Abstract
A TRP5-based reversion system that allows the rates of all possible base pair substitutions to be measured when the TRP5 locus is in both orientations relative to a defined origin of replication has been developed. This system should be useful for a wide variety of mutation and repair studies in yeast.
REVERSION assays that can detect specific base pair substitutions have proven extremely useful. One of the best known is the set of Escherichia coli lacZ alleles that can individually detect all possible base pair substitutions (Cupples and Miller 1989). Hampsey (1991) developed a comparable tester system for yeast that can detect all possible base pair substitutions by reversion of various point mutations at the essential Cys-22 of the CYC1 gene. Although the cyc1 reversion system has proven quite useful in a number of studies (Thomas et al. 1997; Bruner et al. 1998; Earley and Crouse 1998; Scott et al. 1999; Brachman and Kmiec 2003), there are several problems with this assay. As noted in the original article, the reversion assay requires diploid strains (Hampsey 1991) for reasons that are not entirely clear. When genetic manipulation of the strain background is desired, the requirement for diploidy substantially increases the difficulty of strain construction. In addition to a hemizygous cyc1 mutant gene, it is necessary to perform the reversion analysis in a cyc7 deletion background to avoid gene conversion of the CYC1 gene from the similar CYC7 gene (Hampsey 1991). In work subsequent to our published work with this system, we observed strain isolates that would not revert under any circumstances (results not shown). The reason for this problem remains unknown, but the appearance of such strains greatly complicated the use of this system for genetic analysis. We report here the development of a series of strains that together can detect any possible base pair substitution in the Glu-50 residue of TRP5. Each strain reverts only via a true reversion event and revertants are easily assayed by plating on media lacking tryptophan. The only genetic requirement for the assay is that all genes of the Trp pathway other than TRP5 be functional, and either haploid or diploid strains can be used. Moreover, we have placed the mutant trp5 gene in both orientations close to a dependable origin of replication, so that the effect of replication orientation on mutagenesis can be studied. We show here that these strains can be used to determine very easily the mutagenic specificity of various mutagens.
The difficulty in developing a specific reversion assay is finding an absolutely essential residue in a gene whose reversion can be easily scored. In addition, to be able to study all possible base pair reversion events in a similar sequence context, the residue needs to have a codon with unique first and second nucleotides. The Saccharomyces cerevisiae TRP5 gene is homologous to the E. coli tryptophan synthase α- and β-chains, encoded by trpA and trpB, respectively (Zalkin and Yanofsky 1982). It had been shown that the Glu-49 residue of E. coli trpA was essential for function and could not be substituted by any other amino acid (Yutani et al. 1987). An alignment of a variety of fungal and bacterial tryptophan synthases indicated that the residue corresponding to E. coli Glu-49 was conserved in all species and therefore was likely essential in the other enzymes (Figure 1A). The S. cerevisiae TRP5 residue homologous to E. coli trpA Glu-49 is Glu-50. A convenient feature of the S. cerevisiae sequence is that this codon is part of a TaqI restriction site, which is destroyed by any change in positions 1 or 2 of the codon. Thus any mutation in nucleotide sequence would presumably give a strain that would be Trp− and that would lack the TaqI site (Figure 1B). Correspondingly, any true revertant should restore the TaqI site. To place the mutant gene in a region near a reliable origin of replication, the TRP5 gene was first deleted from the genome and then inserted in both orientations near the ARS306 origin, which has been well characterized (Deshpande and Newlon 1992; Theis et al. 1999; Poloumienko et al. 2001). All strains with the introduced trp5 mutations were phenotypically Trp−, indicating the essential nature of the Glu-50 residue.
