Abstract
Caenorhabditis elegans EGO-1, a putative cellular RNA-directed RNA polymerase, promotes several aspects of germline development, including proliferation, meiosis, and gametogenesis, and ensures a robust response to RNA interference. In C. elegans, GLP-1/Notch signaling from the somatic gonad maintains a population of proliferating germ cells, while entry of germ cells into meiosis is triggered by the GLD-1 and GLD-2 pathways. GLP-1 signaling prevents germ cells from entering meiosis by inhibiting GLD-1 and GLD-2 activity. We originally identified the ego-1 gene on the basis of a genetic interaction with glp-1. Here, we investigate the role of ego-1 in germline proliferation. Our data indicate that EGO-1 does not positively regulate GLP-1 protein levels or GLP-1 signaling activity. Moreover, GLP-1 signaling does not positively regulate EGO-1 activity. EGO-1 does not inhibit expression of GLD-1 protein in the distal germline. Instead, EGO-1 acts in parallel with GLP-1 signaling to influence the proliferation vs. meiosis fate choice. Moreover, EGO-1 and GLD-1 act in parallel to ensure germline health. Finally, the size and distribution of nuclear pore complexes and perinuclear P granules are altered in the absence of EGO-1, effects that disrupt germ cell biology per se and probably limit germline growth.
EGO-1, a putative cellular RNA-directed RNA polymerase (RdRP), is required for diverse aspects of germline function in Caenorhabditis elegans (Qiao et al. 1995; Smardon et al. 2000). The ego-1 gene was first identified because it interacts genetically with the Notch/GLP-1 signaling pathway that maintains germline proliferation (Qiao et al. 1995). However, ego-1 mutations affect not only germline proliferation, but also early meiosis and gametogenesis, suggesting that ego-1 activity is important for a variety of germline processes (Qiao et al. 1995; Smardon et al. 2000).
Members of the RdRP family are implicated in RNA silencing phenomena in diverse organisms and in assembly of heterochromatin (reviewed by Grewal and Rice 2004; Lippman and Martienssen 2004; Meister and Tuschl 2004). The specific role of RdRP in these processes remains unclear. In vitro RdRP activity has been demonstrated for Neurospora QDE-1 and Schizosaccharomyces pombe RdP1 (Makeyev and Bamford 2002; Motamedi et al. 2004). Oher RdRPs, including EGO-1, are assumed to have a similar activity. During RNA silencing, RdRPs may amplify the “trigger” RNA that directs the RNA-induced silencing complex (RISC) to mRNA targets and/or amplify siRNA to accelerate the mRNA degradation process (see Meister and Tuschl 2004). During chromatin modification, RdRPs may synthesize/amplify guide molecules that direct chromatin-modifying machinery and/or the RNA-induced transcriptional silencing (RITS) complex to proper chromosomal sites (see Grewal and Rice 2004; Motamedi et al. 2004).
EGO-1 may act in the synthesis of dsRNAs that promote specific aspects of germline development. Here, we have focused on the earliest germline developmental defect associated with ego-1 mutants: premature entry of distal germ cells into meiosis (Smardon et al. 2000). In the C. elegans adult germline, a signal from the somatic distal tip cell (DTC) maintains proliferation of the distal germline (see Seydoux and Schedl 2001). DTC-to-germline signaling is mediated by the GLP-1/Notch pathway (Baron 2003; Lai 2004; Schweisguth 2004), which actively prevents germ cells from entering meiosis (Seydoux and Schedl 2001; Crittenden et al. 2003). GLP-1 signaling in the germline represses the activities of two redundant pathways. The founding members of these pathways, GLD-1 and GLD-2, are translational regulators with different biochemical functions (Francis et al. 1995a,b; Jones and Schedl 1995; Jones et al. 1996; Kadyk and Kimble 1998; Wang et al. 2002). GLP-1 also appears to inhibit a third meiotic entry pathway (Hansen et al. 2004b; Maine et al. 2004).
ego-1 loss-of-function (lf) mutations enhance a weak loss of GLP-1 signaling activity (Qiao et al. 1995; Smardon et al. 2000). Furthermore, in an ego-1(0); glp-1(+) background, germ cells enter meiosis earlier in development than in wild-type animals, indicating a shift in the balance between proliferation and meiotic entry (Smardon et al. 2000). ego-1 mutant germ cells exhibit a series of other defects, as follows. Once germ cells enter meiosis, they are slow to progress through early meiotic prophase (leptotene-zygotene stages); univalents are often observed at diakinesis. Some distal nuclei are enlarged/diffuse, perhaps due to polyploidy. The switch from spermatogenesis to oogenesis is delayed, and small, abnormal (perhaps intersexual) gametes are produced prior to formation of oocytes. Oocytes are small, variably sized, and poorly ovulated, sometimes taking on an endomitotic (Emo) phenotype. Although oocytes can be fertilized, the embryos undergo only a few rounds of cell divisions before arresting. We were unable to obtain cross-progeny from ego-1(−) males, although they produce and transfer sperm; therefore, ego-1 male sperm appear to be fertilization defective. This mutant phenotype is consistent with ego-1 being required throughout most of larval development and adulthood. On the basis of analysis of glp-1 conditional and partial lf mutations, GLP-1 has no essential function in meiotic progression or sex determination (Austin and Kimble 1987; Kodoyianni et al. 1992; Berry et al. 1997). Therefore, we hypothesize that the premature meiotic entry defect in ego-1 mutants is responsible for enhancement of glp-1(lf), whereas other defects reveal a requirement for EGO-1 activity in additional aspects of germline development independent of glp-1.
Here, we investigated the relationship between EGO-1 activity and the meiotic entry pathways. We also characterized the developmental pattern of ego-1 expression. We show that ego-1 mRNA and protein are first detected in mid-to-late larvae and increase in levels as the germline grows. EGO-1 does not regulate the global distribution of GLP-1 or GLD-1. Instead, EGO-1 acts (at least in part) in parallel with GLP-1 signaling to repress meiosis and/or promote proliferation. We also demonstrate that EGO-1 activity influences the assembly/distribution of nuclear pore complexes (NPCs) and germ (P) granules. Therefore, the loss of EGO-1 activity affects the basic cell biology of the germline. Finally, we discuss models for how EGO-1 activity promotes germline proliferation. Together, our findings suggest that EGO-1 acts in two ways to promote proliferation, by affecting (i) the proliferation vs. meiosis fate choice specifically and (ii) basal cellular processes, e.g., germ granule and NPC formation.
MATERIALS AND METHODS
Genetics:
Standard culture conditions were used (Epstein and Shakes 1995). Wild-type strain C. elegans variant Bristol (N2) and mutations used are as described by Chen et al. (2003) or as indicated in the text. Mutations used were: linkage group (LG) I, gld-1(q485), gld-2(q497), unc-13(e51), ego-1(om54, om58, om71, om84, and om97), and hT2 gfp; LGII, rrf-3(pk1426); LGIII, dcr-1(ok247) and glp-1(q175); and LGV, him-5(e1467ts). The following mutations are known to be null: ego-1(om84), ego-1(om97) (this work), gld-1(q485), gld-2(q497), and glp-1(q175) (see Hansen et al. 2004b). The ego-1(om84) deletion allele was used in constructing the ego-1(0) gld-1(0) and gld-2(0) ego-1(0) gld-1(0) strains. PCR was used to verify the presence of the om84 deletion in each strain.
Indirect immunofluorescence:
Experiments were done using fixative and incubation conditions appropriate for the antibody (or antibodies) in question. Monoclonal antibody (mAb) K76 is an IgM; therefore, tissue was prepared by the freeze-crack method of Strome and Wood (1983). All other antibodies were used to label dissected gonads, as follows. Tissue was fixed with paraformaldehyde and/or −20° methanol as appropriate for each antigen, washed in PBS/Tween-20, blocked in PBS with 30% goat serum, and incubated overnight with antibody at 4° in PBS/30% goat serum. Tissue was washed several times, incubated with the appropriate dilution of secondary antibody, washed again, stained with DAPI to visualize DNA, and mounted in Vectashield (Vector Laboratories, Burlingame, CA). Specific references for the antibodies are: GLD-1, Jones et al. (1996); PGL-1, Kawasaki et al. (1998); mAb K76, Strome and Wood (1983); mAb 414, Covance (see Pitt et al. 2000); RME-2, Grant and Hirsh (1999); GLH-1, Gruidl et al. (1996); HIM-3, Zetka et al. (1999); REC-8, Pasierbek et al. (2001)(see Hansen et al. 2004b); and phospho-H3, Hendzel et al. (1997).
Molecular methods:
Nucleic acids were isolated and manipulated using standard methods (Epstein and Shakes 1995; Sambrook and Russell 2001). The developmental RNA blot was prepared using total RNA isolated from staged populations of animals. ego-1-specific probe was prepared as described (Smardon et al. 2000).
