Abstract
Pyrethroid insecticides are known for their potential toxicity to aquatic invertebrates and many fish species. A significant problem in the study of pyrethroid toxicity is their extreme hydrophobicity. They can adsorb to test container surfaces and many studies, therefore, report pyrethroid levels as nominal water concentrations. In this study, pyrethroid adsorption to sampling and test containers was measured and several container treatments were examined for their ability to decrease pyrethroid adsorption. None of the chemical treatments were successful at preventing pyrethroid loss from aqueous samples, but vortexing of containers served to resuspend pyrethroids. The effects of the observed adsorption on Ceriodaphnia dubia and Hyalella azteca permethrin toxicity were examined. Species-specific results showed a time-dependent decrease in toxicity following pyrethroid adsorption to test containers for C. dubia, but not for H. azteca. These results demonstrate that pyrethroid adsorption to containers can significantly affect the observed outcome in toxicity-testing and serves as a caution for researchers and testing laboratories.
Keywords: Pyrethroid, Toxicity testing, Adsorption, Ceriodaphnia dubia, Hyalella azteca
The use of pyrethroids has steadily increased since their introduction into the pesticide market due to a number of desirable physical properties including low mammalian toxicity and reduced environmental persistence (Casida and Quistad, 1998). This increase has resulted in a growing need to analyze aquatic samples for the presence and potential toxic effects of pyrethroids. However, one major difficulty in conducting toxicity studies with pyrethroids is their extreme hydrophobicity (Lee et al., 2002). The compounds readily adsorb to test containers resulting in many researchers reporting aqueous levels as “nominal concentrations”. Given the propensity of pyrethroids to adsorb to sampling and testing container surfaces, it is likely that nominal water concentrations are often lower than expected. This fact could affect the outcome of toxicity-testing in that organisms may be exposed to lower pyrethroid concentrations then estimated. In addition, field-collected samples could lose a large proportion of their pyrethroids (and other hydrophobic compounds) through adsorption to sampling and testing containers thereby underreporting observed pyrethroid toxicity. It is important that this effect be examined to determine whether these potential losses can affect the outcome of toxicity testing.
The tendency of pyrethroids to adsorb to container surfaces was examined using containers with varying volumes and compositions. Water samples were spiked with 14C λ-cyhalothrin (provided by Syngenta; Berkshire, UK) at 4 ng/ml containing approximately 1000 dpm/ml. Containers tested were chosen based upon their use in either sample collection or in toxicity-testing and included a 2.5 gallon low-density polyethylene cubitainer® (B & A Products; Bunch, OK), a 1 gallon amber glass jar, and a 1-l amber glass jar. Sample containers were shaken for ∼30–60 s before samples were taken for analysis. Several open containers were also examined, including 400 and 1000 ml beakers (both Pyrex™ glass and Nalgene™ plastic). The effects of aeration upon pyrethroid loss were examined by bubbling a constant stream of air through the samples (flow rate ∼5–10 ml/min; 48 h).
Smaller containers were used to study pyrethroid loss in toxicity-testing systems. A series of 25, 50, and 250 ml beakers (Pyrex™ glass and Teflon®) were examined as well as 20 ml plastic high-density polyethylene (HDPE) vials and treated and non-treated borosilicate glass scintillation vials. One set of glass scintillation vials was treated with an aqueous 5% polyethylene glycol (PEG; 20,000 MW; Avocado Research Chemicals Ltd.; Heysham, UK) solution according to Hawk et al. (1972). Vials were filled with the PEG solution for 5 min, drained, and then dried at 120°C overnight under vacuum. The vials were then washed repeatedly with distilled water and dried. “Vortex” vials were untreated scintillation vials that were vortexed for 30 s before sampling. Silylated vials were treated with dimethyldichlorosilane (DMDCS) in 5% toluene according to the supplier's instructions (Supelco; Bellefonte, PA). The amount of 14C λ-cyhalothrin adsorbed to the container was determined by taking 1 ml aliquots at given time points and counting the remaining radioactivity on a Wallac 1409 liquid scintillation counter (Wallac; Turku, Finland).
