450 catalyzed O-deethylation using a novel coumarin analog. Biochem. Pharmacol. 1988;37:1731–1739. doi: 10.1016/0006-2952(88)90436-4. [DOI] [PubMed] [Google Scholar]
- 10.Greenlee WF, Poland A. An improved assay of 7-ethoxycoumarin O-deethylase activity: Induction of hepatic enzyme-activity in C57BL-6J and DBA-2J mice by phenobarbital, 3-methylcholanthrene, and 2,3,7,8-tetrachlorodibenzo-para-dioxin. J. Pharmacol. Exp. Ther. 1978;205:596–605. [PubMed] [Google Scholar]
- 11.White INH. A continuous fluorometric assay for cytochrome-P-450-dependent mixed-function oxidases using 3-cyano-7-ethoxycoumarin. Anal. Biochem. 1988;172:304–310. doi: 10.1016/0003-2697(88)90449-6. [DOI] [PubMed] [Google Scholar]
- 12.Shan GM, Hammock BD. Development of sensitive esterase assays based on α-cyano-containing esters. Anal. Biochem. 2001;299:54–62. doi: 10.1006/abio.2001.5388. [DOI] [PubMed] [Google Scholar]
- 13.Wheelock CE, Wheelock AM, Zhang R, Stok JE, Morisseau C, Le Valley SE, Green CE, Hammock BD. Evaluation of α-cyanoesters as fluorescent substrates for examining interindividual variation in general and pyrethroid-selective esterases in human liver microsomes. Anal. Biochem. 2003;315:208–222. doi: 10.1016/s0003-2697(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhang R, Kang K-D, Shan GM, Hammock BD. Design, synthesis, and evaluation of novel P450 fluorescent probes bearing α-cyanoether. Tetrahedron Lett. 2003;44:4331–4334. [Google Scholar]
- 15.Wheelock CE, Severson TF, Hammock BD. Synthesis of new carboxylesterase inhibitors and evaluation of potency and water solubility. Chem. Res. Toxicol. 2001;14:1563–1572. doi: 10.1021/tx015508+. [DOI] [PubMed] [Google Scholar]
- 16.Grant DF, Greene JF, Pinot F, Borhan B, Moghaddam MF, Hammock BD, McCutchen B, Ohkawa H, Luo G, Guenthner TM. Development of an in situ toxicity assay system using recombinant baculoviruses. Biochem. Pharmacol. 1996;51:503–515. doi: 10.1016/0006-2952(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 17.Weber G, Farris FJ. Synthesis and spectral properties of a hydrophobic fluorescent-probe: 6-Propionyl-2-(dimethylamino)naphthalene. Biochemistry. 1979;18:3075–3078. doi: 10.1021/bi00581a025. [DOI] [PubMed] [Google Scholar]
- 18.Nellaiah H, Morisseau C, Archelas A, Furstoss R, Baratti JC. Enantioselective hydrolysis of p-nitrostyrene oxide by an epoxide hydrolase preparation from Aspergillus niger. Biotechnol. Bioeng. 1996;49:70–77. doi: 10.1002/(SICI)1097-0290(19960105)49:1<70::AID-BIT9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Hennig GE, Whiteley HE, Corton JC, Manautou JE. Peroxisome proliferator-activated receptor alpha-null mice lack resistance to acetaminophen hepatotoxicity following clofibrate exposure. Toxicol. Sci. 2000;57:338–344. doi: 10.1093/toxsci/57.2.338. [DOI] [PubMed] [Google Scholar]
- 20.Bradford MM. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265. [PubMed] [Google Scholar]
- 22.Buters JTM, Schiller CD, Chou RC. A highly sensitive tool for the assay of cytochrome-P450 enzyme activity in rat, dog, and man: Direct fluorescence monitoring of the deethylation of 7-ethoxy-4-trifluoromethylcoumarin. Biochem. Pharmacol. 1993;46:1577–1584. doi: 10.1016/0006-2952(93)90326-r. [DOI] [PubMed] [Google Scholar]
- 23.Burke MD, Thompson S, Elcombe CR, Halpert J, Haaparanta T, Mayer RT. Ethoxyphenoxazones, pentoxyphenoxazones, and benzyloxyphenoxazones and homologs: A series of substrates to distinguish between different induced cytochromes-P-450. Biochem. Pharmacol. 1985;34:3337–3345. doi: 10.1016/0006-2952(85)90355-7. [DOI] [PubMed] [Google Scholar]
- 24.Wroczynski P, Wierzchowski J. Aromatic aldehydes as fluorogenic indicators for human aldehyde dehydrogenases and oxidases: Substrate and isozyme specificity. Analyst. 2000;125:511–516. doi: 10.1039/a906962c. [DOI] [PubMed] [Google Scholar]
- 25.Godden PMM, Kass G, Mayer RT, Burke MD. The effects of cigarette smoke compared to 3-methylcholanthrene and phenobarbitone on alkoxyresorufin metabolism by lung and liver-microsomes from rats. Biochem. Pharmacol. 1987;36:3393–3398. doi: 10.1016/0006-2952(87)90316-9. [DOI] [PubMed] [Google Scholar]
- 26.Poland A, Mak I, Glover E, Boatman RJ, Ebetino FH, Kende AS. 1,4-bis[2-(3,5-Dichloropyridyloxy)]benzene, a potent phenobarbital-like inducer of microsomal mono-oxygenase activity. Mol. Pharmacol. 1980;18:571–580. [PubMed] [Google Scholar]
- 27.Segel IH. Biochemical Calculations: How to Solve Mathematical Problems in General Biochemistry. John Wiley; New York: 1976. [Google Scholar]