Rabbit anti-EGO-1 antibodies were raised against a peptide corresponding to amino acids 253–269 and affinity purified against that same peptide (Quality Controlled Biochemicals, Hopkinton, MA). The peptide was chosen on the basis of lack of conservation with other C. elegans proteins and location toward the amino terminus of EGO-1. Total worm protein extract was isolated using standard methods, as follows (Epstein and Shakes 1995). One hundred staged worms were washed in M9 medium and diluted 1:2 in 2× SDS-PAGE buffer (total volume, 60 μl). The tube was placed at 100° for 3 min, vortexed 1 min, placed at 100° for another 7 min, and placed on ice. Material was spun at 6000 rpm for 1 min to pellet nucleic acid, and supernatant was removed to a clean tube. Twenty microliters of extract was loaded per well onto a 5% acrylamide gel for SDS-PAGE. Anti-myosin monoclonal antibody was used as a control, since myosin and EGO-1 are similar in size (∼200 kDa vs. ∼179 kDa, respectively). Protein transfer was done for 2 hr (with 5 amps at 4°) to optimize for large proteins. Anti-EGO-1 antibody was diluted 1:125, and anti-rabbit secondary antibody (Pierce, Rockford, IL) was diluted 1:10,000.
RESULTS
Developmental expression pattern of ego-1:
We previously demonstrated that adults with very few germ cells [glp-4(bn2ts) mutants raised at restrictive temperature] have very little ego-1 mRNA compared with wild-type animals (Smardon et al. 2000). Therefore, ego-1 mRNA is highly enriched and perhaps exclusively expressed in the germline (at least in adults), consistent with the mutant phenotype. Here, we analyzed the developmental ego-1 transcript pattern using RNA isolated from staged populations of animals using a development RNA blot (see materials and methods). ego-1 mRNA was not detected in L1–L2 larvae. It was detected at a very low level in L3 larvae, a substantially higher level in L4 larvae, and most prominently in adults (data not shown). In situ expression data obtained from the Nematode Expression Pattern Database (http://nematode.lab.nig.ac.jp/) are consistent with this pattern; a low level of ego-1 mRNA was detected in the L4 and adult germline. A very low level of mRNA, which is presumably maternal, was also visible in young embryos. The pattern of mRNA accumulation during larval development matches the phenotype: mRNA is first detected at the L3 stage, when mutant defects are first seen.
To analyze EGO-1 protein expression, we generated antibodies against an EGO-1 peptide (see materials and methods). Using affinity-purified antiserum, we detected an ∼179-kDa amino acid product in wild-type protein extracts that was absent in extracts from ego-1 putative null alleles, om84 and om97 (Figure 1A; data not shown). ego-1(om84) and ego-1(om97) are predicted to encode severely truncated proteins and be null for function (Smardon et al. 2000). We concluded that the ∼179-kDa band is EGO-1 protein. We then used the anti-EGO-1 antibody to analyze EGO-1 protein levels during development. We prepared total protein extracts from staged populations of animals and analyzed them by protein blot. We detected EGO-1 at a very low level in L3 larvae and subsequently at higher levels in L4 and adult animals (Figure 1C). EGO-1 abundance was extremely low compared with controls such as myosin and nuclear lamin (Figure 1, B and D; data not shown); therefore, we hypothesize that the very few germ cells in L1–L2 larvae may preclude detection of ego-1 gene product at these stages. Despite concerted effort, we were unable to use these antibodies to detect EGO-1 in fixed tissue by indirect immunofluorescence.
Figure 1.—
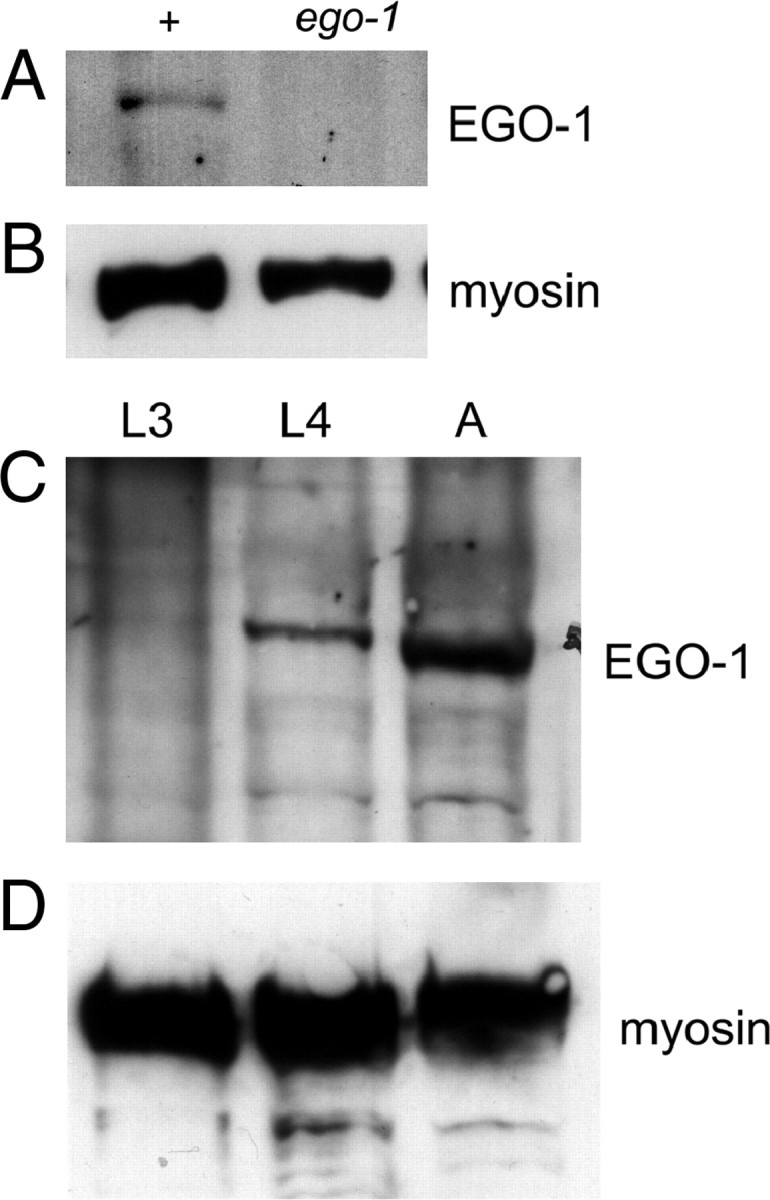
Developmental pattern of EGO-1 expression. (A) An ∼179-kDa protein is detected in extracts from wild-type (N2) adults but absent in extracts from ego-1(om97) adults. The 179-kDa band was also not detected in ego-1(om84) adults. ego-1(om97) has a premature stop codon and ego-1(om84) has a deletion; each mutation is predicted to encode a truncated protein (Smardon et al. 2000). (B) Myosin was used as a positive control. (C) EGO-1 protein is barely detectable in late L2/L3 larvae (L3). The level is higher in L4 larvae (L4) and in adults (A). This pattern mirrors the mRNA expression pattern. (D) Myosin was detected at approximately equivalent levels in each sample. See text.
GLP-1 distribution in the ego-1 mutant germline:
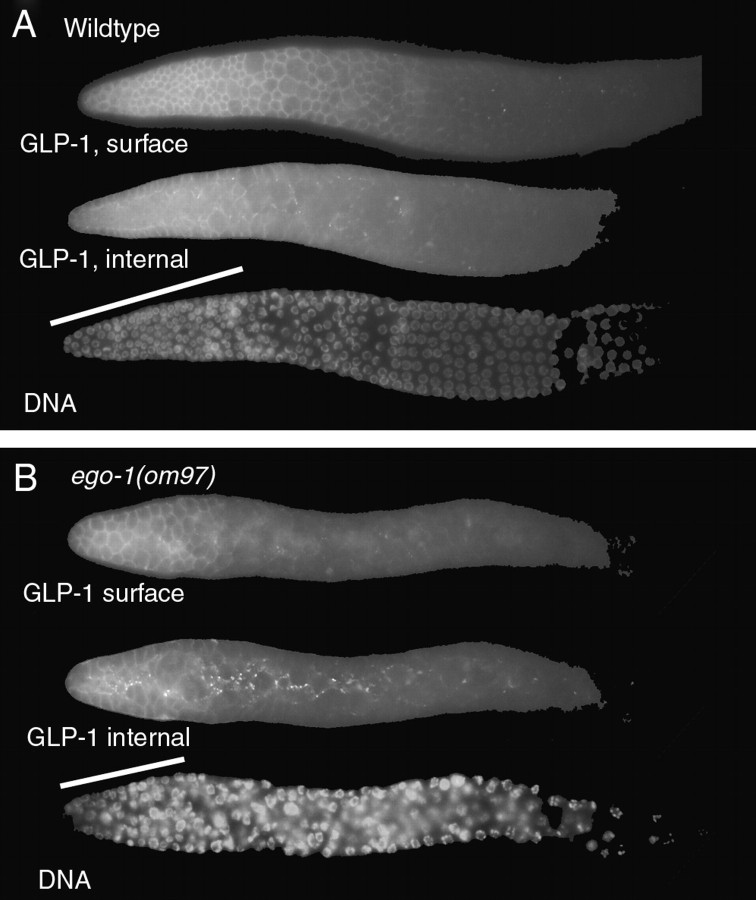
We hypothesized that EGO-1 might promote proliferation by positively regulating GLP-1 expression. To investigate this idea, we used indirect immunofluorescence to characterize the distribution of GLP-1 protein in the ego-1 mutant germline. We used antiserum against an extracellular domain of GLP-1, the LNG repeats (Crittenden et al. 1994). In wild-type adult germlines, the GLP-1 level is relatively high in the distal proliferating region and decreases as germ cells enter meiosis (Crittenden et al. 1994) (Figure 2A). GLP-1 is detected predominantly at the plasma membrane, consistent with its role as a Notch-type receptor. In addition, some punctate GLP-1 foci are detected within the cytoplasmic core of the syncytial germline. These foci may correspond to non-ligand-bound receptor that is being recycled from the cell surface (Figure 2A, GLP-1 internal) (Crittenden et al. 1994; see Baron 2003). The global distribution of GLP-1 is normal in the ego-1 mutant germline (Figure 2B). However, ego-1 mutants have an elevated level of GLP-1 puncta within the cytoplasmic core relative to wild-type controls (Figure 2A vs. 2B). These puncta are located proximal to the mitotic region and may indicate that protein trafficking is impaired in the ego-1 mutant germline. Defects in protein trafficking in general, and GLP-1 trafficking in particular, are likely to contribute to the ego-1 mutant phenotype.