The toxicity of bioavailable permethrin was assessed with the cladoceran Ceriodaphnia dubia, and the amphipod Hyalella azteca. Permethrin was used for the toxicity studies as it possesses similar physical properties to λ-cyhalothrin and is often the target of toxicity testing. The log P-values for permethrin and 14C λ-cyhalothrin are 6.10 and 7.00, respectively, and the water solubilities are 5.5 and 5.0 μg/l, respectively (Laskowski, 2002). C. dubia neonates (<24 h old) were obtained from cultures maintained at AQUA-Science (25°C; Davis, CA). The 48-h toxicity test procedures followed those outlined by the USEPA (1993) and are described in detail in Wheelock et al. (2004). H. azteca from Chesapeake Cultures (Hayes, VA) were obtained 48 h prior to test initiation. Exposures were conducted for 96 h at 23°C according to published protocols (USEPA, 2002). Permethrin standards (99% a.i., 100 μg/l in methanol) were obtained from AccuStandard (New Haven, CT). Permethrin-spike test solutions were prepared and 18 ml (for C. dubia) or 15ml (for H. azteca) were added to each 20 ml borosilicate glass scintillation vial at the concentrations shown in Tables 1 and 2. The vials stood for the indicated time intervals shown in both Tables before addition of the test organisms. At 240 min, one set of vials was vortexed for 1 min and then organisms were added identically as for the other time points.
Table 1.
Effect of time to test initiation on 48 h acute toxicity of permethrin to C. dubia
| Time to test initiation (min)b | 48-h percent mortalitya |
|||||
|---|---|---|---|---|---|---|
| Permethrin concentration (ng/l)c |
||||||
| 0 | 125 | 250 | 375 | LC50d | 95% CIe | |
| 15 | 0 ± 0 | 90 ± 12 | 100 ± 0 | 100 ± 0 | 65.8 | 60.5–78.2 |
| 30 | 5 ± 10 | 85 ± 19 | 100 ± 0 | 100 ± 0 | 74.2 | 55.4–105.7 |
| 60 | 0 ± 0 | 80 ± 16 | 100 ± 0 | 100 ± 0 | 78.1 | 58.4–107.0 |
| 120 | 0 ± 0 | 70 ± 26 | 100 ± 0 | 100 ± 0 | 89.3 | 57.5–146.4 |
| 240 | 0 ± 0 | 40 ± 23 | 100 ± 0 | 100 ± 0 | 140.2 | 106.4–167.9 |
Four replicates of five neonate C. dubia per treatment.
Test solutions were prepared at given permethrin concentrations and placed into test containers followed by the addition of C. dubia at the time intervals indicated.
Nominal water concentration.
LC50 (concentration to cause 50% lethality) values are reported in ng/l. Values were calculated using Spearman–Karber analysis.
95% confidence interval (CI) of the LC50 results.
Table 2.
Effect of time to test initiation on 96 h acute toxicity of permethrin to H. azteca
| Time to test initiation (min)b | 96-h percent mortalitya |
|||||
|---|---|---|---|---|---|---|
| Permethrin concentration (ng/l)c |
||||||
| 0 | 25 | 50 | 75 | LC50d | 95% CIe | |
| 15 | 13 ± 12 | 13 ± 12 | 87 ± 12 | 87 ± 12 | 39.5 | 34.2–45.6 |
| 30 | 6 ± 10 | 27 ± 23 | 73 ± 23 | 87 ± 12 | 35.4 | 27.8–44.9 |
| 60 | 7 ± 12 | 13 ± 12 | 93 ± 12 | 100 ± 0 | 35.4 | 32.8–38.1 |
| 120 | 13 ± 12 | 33 ± 23 | 73 ± 12 | 93 ± 12 | 37.5 | 29.3–48.0 |
| 240 | 13 ± 12 | 0 ± 0 | 53 ± 12 | 93 ± 12 | 47.7 | 41.1–55.4 |
Three replicates of five H. azteca per treatment.
Test solutions were prepared at given permethrin concentrations and placed into test containers followed by the addition of H. azteca at the time intervals indicated.