Figure 1.—
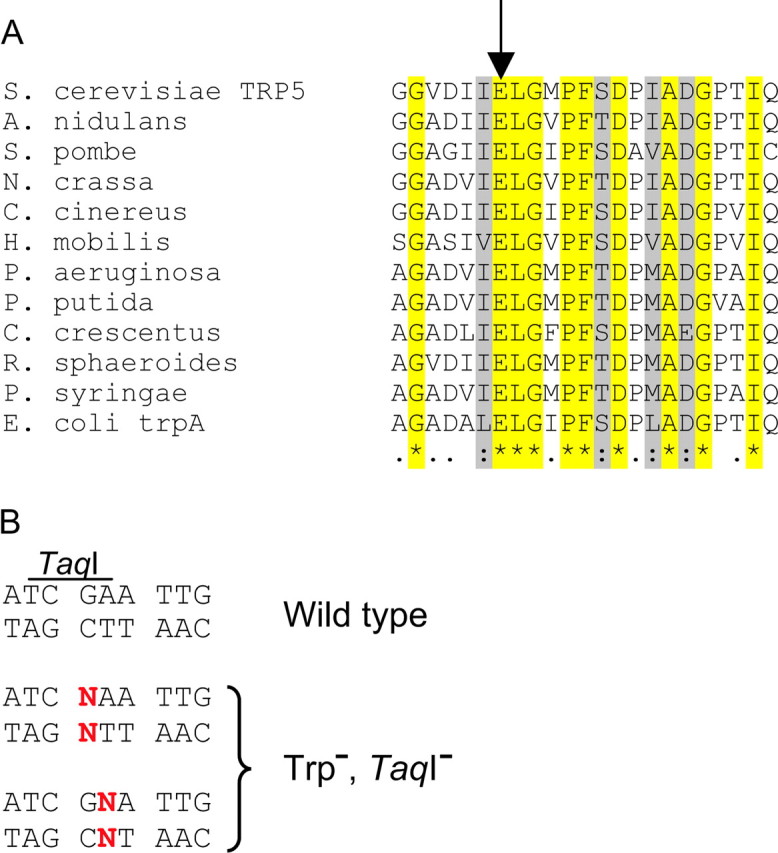
(A) Alignment of the most conserved region of the S. cerevisiae TRP5 protein with various fungal and bacterial homologs. Alignment was done using CLUSTAL W (1.74) multiple sequence alignment (Thompson et al. 1994). The shaded residues are similar, and those in yellow are identical in all species compared. The arrow marks the Glu-50 residue chosen for mutagenesis in this study. (B) Sequence surrounding the Glu-50 codon (GAA) of Trp5. The TRP5 wild-type sequence has a TaqI site overlapping the Glu-50 codon, but replacement of the base at nucleotide position 148 or 149 by any base other than wild type (N) destroys the restriction site, as well as renders the strain Trp−.
Various trp5 strains were grown and plated on Trp− plates, either with or without prior mutagen treatment. Revertants were chosen at random from a variety of strains and mutagen treatments and the region surrounding the introduced mutation was amplified by PCR and digested with TaqI to determine if the revertant were a true revertant or whether reversion could be obtained by second-site mutations. Of the 119 revertants tested, none did not gain the TaqI site, indicating that reversion required the restoration of the Glu-50 residue. However, 12% of the tested revertants gave a PCR product that did not cut completely, even after repurification of the revertant colony. The partial digestion was not due to insufficient restriction enzyme or impurities in the DNA; in addition, there was usually an approximately equal amount of digested and undigested product (results not shown). Sequence of two of these revertants showed approximately equal amounts of the original mutant sequence and the reverted wild-type sequence. Such revertants were rarely seen upon methyl methanesulfonate (MMS) treatment, more frequently with UV treatment, and none were observed under other conditions. Therefore it may be that replication-blocking lesions can result in gene duplication events prior to reversion, but more work will have to be done to clarify the cause and mechanism of these reversion events.