Figure 2.—
GLP-1 expression in the distal ego-1 germline. The distal portion of a dissected gonad arm stained with anti-GLP-1 antibody is shown. For each genotype, two focal planes are shown: a surface view and an internal view focusing through the cytoplasmic core of the gonad. Nuclear morphology (DNA) is visualized by counterstaining with DAPI; the focal plane is the same as GLP-1, internal. The mitotic region is indicated with a bar. (A) Wild-type GLP-1 distribution in the mitotic and distal meiotic region of the germline. The GLP-1 level is relatively high in the mitotic region and decreases as germ cells enter leptotene/zygotene (just right of the bar). GLP-1 surface view shows protein is associated with the plasma membrane surrounding each nucleus and cytoplasmic alcove, producing a honeycomb pattern. GLP-1 internal view shows a few puncta in the cytoplasmic core. (B) The distribution of GLP-1 with respect to mitotic vs. meiotic germ cells is normal in ego-1(om97) mutants. However, punctate GLP-1 foci (GLP-1 internal view) are prominent throughout the cytoplasmic core. A similar staining pattern was seen in ego-1(om84) mutants.
GLD-1 distribution in the ego-1 mutant germline:
We next investigated whether EGO-1 inhibits expression of GLD-1 protein in the distal germline. We evaluated the distribution of GLD-1 protein in the ego-1 mutant germline using a functional GLD-1::GFP fusion transgene and antiserum against GLD-1 (Jones et al. 1996; Lee and Schedl 2001; Schumacher et al. 2005). In wild-type animals, GLD-1 is present at a low level in the cytoplasm in distal mitotic germ cells, increases in concentration within proximal mitotic germ cells, and peaks in concentration in early meiotic prophase (leptotene-zygotene stage) (Jones et al. 1996) (Figure 3A). GLD-1 levels remain high through the pachytene region and decrease rapidly as germ cells progress to diplotene stage. In the embryo, GLD-1 is associated with germline-specific ribonucleoprotein particles, called P granules (Jones et al. 1996). In ego-1 mutants, the relative distribution of GLD-1 in proliferating vs. meiotic cells was normal (Figure 3, B and C). A similar pattern of GLD-1 staining was seen in several different null mutants [ego-1(om84), ego-1(om97), and ego-1(om58)] as well as a partial lf allele, ego-1(om54). In addition, costaining with antibodies against GLD-1 and a phosphorylated form of histone H3 that is present in cells immediately prior to and during mitosis (Hendzel et al. 1997) confirmed that GLD-1 levels in the proliferating region of the ego-1 germ line are normal (data not shown). Therefore, EGO-1 does not repress gld-1 expression in the distal germline. Although the overall distribution of GLD-1 was normal, we did note a change in subcellular distribution that led us to investigate P-granule morphology (see below).
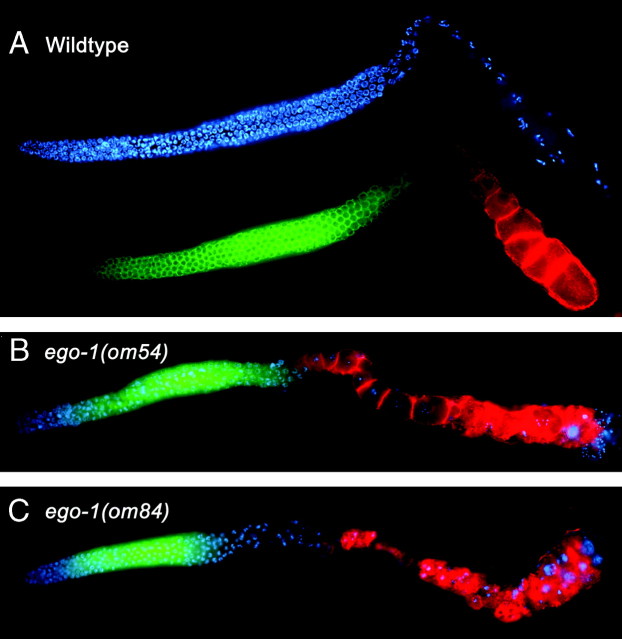
Figure 3.—
Global GLD-1 distribution is normal in the ego-1 germline. One arm of the gonad is shown in (A–C). (A) Wild type, (B) ego-1(om54) partial loss-of-function, and (C) ego-1(om84) null germlines were labeled with antibodies against GLD-1 (green) and the yolk receptor, RME-2 (red). DNA is indicated in blue. In all three cases, GLD-1 is detected in the proximal mitotic region and in leptotene/zygotene and pachytene stages of early meiotic prophase. RME-2 is detected in late-stage oocytes in diakinesis. No overlap in RME-2 and GLD-1 expression is evident, consistent with translational repression of rme-2 mRNA by GLD-1. Thus, EGO-1 does not appear to be involved in repression of GLD-1 or to regulate (via GLD-1) RME-2 expression.
GLD-1 regulates germline processes by repressing translation of certain mRNAs (Jan et al. 1999; Clifford et al. 2000; Lee and Schedl 2001, 2004; Xu et al. 2001; Marin and Evans 2003; Mootz et al. 2004). For example, one target is rme-2 mRNA, which encodes the yolk receptor (Grant and Hirsh 1999). GLD-1 represses translation of rme-2 mRNA in early meiotic prophase, and RME-2 accumulates in late-stage oocytes, where GLD-1 is not present (Figure 3A). RME-2 expression was also normal in ego-1 mutant germlines (Figure 3, B and C), suggesting that GLD-1 correctly regulates rme-2 translation in an ego-1(0) mutant. We also monitored the uptake of yolk protein, YP170, in ego-1 mutants using a GFP-tagged transgene, YP170::GFP (Grant and Hirsh 1999). YP170::GFP was taken up into the ego-1 oocytes, suggesting the RME-2 was functioning normally (data not shown). Therefore, EGO-1 does not influence expression of RME-2 or its regulation by GLD-1.
ego-1 and gld-1 interact synergistically:
On the basis of the immunolabeling experiments described above, EGO-1 does not regulate the global distribution of GLP-1 or GLD-1 proteins. We next turned to genetics to investigate further how EGO-1 functions relative to the GLP-1 signaling pathway and other regulators of the proliferation vs. meiotic entry decision. We first investigated the interaction between ego-1 and gld-1. The earliest known role for GLD-1 in the larval germline is to promote meiotic entry; in this capacity, GLD-1 functions redundantly with GLD-2 (Kadyk and Kimble 1998). GLD-1 regulates several additional germline processes (Francis et al. 1995a,b), and there is no evidence that GLD-2 is redundant with GLD-1 in any of these other events (Kadyk and Kimble 1998). In the gld-1(0) single mutant, germ cells that are female (i.e., should become oocytes) are defective in meiotic progression (Francis et al. 1995a). They enter meiosis and progress to the pachytene stage, but then exit meiosis and resume mitotic proliferation. This ectopic (“postmeiotic”) proliferation does not require an active GLP-1 signaling pathway or the distal tip cell and leads to production of a germline tumor in the proximal gonad (Francis et al. 1995b) (Figure 4B).