Nominal water concentration.
LC50 (concentration to cause 50% lethality) values are reported in ng/l. Values were calculated using Spearman–Karber analysis.
95% confidence interval (CI) of the LC50 results.
Results showed that regardless of the container surface, λ-cyhalothrin was quickly removed from an aqueous environment (Fig. 1). Larger volume containers generally exhibited less pyrethroid removal from solution. This trend decreased with smaller volumes as observed with the 25 and 50 ml glass containers in Fig. 1C, which displayed nearly identical pyrethroid loss by 48 h. Studies with 1-l glass containers saw a 20% reduction in aqueous pyrethroid levels over 96 h, with the majority of the loss occurring in the first 24 h. In all cases HDPE plastic adsorbed more pyrethroid than glass, and Teflon® adsorbed the largest quantities of pyrethroid with >75% of the compound removed from solution over 48 h. Aerating the sample increased the initial rate of loss, but by 48 h, the percentage of pyrethroid lost was essentially equal to non-aerated samples.
Fig. 1.
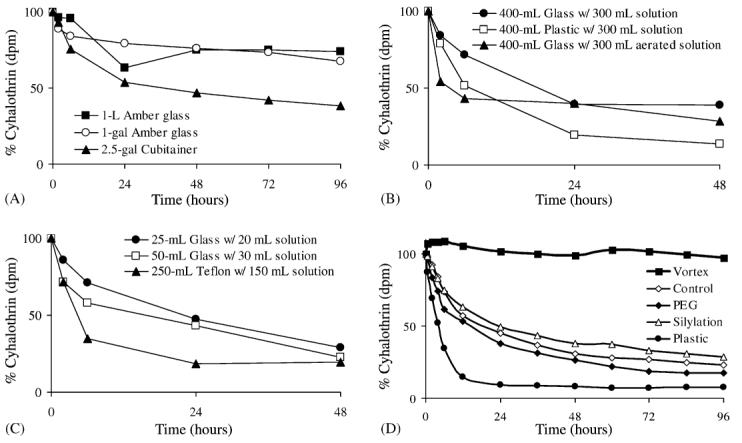
Containers were spiked with approximately 4 ng/ml of λ 14cyhalothrin containing ∼1000 dpm/ml. Sampling volume was 1 ml, which was counted in 19 ml of Scintiverse scintillation cocktail. (A) For the 2.5 gallon (gal) cubitainer®, 1-gal amber glass and 1 L amber glass, the water was added to the container followed by the λ 14cyhalothrin. The container was shaken for 30–60 s and then the initial sample was taken. (B) λ 14Cyhalothrin was added to either glass or plastic beakers and sampled until 48 h. Similar experiments were performed with 1 L beakers and experimental results were nearly identical. (C) λ 14Cyhalothrin was added to either glass or Teflon® beakers and sampled until 48 h. (D) Samples were spiked into 20 ml of Nanopure™ water in borosilicate glass scintillation vials (except where noted). “Vortex” samples were vortexed for 30 s before sampling; “Control” samples were not treated; “PEG” samples were coated with polyethyleneglycol; “Silylation” samples were treated with dimethyldichlorosilane (DMDCS); “Plastic” samples were taken from 20 ml high-density polyethylene vials (HDPE). Following the 96 h time-point, all samples were vortexed for 30 s to examine recovery: control (115±7%), PEG (95±7%), silylation (95±8%), plastic (8±1%). For (A–C), n = 2 and for (D), n=3.
Studies with the 20-ml glass vials showed that ∼50% of the pyrethroid was removed from solution in the first 24 h, with up to 75% removed after 96 h (Fig. 1). Treatment with PEG or silylation had no significant effect upon pyrethroid loss from the water (P > 0.05 with Students' t-test). However, vortexing the untreated glass vial for 30 s before sampling served to eliminate pyrethroid loss out to 96 h. HDPE plastic vials resulted in fast removal of the pyrethroid from the water, with almost complete removal within 24 h. All vials and treatments were vortexed for 30 s after the 96 h sampling, giving essentially 100% recovery of 14C λ-cyhalothrin (except for the plastic containers as shown in Fig. 1D).