Results of the treatment of the trp5 strains with various mutagens are displayed in Figure 2. Spontaneous reversion of the mutant strains is very low; in some cases no revertants were seen in multiple cultures. UV damage caused primarily GC → AT transitions, although there were increases in all types of mutations. UV targets primarily dipyrimidines (Friedberg et al. 1995). Thus, UV was a poor mutagen for reverting the cyc1 mutations in the Hampsey strains, because the proper sequence context was missing (Hampsey 1991). Here, however, the A149G strain provides a sequence context that matches a prime target for UV mutagenesis and thus gives a high frequency of reversion. 5-Azacytidine (5-AZ) was the most specific mutagen, giving almost exclusively CG → GC transversions, as also seen in the cyc1 gene (Hampsey 1991). Ethyl methanesulfonate (EMS) is known to be highly specific in its mutagenicity, and this was seen in these experiments, as the only significant reversion frequencies were GC → AT transitions, as also observed in cyc1 reversion (Hampsey 1991). MMS gave significant numbers of several different types of revertants, with the greatest number being AT → GC transitions. Although similar in many aspects of the reversion spectrum, the relative sensitivities of cyc1 and trp5 to MMS-induced reversion by GC → AT transitions and GC → TA transversions differed. Many of the mutations caused by MMS are likely due to error-prone synthesis across abasic sites (Glaab et al. 1999; Xiao et al. 2001); the most likely explanation for the difference in MMS mutation specificity between the two assays is sequence context effects. The base analog 6-N-hydroxylaminopurine (HAP) gave both AT → GC and GC → AT transitions, as has been found previously (Shcherbakova and Pavlov 1993; Pavlov et al. 1996; Kulikov et al. 2001), although in a different ratio. In E. coli, it was observed that HAP induced equal numbers of AT → GC and GC → AT transitions in the lacI gene (Pavlov et al. 1996). In yeast, in the URA3 gene, 21 of 29 sequenced HAP mutations were GC → AT transitions and 5 of 29 were AT → GC transitions (Shcherbakova and Pavlov 1993); in the LYS2 gene all transitions were GC → AT (Kulikov et al. 2001). In the present reversion assay, there were substantially more AT → GC transitions than GC → AT transitions. A strand and sequence context bias has been previously noted for HAP mutagenesis (Kulikov et al. 2001) and the differences in spectrum that we see here are yet another indication of this bias.
Figure 2.—
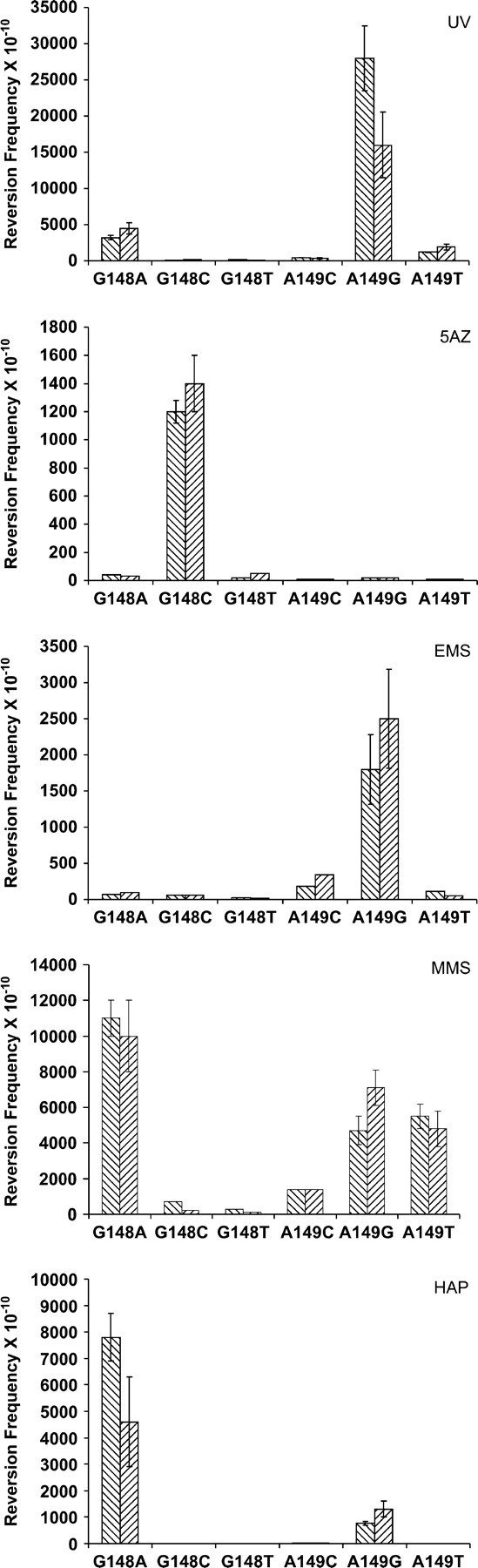
Reversion frequencies of the various trp5 mutant strains when treated with different mutagens. All strains were derived from the S288C derivative SJR828a (from Sue Jinks-Robertson), MATα his3Δ200 ura3-52 leu2Δ1. Gene deletion and mutagenesis was done using the pCORE plasmid and delitto perfetto strategy (Storici et al. 2001). The TRP5 gene was first deleted from its normal location in the genome; a PCR product of the TRP5 gene was then used to replace RNQ1, located near ARS306, in either orientation, after which the desired trp5 mutations were introduced. For measuring revertants, after mutagenesis strains were plated on SD media lacking tryptophan (SD-trp). Usually <1–2 × 108 cells/100-mm-diameter plate were plated, but plating at twice that density did not seem to interfere with the number of revertant colonies obtained. For measurement of UV mutagenesis, strains were plated on SD-trp plates at a density of ∼5 × 107 cells/100-mm-diameter plate and then exposed to 20 J/m2 UV light in an Ultra-Lum ultraviolet crosslinker (for an average survival of 74 ± 9%). For 5-AZ mutagenesis, cultures were grown overnight in YPD medium and ∼2 × 107 cells were transferred to SD-complete medium containing 5 mg/ml 5-AZ, grown overnight, and then plated on SD-trp plates. For the MMS and EMS mutagenesis assays, cells were washed and resuspended three times in the original culture volume of 50 mm potassium phosphate buffer, pH 7. At that point, EMS was added to a final concentration of 2.0% (for an average survival of 79 ± 11%) or MMS was added to a final concentration of 0.5% (for an average survival of 11 ± 4%) and cells