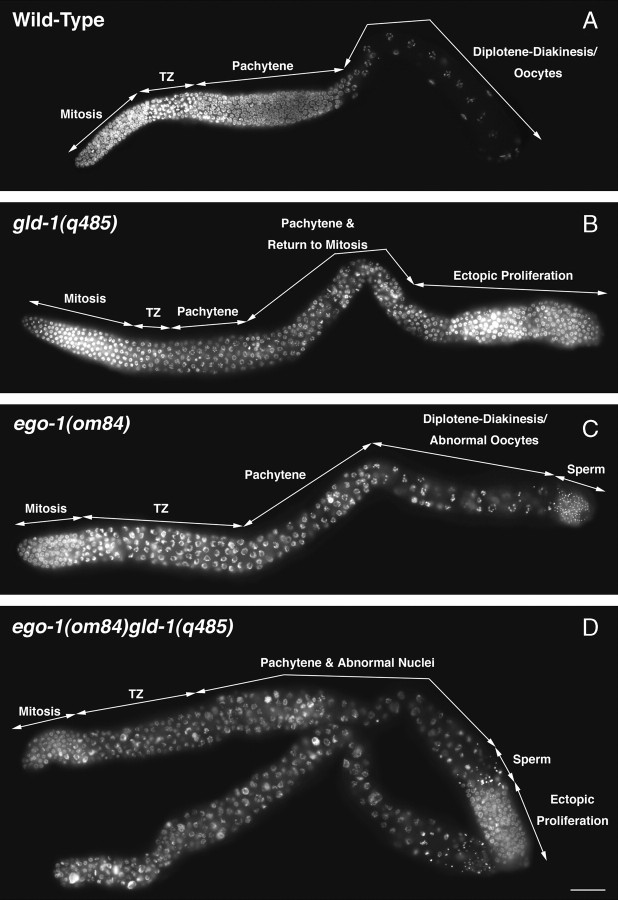
Figure 4.—
ego-1(0) severely reduces the level of ectopic proliferation in the gld-1(0) germline. One arm of the hermaphrodite germline is shown. Tissue has been stained with DAPI to visualize DNA. Regions of the germline with cells in mitosis or different stages of meiotic prophase are indicated. (A) Wild-type germline with proliferating cells at the distal end. (B) The gld-1(q485) germline with mitotic cells at the proximal end. Germ cells have entered meiosis and then returned to mitosis as they moved proximally within the gonad. Sperm are absent. (C) In the ego-1(om84) germline, mitotic cells are present only at the distal end (as in wild type). The leptotene/zygotene “transition” zone is enlarged relative to wild type and oocyte nuclei are crowded together. (D) The ego-1(om84) gld-1(q485) germline contains a large region of intermixed pachytene and abnormal nuclei. Two gonad arms are shown. A small number of sperm are present in each arm. The top gonad arm contains a small region of ectopic proliferation, whereas the bottom arm lacks ectopic proliferation altogether.
We examined the ego-1(om84) gld-1(q485) double mutant to determine whether the loss of EGO-1 activity could restore meiotic progression and/or reduce ectopic proliferation. Ectopic proliferation was absent from 74% of ego-1 gld-1 germlines and severely reduced in the remaining 26% of germlines (n = 23). Figure 4D shows examples of an ego-1 gld-1 germline without and with reduced ectopic proliferation. Many abnormal nuclei are present, and the germline is disorganized compared with wild-type and ego-1 single mutants (Figure 4, A and C vs. D). Meiotic germ cells do not progress beyond pachytene stage. Therefore, ego-1 does not suppress the gld-1 meiotic progression defect, although it does suppress the ectopic proliferation.
We hypothesize that the synergistic ego-1 gld-1 phenotype may reflect the cumulative effect of misregulation of a large number of genes. Several chromatin regulators are included among the many GLD-1 targets (Xu et al. 2001; M.-H. Lee, V. Reinke and T. Schedl, unpublished data), and EGO-1 activity also regulates chromatin structure (see discussion). Therefore, in the ego-1 gld-1 double mutant, the cumulative effect may be to misexpress a large number of genes, leading to the unhealthy germline that we observe. We hypothesize that one consequence of the very abnormal germline is suppression of ectopic proliferation.
ego-1(0) partially suppresses the gld-2 gld-1 meiotic entry defect:
We next investigated the function of EGO-1 relative to GLD-1 and GLD-2 in the meiotic entry decision. gld-2 gld-1 double null mutants are tumorous, containing mostly mitotic cells and a few meiotic cells that never progress beyond the leptotene-zygotene stage (Kadyk and Kimble 1998; Hansen et al. 2004a). Note that the gld-2 gld-1 tumor forms because germ cells fail to enter meiosis, whereas the tumor in the gld-1 single mutant forms because meiotic germ cells return to mitosis.
We constructed a gld-2(q497) ego-1(om84) gld-1(q485) triple null mutant to determine whether ego-1 suppresses the gld-2 gld-1 tumor. Nuclear morphology and the chromosomal association of REC-8 and HIM-3 proteins were used to distinguish mitotic from meiotic cells. HIM-3 is a component of the proteinaceous core between sister meiotic chromatids (Zetka et al. 1999). REC-8 is a cohesin component that is present in the nucleoplasm and on chromatin of mitotic cells and becomes associated with meiotic chromosomal axial elements (Pasierbek et al. 2001). Under our fixation and staining conditions, REC-8 is visible in the nucleoplasm and on chromatin of mitotic nuclei, but is difficult to detect when associated with meiotic chromosomes; this difference allows us to use strong chromosomal REC-8 staining as a mitotic marker (see Hansen et al. 2004b; Maine et al. 2004).
gld-2 ego-1 gld-1 germlines had, on average, 10-fold more HIM-3-positive, early meiotic (leptotene-zygotene) nuclei at the L4/adult molt compared with gld-2 gld-1 germlines (Figure 5A vs. 5B; Table 1). Therefore, ego-1 partially suppresses the gld-2 gld-1 meiotic entry defect. This result indicates that EGO-1 activity does not regulate activities of gld-1 and/or gld-2, but instead acts downstream of or in parallel with them. EGO-1 activity may be antagonistic to GLD-1 and GLD-2, for example, regulating expression of common targets (or genes that act together with GLD-1 and GLD-2 targets). Alternatively, EGO-1 may repress a third meiotic entry pathway; when ego-1 is inactivated, this pathway would become hyperactive and more meiosis would occur (see Hansen et al. 2004b; Maine et al. 2004). These effects may be either a direct or an indirect consequence of a more global role for EGO-1 in regulating genome function, e.g., via chromatin structure and/or RNA metabolism.
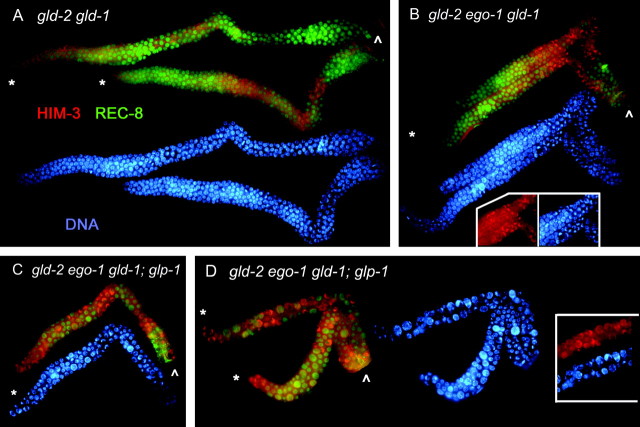
Figure 5.—
ego-1 suppresses the gld-2 gld-1 meiotic entry defect. Dissected hermaphrodite gonads are shown. REC-8 (green) strongly associates with chromosomes in mitotic germ cells, and HIM-3 (red) is associated with paired meiotic chromosomes. Each top image in A–D shows REC-8 and HIM-3 staining; each bottom image shows DNA stained with DAPI. The asterisk indicates the distal end of each germline, and the arrowhead indicates the proximal end. (A) Two gld-2(q497) gld-1(q485) germlines are shown. Both are tumorous, containing extensive mitotic nuclei and very few meiotic nuclei. (B) Two gld-2(q497) ego-1(om84) gld-1(q485) germlines are shown. The gld-2 gld-1 tumor is partially suppressed by ego-1. Note that a higher proportion of HIM-3-positive nuclei are present than in A. (See Table 1.) The inset compares HIM-3 and DNA (without REC-8) to emphasize the leptotene/zygotene morphology. (C and D) The gld-2(497) gld-1(485); glp-1(q175) tumor is strongly suppressed by ego-1(om84). Three gonad arms are shown. Note the increased proportion of meiotic nuclei compared with that in A and B. Note that germ cells did not progress through meiotic prophase, suggesting ego-1 does not suppress the gld-1 or gld-2 meiotic progression defect (Francis et al. 1995a; Kadyk and Kimble 1998).
TABLE 1 .
Tests for suppression of meiotic entry defects
| Genotype | No. meiotic germ cellsa |
N |
|---|---|---|
| gld-2(0) gld-1(0) | 2 ± 4 | 30 |
| gld-2(0) ego-1(0) gld-1(0) | 20 ± 10 | 30 |
| gld-2(0) ego-1(0) gld-1(0); glp-1(0) | 31 ± 13 | 30 |
The number of meiotic germ cells present at the L4/adult molt. We chose this early time point, where there is little meiotic entry (Hansen et al. 2004b), to maximize the ability to detect increased meiotic entry. Standard deviation is indicated. N, number of germlines assayed. Alleles used to construct these strains were ego-1(om84), gld-1(q485), gld-2(q497), and glp-1(q175).