In the toxicity studies, increasing sample incubation time before C. dubia addition resulted in lower mortality over 48 h (Table 1). Incubating the vials for 4 h resulted in a ∼50% reduction in the 48-h percent mortality in the 125 ng/l concentration, with the 48-h LC50 increasing from 65.8 ng/l in the 15 min treatment to 140.2 ng/l in the 240 min treatment. This reduction in toxicity was statistically significant at 240 min, but not at lesser time intervals (see CI values in Table 1). Higher concentrations exhibited 100% mortality illustrating the narrow dose-response relationships that C. dubia have for pyrethroids. Vortexing of the 4-h sample served to increase the toxicity to nearly initial levels with 48-h mortality being 85 ± 15% (data not shown).
H. azteca toxicity did not decrease ± as drastically as that of C. dubia. The 96-h LC50 only increased from 39.5 ng/l in the 15 min treatment to 47.7 ng/l in the 240 min treatment (Table 2). This small reduction in toxicity was not statistically significant as evidenced by the overlapping CI values as shown in Table 2. The result is supported by the fact that vortexing the 240 min vials did not return the lost toxicity (data not shown). Differences in response between C. dubia and H. azteca might be attributed to organism-specific behavior. C. dubia is a water column organism that is seldom in contact with the walls of the test container, the location of adsorbed permethrin. H. azteca are epibenthic and their association with the bottom of the container could place them in closer contact with the adsorbed pyrethroid. These data suggest that response to pyrethroid adsorption to test containers will be organism and test-specific.
An integral part of toxicity-testing procedures is the containers used for sampling and testing. Due to pesticide lipophilicity, the selection of container type may be critical for pyrethroid testing. One approach to the problem of pyrethroid adsorption to containers is to coat the container with a substance that will prevent adsorption. PEG has been successfully used to prevent adsorption of lipophilic compounds to glass (Hammock et al., 1975). Silylation is another well-known coating material, however, like PEG, the coating is relatively lipophilic and pyrethroids are likely to adhere to the coating as shown in Fig. 1D. It is possible that other treatments could prevent or reduce pyrethroid binding. Treatments should be selected based upon their ability to increase the hydrophilicity of the vessel surface. For example, other silylating materials that have free carboxylic acid or amine groups may be more useful as these should provide a hydrophilic environment, and thus repel the pyrethroid from adsorption to the glass. However, caution should be exercised because coating agents may cause significant toxicity to the test organisms.
The only treatment that proved useful for preventing pyrethroid loss was vortexing of the vials before sampling. This technique did not prevent pyrethroid adsorption, but rather resuspended the pyrethroid showing that it was not covalently bound to the glass surface. Shaking of samples has been shown by other researchers to be an effective tool to resuspend pyrethroid levels in aqueous samples (Sharom and Solomon, 1981; Lee et al., 2002). Although shaking would be a simple method to keep the pyrethroid in solution, integrating this technique into a bioassay that utilizes live organisms is not practical. These results are nevertheless intriguing, and further studies should explore how vigorous the shaking must be in order to release the pyrethroid from the glass.
These results raise an important question regarding the bioavailable fraction of pyrethroid during toxicity testing. Our data show that up to 50% of the pyrethroid can adsorb to the container in 24 h. These results are similar to those of Lee et al. (2002) who reported losses of 58–72% within the first 24 h. Additionally, our results indicated that within 4 h pyrethroid levels had dropped enough to reduce the toxic effect by 50% in the C. dubia studies (Table 1). It is therefore uncertain as to the actual concentration of pyrethroid to which organisms are being exposed. Aquatic toxicity studies often involve the use of several different containers. Water samples are initially collected in one container, aliquoted into smaller containers for dilution preparation, and then aliquoted to exposure chambers. If ∼50% of the pyrethroid adsorbs to each container, then this three step process could result in nearly a 90% loss of pyrethroid. The loss will vary greatly based upon the volume and type of the container used as well as the procedure for sample preparation. Water quality is also an essential variable, with the potential for suspended sediments and colloids to alter test results via similar sorption processes, making results obtained from “field water” substantially different from filtered laboratory water. These data show that until the problem of pyrethroid adsorption is solved, it is necessary to rigorously define sample treatment. In addition, this work demonstrates that it is not sufficient to report nominal water concentrations and that it is critical to provide analytical verification of pyrethroid concentrations in solution. It is also likely that these effects will be similar for other hydrophobic contaminants.