EGO-1 acts independently of the GLP-1 signaling pathway:
We noted that the gld-2 ego-1 gld-1 germline had a higher proportion of meiotic germ cells than the gld-2 gld-1; glp-1 germline described by Hansen et al. (2004b). If EGO-1 were a positive regulator of GLP-1 signaling, then these two phenotypes should have been similar. Since meiosis is more prominent in the gld-2 ego-1 gld-1 germline than in the gld-2 gld-1; glp-1 germline, we conclude EGO-1 is not a positive regulator of glp-1 function. This result is consistent with data that GLP-1 levels are virtually unchanged in an ego-1(0) mutant (see above). The genetic data also indicate that GLP-1 signaling cannot be the sole positive regulator of ego-1 expression. This result is not surprising, given that ego-1 activity impacts many germline processes that are likely to be independent of GLP-1 signaling (e.g., meiotic progression, gametogenesis). Even though GLP-1 could theoretically be a positive regulator of ego-1 expression specifically in the distal germline, this does not appear to be the case.
We next investigated whether ego-1 might be regulated by GLD-1 and/or GLD-2. If EGO-1 acts in a linear pathway downstream of GLD-1/GLD-2, then the loss of GLP-1 should have no effect on the gld-2 ego-1 gld-1 phenotype. However, the gld-2 ego-1 gld-1; glp-1 germlines have considerably more meiotic nuclei and smaller germlines than the gld-2 ego-1 gld-1 germlines (Table 1; Figure 5, C and D vs. B). Some of the nuclei also appear to have progressed beyond leptotene/zygotene to pachytene stage (Figure 5D, inset). In addition, the distalmost nuclei in gld-2 ego-1 gld-1; glp-1 germlines are often meiotic (Figure 5, C and D), in contrast to gld-2 ego-1 gld-1 (Figure 5B) and gld-2 gld-1; glp-1 mutants (Hansen et al. 2004b), where distalmost germ cells are typically mitotic. Because inactivation of GLP-1 and EGO-1 has separable effects on meiotic entry, we conclude that each protein is active in the absence of the other. Hence, GLP-1 is not the sole positive regulator of EGO-1 activity, and EGO-1 does not regulate GLP-1 signaling.
Our results also demonstrate that GLP-1 activity still regulates the proliferation vs. meiosis choice even when GLD-1 and GLD-2 are absent. In other words, since the presence vs. absence of GLP-1 activity has an effect on gld-1 ego-1 gld-1 animals, GLP-1 must regulate something in addition to GLD-1 and GLD-2. This result is consistent with previous studies (Hansen et al. 2004b; Maine et al. 2004).
P-granule assembly and distribution is abnormal in ego-1 mutants:
P granules are cytoplasmic RNP particles that segregate to the germ lineage during embryonic development and are present in the larval and adult germline (Strome and Wood 1983; see Seydoux and Schedl 2001). In the germline, they associate with the nuclear envelope, where each P granule typically spans a nuclear pore (Strome and Wood 1983; Pitt et al. 2000). P granules are fairly uniform in size and distribution around each nucleus (Strome and Wood 1983; also see Gruidl et al. 1996; Kawasaki et al. 1998; Kuznicki et al. 2000; Pitt et al. 2000; Schisa et al. 2001) (see Figure 7A). We investigated the morphology and distribution of P granules in the ego-1 germline using antisera against two core protein components, P-granule component (PGL-1) (Kawasaki et al. 1998) and germline helicase (GLH-1) (Gruidl et al. 1996). We compared the morphology and distribution of P granules in wild-type (N2) and ego-1 mutants by immunostaining with polyclonal or monoclonal antisera against PGL-1 (anti-PGL-1 and mAb K76, respectively; Strome and Wood 1983; Kawasaki et al. 1998) and polyclonal antiserum against GLH-1 (Gruidl et al. 1996) (see materials and methods).
Figure 7.—
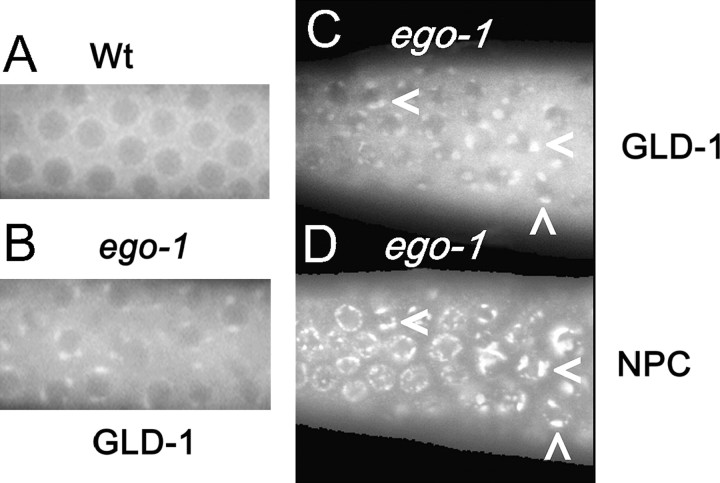
Punctate GLD-1 foci are present in the ego-1(0) germline. (A) Localization of GLD-1::GFP (ozEx50) in wild-type leptotene/zygotene germ cells. GLD-1::GFP is present in the cytoplasm and, at a low level, as perinuclear foci. (B) Localization of GLD-1::GFP in ego-1(om84) leptotene/zygotene germ cells. Note prominent, irregular perinuclear foci. (C and D) Dissected ego-1(om84) gonad that was costained with anti-GLD-1 and mAb414 against nucleoporins. Germ cells in leptotene/zygotene phase are shown. Perinuclear GLD-1 foci (C) are adjacent to brighter regions of nucleoporin staining (D). Arrowheads indicate corresponding GLD-1 and nucleoporin foci.
In wild-type adult germlines, we detected diffuse PGL-1 staining throughout the cytoplasm in the mitotic and leptotene/zygotene regions; we also observed perinuclear PGL-1 foci (corresponding to P granules) that were fairly uniform in size and distribution, as has been reported (Figure 6A). In the pachytene region, the diffuse cytoplasmic staining was sharply reduced, and perinuclear foci were prominent (Figure 6C). In the ego-1(0) germline, we saw a similar global distribution of PGL-1, but the morphology and distribution of perinuclear PGL-1 foci were consistently altered (Figure 6, B and D). A similar effect on PGL-1 distribution was seen in both ego-1(om84) and ego-1(om97) mutants. In particular, P granules were irregularly distributed and highly variable in size. This effect was most pronounced in the pachytene region where some P granules were much larger than wild type (Figure 6D). We saw similar changes in the size and distribution of GLH-1 foci in wild-type vs. ego-1(om84) germlines; e.g., size and distribution of perinuclear GLH-1 foci was fairly uniform in wild-type germlines and irregular in ego-1(om84) germlines (data not shown). We conclude that ego-1 activity promotes the normal assembly/distribution of germline P granules.
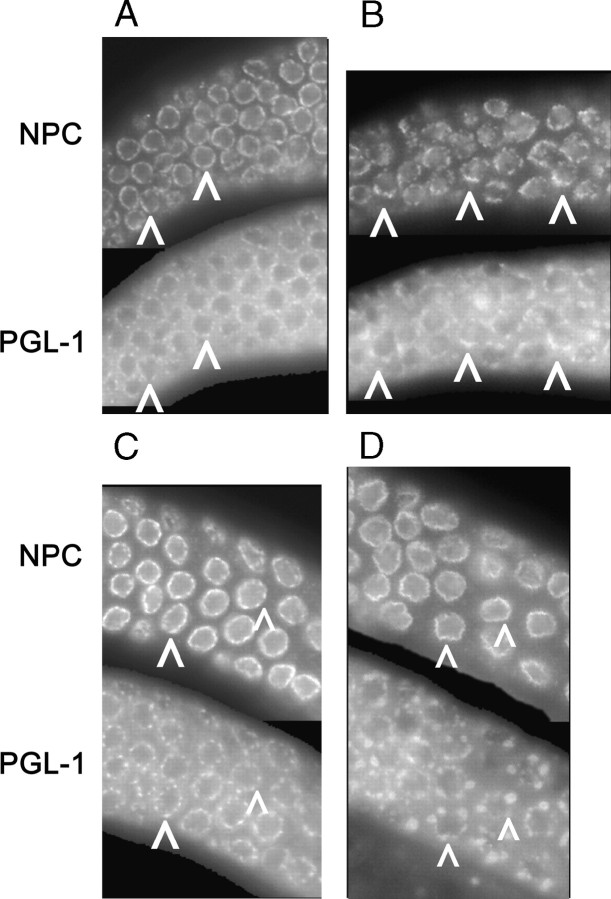
Figure 6.—
Distribution of P granules and nuclear pore complexes is altered in ego-1 mutants. Tissue was costained with anti-PGL-1 to visualize P granules and anti-nucleoporin monoclonal antibody, mAb414, to visualize nuclear pore complexes (NPC). Arrowheads indicate corresponding PGL-1 and mAb414 foci. (A) Wild-type leptotene/zygotene tissue. Diffuse cytoplasmic PGL-1 and perinuclear PGL-1 foci are visible. (B) ego-1(om84) leptotene/zygotene tissue. PGL-1 is again visible in the cytoplasm and as perinuclear foci. Note the large, adjacent PGL-1 foci and NPCs. (C) Wild-type pachytene tissue. Note that diffuse cytoplasmic PGL-1 is reduced and NPCs are more pronounced than in A. (D) ego-1(om84) pachytene tissue. P-granule size is very large compared with wild type (B) and distribution is less uniform.