The overall bioavailability of pyrethroids in aquatic toxicity-testing remains in question. Studies involving monitoring of pyrethroid levels in aquatic systems need to be aware of the hydrophobic nature of pyrethroids. This study as well as those of other researchers have shown that pyrethroids will adsorb to most containers, resulting in >50% loss of pyrethroid over 24 h. The use of multiple containers for sampling and toxicity-testing can result in a severe decrease in pyrethroid levels. However, vigorous shaking of the container appears to be sufficient to resuspend the pyrethroid, at least in small containers. Further work should rigorously define the effects of container shaking and agitation on resuspension rates and levels for pyrethroid sampling and toxicity testing. Results showed that pyrethroid adsorption to the toxicity-testing container can cause significant reductions in the observed toxicity, but that it is dependent upon the organisms and system being employed.
Acknowledgements
C.E.W. was supported by NIH post-doctoral training grant T32 DK07355-22. This work was supported in part by SWRCB Contract No. 0-079-250-0, NIEHS Grant R37 ES02710, NIEHS Superfund Grant P42 ES04699, NIEHS Center for Environmental Health Sciences Grant P30 ES05707, and NIH/NIAID Grant U01 AI058267.
References
- Casida JE, Quistad GB. Golden age of insecticide research: past, present, or future? Annu. Rev. Entomol. 1998;43:1–16. doi: 10.1146/annurev.ento.43.1.1. [DOI] [PubMed] [Google Scholar]
- Hammock BD, Nowock J, Goodman W, Stamoudis V, Gilbert LI. The influence of hemolymph-binding protein on juvenile hormone stability and distribution in Manduca sexta fat body and imaginal discs in vitro. Mol. Cell. Endocrinol. 1975;3:167–184. doi: 10.1016/0303-7207(75)90043-x. [DOI] [PubMed] [Google Scholar]
- Hawk GL, Cameron JA, Dufault LB. Chromatography of biological materials on polyethylene glycol treated controlled-pore glass. Prep. Biochem. 1972;2:193–203. doi: 10.1080/00327487208061469. [DOI] [PubMed] [Google Scholar]
- Laskowski DA. Physical and chemical properties of pyrethroids. Rev. Environ. Contam. Toxicol. 2002;174:49–170. doi: 10.1007/978-1-4757-4260-2_3. [DOI] [PubMed] [Google Scholar]
- Lee S, Gan J, Kabashima J. Recovery of synthetic pyrethroids in water samples during storage and extraction. J. Agric. Food Chem. 2002;50:7194–7198. doi: 10.1021/jf0258353. [DOI] [PubMed] [Google Scholar]
- Sharom MS, Solomon KR. Adsorption and desorption of permethrin and other pesticides on glass and plastic materials used in bioassay procedures. Can. J. Fish. Aquat. Sci. 1981;38:199–204. [Google Scholar]
- US Environmental Protection Agency . Methods for measuring the acute toxicity of effluent and receiving waters to freshwater and marine organisms. Office of Research and Development; Duluth, MN, USA: 1993. EPA-600/4-90/027F. [Google Scholar]
- US Environmental Protection Agency . Methods for measuring acute toxicity of effluents and receiving water to freshwater and marine organisms. Office of Research and Development; Washington, DC: 2002. EPA-821-R-02-021. [Google Scholar]
- Wheelock CE, Miller JL, Miller MG, Shan G, Gee SJ, Hammock BD. Development of Toxicity Identification Evaluation (TIE) procedures for pyrethroid detection using esterase activity. Environ. Toxicol. Chem. 2004;23:2699–2708. doi: 10.1897/03-544. [DOI] [PubMed] [Google Scholar]