Consistent with the enlarged P-granule morphology, we saw stronger GLD-1 staining of germline P granules in ego-1 mutants (Figure 7). In wild-type germlines, GLD-1 association with P granules is not prominent until embryogenesis (Jones et al. 1996; S. Nayak, personal communication) (Figure 7A). In ego-1 mutants, we observed very distinct perinuclear GLD-1 puncta in the distal germline (Figure 7B). The effect is striking in ego-1(om84), ego-1(om97), and ego-1(om58) mutants and less severe in the ego-1(om54) partial lf mutant. To confirm that these GLD-1 puncta are associated with P granules, we colabeled tissue with antibodies against GLD-1 and PGL-1. The GLD-1 puncta consistently colocalized with PGL-1 in all germlines (data not shown), indicating that either elevated levels of GLD-1 associate with P granules in ego-1 mutants or P-granule structure is altered so as to allow greater accessibility of the anti-GLD-1 antibody.
Changes in P-granule morphology are not a general feature of mutants with altered RNAi:
We asked whether mutations that affect RNAi, in general, cause changes in P-granule morphology by examining PGL-1 distribution in adult dcr-1(0) and rrf-3(0) gonads. dcr-1 encodes the Dicer nuclease that generates siRNAs from the dsRNA precursor (Grishok et al. 2001; Ketting et al. 2001; Knight and Bass 2001). No changes in the gross morphology or distribution of PGL-1 in dcr-1 mutants were seen under our conditions (data not shown). Therefore, the RNAi machinery per se does not appear to be required for P-granule assembly. [One caveat is that the dcr-1(0) animals were derived from dcr-1(+/0) mothers; therefore, one cannot rule out a maternal rescue effect.] rrf-3 encodes a putative RdRP that is active in both germline and soma (Sijen et al. 2001; Simmer et al. 2002). Mutations in rrf-3(0) produce a hypersensitive or “enhanced” RNAi phenotype and cause temperature-sensitive developmental defects in the germline (Simmer et al. 2002). P-granule morphology appeared normal in rrf-3(0) adults raised at restrictive temperature (data not shown).
Nucleoporin distribution is abnormal in ego-1 mutants:
Since P granules are typically associated with clusters of nuclear pores (Pitt et al. 2000), we next investigated nuclear pore distribution and the relationship between nuclear pore and P-granule location in ego-1(0) germlines. Nuclear pore complexes contain integral membrane proteins and a number of related proteins, called nucleoporins (Vasu and Forbes 2001). The monoclonal antibody, mAb414 (Davis and Blobel 1986), recognizes an epitope present in several nucleoporins and is therefore often used to visualize nuclear pore distribution in diverse tissues and species (see Pitt et al. 2000; Liu et al. 2000; Vasu and Forbes 2001; Taddei et al. 2004). In wild-type germlines, P granules are detected in close proximity to clusters of NPCs by electron microscopy and by indirect immunofluorescence with mAb414 (although not every nuclear pore is associated with a P granule) (Pitt et al. 2000). At the level of detection by immunostaining, wild-type germ cells have distinct nucleoporin foci that are fairly evenly distributed on the nuclear envelope during mitosis and very early (leptotene/zygotene) meiotic prophase; staining increases and becomes more continuous during pachytene stage (Figure 6, A and C). In ego-1(0) mutants, in contrast, nucleoporin foci have a patchy distribution in the mitotic and leptotene/zygotene regions (Figure 6B). Nucleoporin staining intensity increases as cells enter pachytene stage, but remains patchy (Figure 6D). Therefore, EGO-1 activity influences nucleoporin distribution, and presumably this reflects a change in NPC assembly and distribution.
We hypothesized that ego-1 P granules might be irregular in part because their assembly might be dissociated from nuclear pores. To investigate this possibility, we co-immunolabeled tissue with mAb414 and anti-PGL-1, mAb K76, or anti-GLD-1. In mitotic and leptotene/zygotene cells, perinuclear PGL-1 and GLD-1 foci were consistently detected adjacent to nucleoporin foci (Figures 6 and 7, C and D). The relative size of the PGL-1 and GLD-1 foci reflected the relative size of the nucleoporin foci; i.e., large PGL-1 and GLD-1 foci were associated with large nucleoporin foci. Within pachytene tissue, relatively high levels of nucleoporin staining again correlated with PGL-1 (Figure 6D) and GLD-1 foci. Lower levels of nucleoporin staining were visible that frequently were not associated with a P granule. On the basis of these data, we conclude that the association between P granules and NPCs is maintained in ego-1 mutants. We hypothesize that the abnormal distribution (and, perhaps, composition) of P granules in ego-1 germ cells may reflect the underlying defect in nuclear pore distribution.
DISCUSSION
Cellular RdRPs have generated a great deal of interest because they are implicated in RNA silencing (reviewed by Huang et al. 2003; Meister and Tuschl 2004), development (Smardon et al. 2000; Shiu et al. 2001; Simmer et al. 2002; Peragrine et al. 2004), and chromatin regulation (Volpe et al. 2002, 2003; reviewed by Grewal and Rice 2004; Lippman and Martienssen 2004) in diverse organisms. We originally identified ego-1 on the basis of genetic interactions with the GLP-1/Notch signaling pathway, which maintains germline proliferation, and subsequently showed that ego-1 promotes several other aspects of germline development (Qiao et al. 1995; Smardon et al. 2000). Here, we investigated how EGO-1 functions relative to the regulatory pathways that promote meiotic entry. We show that the global distribution of GLP-1 and GLD-1 proteins is normal in the ego-1 mutant germline, indicating that EGO-1 does not regulate the level of either protein. Using genetic analysis, we show that EGO-1 is likely to function in parallel with GLP-1 signaling to regulate the balance between proliferation and meiotic entry. We also show that ego-1 interacts synergistically with gld-1 to promote germline health. Finally, we demonstrate that assembly/distribution of nuclear pore complexes and P granules, structures critical to germ cell biology, relies on EGO-1 activity. We suspect that the ego-1 proliferation defect (and other aspects of the phenotype) results from a combination of these cell biological defects and the misregulation of gene expression. EGO-1 functions in chromatin assembly (E. Maine, J. Hauth, T. Ratliff and W. Kelly, unpublished data), and preliminary microarray analysis suggests that a large number of genes are misregulated in the ego-1(0) mutant (V. Vought, V. Reinke and E. Maine, unpublished data).
EGO-1 acts in parallel with GLP-1 to regulate the proliferation vs. meiosis choice:
EGO-1 influences the balance between the proliferative and meiotic fates (Smardon et al. 2000; this study). GLP-1 activity is required for maintenance of germline proliferation (Austin and Kimble 1987). GLP-1 signaling restricts activity of the GLD-1 meiotic entry pathway and is suspected to restrict activity of the GLD-2 meiotic entry pathway, as well (Francis et al. 1995a; Kadyk and Kimble 1998; Hansen et al. 2004a,b). Our data clearly indicate that EGO-1 is not a positive regulator of GLP-1 or a negative regulator of GLD-1 or GLD-2. Moreover, EGO-1 is active in the absence of GLP-1 signaling, indicating that GLP-1 cannot be the sole positive regulator of ego-1. Consequently, we propose that EGO-1 acts independently of GLP-1 to promote proliferation and/or inhibit meiotic entry.
The ego-1 proliferation phenotype may result from several different defects, e.g., changes in gene expression level compounded by the impaired NPC and/or P-granule function. On the basis of our genetic data, EGO-1 may act in any of several ways to antagonize meiotic entry. For example, EGO-1 may antagonize GLD-1 and GLD-2 by repressing expression of factors that promote meiosis and/or increasing expression of factors that promote proliferation. These factors might be GLD-1 and GLD-2 targets and/or factors that interact with those targets. In addition, EGO-1 may act in parallel with GLP-1 to repress the proposed third meiotic entry pathway. Finally, EGO-1 activity may influence the activity of other proliferation factors acting in parallel with GLP-1, e.g., ATX-2 (Maine et al. 2004). These alternatives are not mutually exclusive.
EGO-1 activity is important for nuclear pore and germ granule assembly:
Nuclear pores are critical for normal cellular functions, and changes in their structure are likely to have many phenotypic consequences in the C. elegans germline (Vasu and Forbes 2001; Erkmann and Kutay 2004; Taddei et al. 2004). Changes in nuclear pore structure can alter the import/export of proteins, RNAs, and other molecules and have been shown to affect the size exclusion limit of the pore. Clumping of nuclear pore complexes probably contributes to the irregular P-granule assembly/distribution that we see in ego-1(0) mutants. P granules contain a large, dynamic population of mRNAs (Schisa et al. 2001), and core protein components (e.g., PGL-1, the GLHs) promote germline proliferation and gametogenesis (Gruidl et al. 1996; Kawasaki et al. 1998; Kuznicki et al. 2000). We propose that changes in nuclear pore and P-granule assembly/distribution present in ego-1 mutants are likely to impair germline function per se, including proliferation.
Interestingly, the ego-1(0) P-granule defect is novel. Previously described mutants, e.g., in pgl-1 and glh-1, have small P granules, presumably because assembly is impaired by the absence or reduced activity of a core component (Schisa et al. 2001). In the ego-1 germline, P-granule assembly appears to be unregulated with respect to size. We propose that P-granule size is regulated by association with nuclear pores, and the clumping of nuclear pore material leads to unregulated P-granule assembly. Elevated levels of GLD-1 protein on P granules in the ego-1 germline may be another feature of this unregulated assembly.
The ego-1 nuclear pore defect is most striking in leptotene/zygotene nuclei (see Figure 6). The nucleus reorganizes during this time, as sister chromosomes pair and the synaptonemal complex forms (Zickler and Kleckner 1998). This stage is notably protracted in ego-1 mutants (Smardon et al. 2000). Pitt et al. (2000) demonstrated that pachytene chromosomes are not located adjacent to the regions of P-granule/nucleoporin staining and hypothesize that pachytene chromosomes may not be able to attach to the nuclear envelope in regions of high pore density. Perhaps the abnormal nuclear pore distribution in ego-1 mutants limits the ability of chromosomes to rearrange at leptotene/zygotene (e.g., by restricting their ability to attach to the nuclear envelope).
Why are nuclear pores abnormal in ego-1 mutants?
It is not clear how nuclear pore number or distribution is regulated, although work in a variety of organisms has identified important contributing factors. Nuclear pore assembly has been linked to nuclear envelope assembly per se and to the function of specific nucleoporins (Vasu and Forbes 2001). For example, two C. elegans nucleoporin genes regulate NPC distribution in the embryo (Galy et al. 2003). Strikingly, when either Nup93 (encoded by npp-13) or Nup205 (encoded by npp-3) is knocked down by RNAi, NPCs in embryonic cells have a patchy distribution very similar to the NPC distribution in ego-1 mutant germlines. Importantly, Galy et al. (2003) showed that changes in nuclear pore function correlate with the altered distribution. Another example is provided by analysis of lamin, a core nuclear envelope component. Loss of lamin (LMN-1) activity in the C. elegans embryo causes NPC clustering similar to what is seen when Nup93 or Nup205 is absent (Liu et al. 2000). In addition to nuclear envelope components, it has been proposed that the underlying chromatin may, in some way, influence NPC distribution (Vasu and Forbes 2001). The nuclear envelope assembles around chromatin, and both nuclear envelope and NPC assembly are directed by proteins (e.g., RAN-GTPase) that associate with chromatin. Heterochromatin tends to associate with the nuclear periphery, and the inner nuclear envelope can bind chromatin (Taddei et al. 2004). Moreover, direct physical links between NPCs and chromatin boundary elements have been demonstrated to be important in regulating gene expression (e.g., Ishi et al. 2002; Oki et al. 2004; see Pai and Corces 2002).
EGO-1 activity might influence nuclear pore distribution/assembly in two general ways. First, EGO-1 activity might promote expression of certain nucleoporins and/or other nuclear envelope components. In the absence of EGO-1 activity, the balance between these components shifts, causing patchy distribution of nuclear pores. Second, EGO-1 may promote a chromatin state that directs nuclear pore distribution. For example, the association of NPCs with heterochromatin boundaries (Ishi et al. 2002) implies that nuclear pore distribution depends on the chromatin structure of underlying chromosomes. Therefore, the change in chromatin structure in ego-1(0) germ cells might alter nuclear pore distribution. This effect might occur directly by loss of specific chromatin modifications required for NPC assembly or indirectly by an overall redistribution of chromatin in the nucleus.
Acknowledgments
We thank Tim Schedl, Karen Bennett, Pam Hoppe, and our colleagues at Syracuse University for helpful discussions in the course of this work and Tim Schedl and Dave Hansen for comments on the manuscript. We thank colleagues who have sent reagents, including Henry Epstein (anti-myosin), Susan Strome (anti-PGL-1), Joseph Loidl (anti-REC-8), Monique Zetka (anti-HIM-3), Sudhir Nayak (ozEx50[gld-1::gfp]), Sarah Crittenden and Judith Kimble (anti-GLP-1), Dave Allis (anti-H3-phos), Karen Bennett (anti-GLH-1), and Barth Grant (anti-RME-2, yp170::gfp). We acknowledge Anne Smardon for the ego-1 developmental RNA blot experiment. Some strains used in this study were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources. This work was supported by funding from the National Science Foundation and Syracuse University (to E.M.). The work of M.O. and M-H.L. in Tim Schedl's lab was supported by NIH grant GM63310.
References
- Austin, J., and J. Kimble, 1987. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell 51 589–599. [DOI] [PubMed] [Google Scholar]
- Baron, M., 2003. An overview of the Notch signaling pathway. Semin. Cell Dev. Biol. 14 113–119. [DOI] [PubMed] [Google Scholar]
- Berry, L. W., B. Westlund and T. Schedl, 1997. Germ-line tumor formation caused by activation of glp-1, a Caenorhabditis elegans member of the Notch family of receptors. Development 124 925–936. [DOI] [PubMed] [Google Scholar]
- Chen, C. K., K. Bradnam, R. Durbin and J. Hodgkin, 2003 Genetic Map of Caenorhabditis elegans. Caenorhabditis Genetics Center, St. Paul, MN.
- Clifford, R., M. H. Lee, S. Nayak, M. Ohmachi, F. Giorgini et al., 2000. FOG-2, a novel F-box containing protein, associates with the GLD-1 RNA binding protein and directs male sex determination in the C. elegans hermaphrodite germline. Development 127 5265–5276. [DOI] [PubMed] [Google Scholar]
- Crittenden, S. L., E. R. Troemel, T. C. Evans and J. Kimble, 1994. GLP-1 is localized to the mitotic region of the C. elegans germ line. Development 190 2901–2911. [DOI] [PubMed] [Google Scholar]
- Crittenden, S. L., C. R. Eckmann, L. Wang, D. S. Bernstein, M. Wickens et al., 2003. Regulation of the mitosis/meiosis decision in the Caenorhabditis elegans germline. Philos. Trans. R. Soc. Lond. 358 1359–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, L. I., and G. Blobel, 1986. Identification and characterization of a nuclear pore complex protein. Cell 45 699–709. [DOI] [PubMed] [Google Scholar]
- Epstein, H. F., and D. C. Shakes, 1995. Caenorhabiditis elegans: biological analysis of an organism. Methods Cell Biol. 48 4–29, 513–519. [Google Scholar]
- Erkmann, J. A., and U. Kutay, 2004. Nuclear export of mRNA: from the site of transcription to the cytoplasm. Exp. Cell Res. 296 12–20. [DOI] [PubMed] [Google Scholar]
- Francis, R., M. K. Barton, J. Kimble and T. Schedl, 1995. a gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics 139 579–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, R., E. Maine and T. Schedl, 1995. b Analysis of the multiple roles of gld-1 in germline development: interactions with the sex determination cascade and the glp-1 signaling pathway. Genetics 139 607–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy, V., I. W. Mattaj and P. Askjaer, 2003. Caenorhabditis elegans nucleoporins Nup93 and Nup205 determine the limit of nuclear pore complex size exclusion in vivo. Mol. Biol. Cell 14 5104–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, B., and D. Hirsh, 1999. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 10 4311–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal, S. I. S., and J. C. Rice, 2004. Regulation of heterochromatin by histone methylation and small RNAs. Curr. Opin. Cell Biol. 16 230–238. [DOI] [PubMed] [Google Scholar]
- Grishok, A., A. E. Pasquinelli, D. Conte, N. Li, S. Parrish et al., 2001. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106 23–34. [DOI] [PubMed] [Google Scholar]
- Gruidl, M. E., P. A. Smith, K. A. Kuznicki, J. S. McCrone, J. Kirchner et al., 1996. Multiple potential germ-line helicases are components of the germ-line-specific P granules of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 93 13837–13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, D., L. Wilson-Berry, T. Dang and T. Schedl, 2004. a Control of the proliferation versus meiotic development decision in C. elegans through regulation of GLD-1 protein accumulation. Development 131 93–104. [DOI] [PubMed] [Google Scholar]
- Hansen, D., E. J. A. Hubbard and T. Schedl, 2004. b Multi-pathway control of the proliferation versus meiotic development decision in the C. elegans germ line. Dev. Biol. 268 342–357. [DOI] [PubMed] [Google Scholar]
- Hendzel, M. J., Y. Wei, M. A. Mancini, A. Van Hooser, T. Ranalli et al., 1997. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106 348–360. [DOI] [PubMed] [Google Scholar]
- Huang, L., J. Gledhill and C. E. Cameron, 2003 RNA-dependent RNA polymerase in gene silencing, pp. 175–203 in RNAi: A Guide to Gene Silencing, edited by G. J. Hannon. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Ishi, K., G. Arib, C. Lin, G. VanHouwe and U. K. Laemmli, 2002. Chromatin boundaries in budding yeast: the nuclear pore connection. Cell 109 551–562. [DOI] [PubMed] [Google Scholar]
- Jan, E., C. K. Motzny, L. E. Graves and E. B. Goodwin, 1999. The STAR protein, GLD-1, is a translational regulator of sexual identity in Caenorhabditis elegans. EMBO J. 18 258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A. R., and T. Schedl, 1995. Mutations in gld-1, a female germ cell-specific tumor suppressor gene in Caenorhabditis elegans, affect a conserved domain also found in Src-associated protein Sam68. Genes Dev. 9 1491–1504. [DOI] [PubMed] [Google Scholar]
- Jones, A. R., R. Francis and T. Schedl, 1996. GLD-1, a cytoplasmic protein essential for oocyte differentiation, shows stage and sex-specific expression during C. elegans germline development. Dev. Biol. 180 165–183. [DOI] [PubMed] [Google Scholar]
- Kadyk, L., and J. Kimble, 1998. Genetic regulation of entry into meiosis in C. elegans. Development 125 1803–1813. [DOI] [PubMed] [Google Scholar]
- Kawasaki, I., Y. H. Shim, J. Kirchner, J. Kaminker, W. B. Wood et al., 1998. PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C. elegans. Cell 94 635–645. [DOI] [PubMed] [Google Scholar]
- Ketting, R. F., S. E. Fischer, E. Bernstein, T. Sijen, G. J. Hannon et al., 2001. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 15 2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, S. W., and B. L. Bass, 2001. A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science 293 2269–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodoyianni, V., E. M. Maine and J. Kimble, 1992. The molecular basis of loss-of-function mutations in the glp-1 gene of C. elegans. Mol. Biol. Cell 3 1199–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznicki, K. A., P. A. Smith, W. M. A. Leung-Chiu, A. O. Estevez, H. C. Scott et al., 2000. Combinatorial RNA interference indicates GLH-4 can compensate for GLH-1; these two P granules components are critical for fertility in C. elegans. Development 127 2907–2916. [DOI] [PubMed] [Google Scholar]
- Lai, E. C., 2004. Notch signaling: control of cell communication and cell fate. Development 131 965–973. [DOI] [PubMed] [Google Scholar]
- Lee, M.-H., and T. Schedl, 2001. Identification of in vivo mRNA targets of GLD-1, a maxi-KH motif containing protein required for C. elegans germ cell development. Genes Dev. 15 2408–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M.-H., and T. Schedl, 2004. Translation repression by GLD-1 protects its mRNA targets from nonsense-mediated mRNA decay in C. elegans. Genes Dev. 18 1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman, Z., and R. Martienssen, 2004. The role of RNA interference in heterochromatic silencing. Nature 431 364–370. [DOI] [PubMed] [Google Scholar]
- Liu, J. K., T. R. Ben-Shahar, D. Riemer, M. Treinin, P. Spann et al., 2000. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol. Biol. Cell 11 3937–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maine, E. M., D. Hansen, D. Springer and V. E. Vought, 2004. Caenorhabditis elegans atx-2 promotes germline proliferation and the oocyte fate. Genetics 168 817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev, E. V., and D. H. Bamford, 2002. Cellular RNA-dependent RNA polymerase involved in posttranscriptional gene silencing has two distinct activity modes. Mol. Cell 10 1417–1427. [DOI] [PubMed] [Google Scholar]
- Marin, V. A., and T. C. Evans, 2003. Translational repression of a C. elegans Notch mRNA by the STAR/KH domain protein GLD-1. Development 130 2623–2632. [DOI] [PubMed] [Google Scholar]
- Meister, G., and T. Tuschl, 2004. Mechanisms of gene silencing by double-stranded RNA. Nature 431 343–349. [DOI] [PubMed] [Google Scholar]
- Mootz, D., D. M. Ho and C. P. Hunter, 2004. The STAR/Maxi-KH domain protein GLD-1 mediates a developmental switch in the translational control of C. elegans PAL-1. Development 131 3263–3272. [DOI] [PubMed] [Google Scholar]
- Motamedi, M. R., A. Verdel, S. U. Colmenares, S. A. Gerber, S. P. Gygi et al., 2004. Two RNAi complexes, RITS and RDRC, physically interact and localize to noncoding centromeric RNAs. Cell 119 789–802. [DOI] [PubMed] [Google Scholar]
- Oki, M., L. Valenzuela, T. Chiba, T. Ito and R. T. Kamakaka, 2004. Barrier proteins remodel and modify chromatin to restrict silenced domains. Mol. Cell. Biol. 24 1956–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai, C. Y., and V. G. Corces, 2002. The nuclear pore complex and chromatin boundaries. Trends Cell Biol. 12 452–455. [DOI] [PubMed] [Google Scholar]
- Pasierbek, P., M. Jantsch, M. Melcher, A. Schleiffer, D. Schweizer et al., 2001. A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes Dev. 15 1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peragrine, A., M. Yoshikawa, G. Wu, H. L. Albrecht and R. S. Poethig, 2004. SGS3 and SGS2/SDE1/RDR6 are required for juvenile development and the production of trans-acting siRNAs in Arabidopsis. Genes Dev. 18 2368–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt, J. N., J. A. Schisa and J. R. Priess, 2000. P granules in the germ cells of Caenorhabditis elegans adults are associated with clusters of nuclear pores and contain RNA. Dev. Biol. 219 315–333. [DOI] [PubMed] [Google Scholar]
- Qiao, L., J. L. Lissemore, P. Shu, A. Smardon, M. Gelber et al., 1995. Enhancers of glp-1, a gene required for cell-signaling in C. elegans, define a set of genes required for germline development. Genetics 141 551–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., and D. W. Russell, 2001 Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Schisa, J. A., J. N. Pitt and J. R. Priess, 2001. Analysis of RNA associated with P granules in germ cells of C. elegans adults. Development 128 1287–1298. [DOI] [PubMed] [Google Scholar]
- Schumacher, B., M. Hanazawa, M.-H. Lee, S. Nayak, K. Volkmann et al., 2005. Translational repression of C. elegans p53 by GLD-1 regulates DNA damage-induced apoptosis. Cell 120 357–368. [DOI] [PubMed] [Google Scholar]
- Schweisguth, F., 2004. Regulation of Notch signaling activity. Curr. Biol. 14 R129–R138. [PubMed] [Google Scholar]
- Seydoux, G., and T. Schedl, 2001. The germline in C. elegans: origins, proliferation, and silencing. Int. Rev. Cytol. 203 139–185. [DOI] [PubMed] [Google Scholar]
- Shiu, P. K., N. B. Raju, D. Zickler and R. L. Metzenberg, 2001. Meiotic silencing by unpaired DNA. Cell 107 905–916. [DOI] [PubMed] [Google Scholar]
- Sijen, T., J. Fleenor, F. Simmer, K. L. Thijssen, S. Parish et al., 2001. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107 465–476. [DOI] [PubMed] [Google Scholar]
- Simmer, F., M. Tijsterman, S. Parrish, S. P. Koushika, M. L. Nonet et al., 2002. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr. Biol. 12 1317–1319. [DOI] [PubMed] [Google Scholar]
- Smardon, A., J. M. Spoerke, S. C. Stacey, M. E. Klein, N. Mackin et al., 2000. EGO-1 is related to RNA-directed RNA polymerase and functions in germline development and RNA interference in C. elegans. Curr. Biol. 10 169–178. [DOI] [PubMed] [Google Scholar]
- Strome, S., and W. B. Wood, 1983. Generation of asymmetry and segregation of germ-line granules in early C. elegans embryos. Cell 35 15–25. [DOI] [PubMed] [Google Scholar]
- Taddei, A., F. Hediger, F. R. Newmann and S. M. Gasser, 2004. The function of nuclear architecture: a genetic approach. Annu. Rev. Genet. 38 305–345. [DOI] [PubMed] [Google Scholar]
- Vasu, S. K., and D. J. Forbes, 2001. Nuclear pores and nuclear assembly. Curr. Opin. Cell Biol. 13 363–375. [DOI] [PubMed] [Google Scholar]
- Volpe, T. A., C. Kidner, I. M. Hall, G. Teng, S. I. Grewal et al., 2002. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297 1833–1837. [DOI] [PubMed] [Google Scholar]
- Volpe, T., V. Schramke, G. L. Hamilton, S. A. White, G. Teng et al., 2003. RNA interference is required for normal centromere function in fission yeast. Chromosome Res. 11 137–146. [DOI] [PubMed] [Google Scholar]
- Wang, L., C. R. Eckmann, L. C. Kadyk, M. Wickens and J. Kimble, 2002. A regulatory cytoplasmic poly(A) polymerase in Caenorhabditis elegans. Nature 419 312–316. [DOI] [PubMed] [Google Scholar]
- Xu, L., J. Paulsen, Y. Yoo, E. B. Goodwin and S. Strome, 2001. Caenorhabditis elegans MES-3 is a target of GLD-1 and functions epigenetically in germline development. Genetics 159 1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetka, M. C., I. Kawasaki, S. Strome and F. Muller, 1999. Synapsis and chiasma formation in Caenorhabditis elegans require HIM-3, a meiotic chromosome core component that functions in chromosome segregation. Genes Dev. 13 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickler, D., and N. Kleckner, 1998. The leptotene-zygotene transition of meiosis. Annu. Rev. Genet. 32 619–697. [DOI] [PubMed] [Google Scholar]