were incubated at room temperature for 1 hr before being plated onto SD-trp plates, after inactivation of the mutagen by the addition of an equal volume of sterile 10% sodium thiosulfate. Mutagenesis with HAP was as described (Shcherbakova et al. 1996), using HAP at a concentration of 100 μg/ml. In all cases, YPD plates for determination of viable cells were counted after 2 days of incubation, and SD-trp plates were counted after 5 days. YPD plates for growth of trp5 strains were routinely supplemented with 40 mg/liter tryptophan. Reversion frequencies were determined from at least three cultures per strain and were averaged. If there were >10 revertants per culture, the standard deviation was calculated and is indicated with error bars on the graph. Forward orientation of the TRP5 gene is indicated by and reverse orientation by . In the forward orientation, TRP5 is oriented left to right on chromosome III and is to the left of ARS306.
Mutagens were tested on the trp5 mutants in both orientations relative to the nearby ARS306 origin of replication; in only two cases were the differences as much as twofold between the two orientations: in strain A149G with UV damage and in strain G148A with HAP. In both cases, although the standard deviations do not overlap, the difference is less than twofold and it would be necessary to perform additional experiments to be sure that the observed differences were a function of orientation of replication. For most of the mutagens, no previous experiments in yeast have shown any difference in mutation due to replication direction. However, it was observed that reversion of a ura3-29 allele by HAP showed a marked strand bias of sevenfold when the gene was in a similar location to our TRP5 gene (Pavlov et al. 2002). As mentioned above, HAP is known to display marked sequence context bias (Kulikov et al. 2001) and it may be that mutagenesis is affected by the replication strand only in certain contexts. The activity of the ARS306 origin is not known to be affected by strain background (Deshpande and Newlon 1992; Zhu et al. 1992) and the ARS306 origin in our strains has been found to fire efficiently (A. Dershowitz and C. Newlon, personal communication). Therefore we believe that the difference that we observed with previous data (Pavlov et al. 2002) is not due to the lack of directional replication through the TRP5 gene.
The set of mutations at the Glu-50 codon of TRP5 can be added to the small list of strains that can show all possible base pair reversions individually. The ease of revertant selection, lack of second-site revertants, and simple genetic requirements should make this assay useful in a number of circumstances. Because a given strain will reveal only one type of reversion event and because the background of spontaneous reversion is so low, it is possible to observe mutational events that occur at such low frequencies that they would rarely be revealed by sequencing of random events. This property makes it possible to study certain rare mutational events, even in the background of other types of events that are much more frequent. For example, given their frequency with UV damage, the mutational events occurring in the A149T strain would be expected to compose <5% of total UV mutational events, yet these events can be studied in isolation in the A149T strain. These events are interesting, for the sequence at the mutant base does not contain the usual dipyrimidine target of UV (Friedberg et al. 1995). One possible explanation for these reversion events is that they are due to error-prone replication by a translesion polymerase engaged to bypass a thymine photoproduct produced at two adjacent thymidine bases just 3′ of this target. Whether or not this explanation is correct, it is suggestive of the type of events that can be studied in such a reversion system that would be difficult to study in other contexts.
Acknowledgments
We are very grateful to Eric Radany for initial discussions concerning reversion assays and for his helpful suggestions. We are especially grateful to Ann Dershowitz and Carol Newlon for examining replication of the ARS306 in our strains and to Carol Newlon and Sue Jinks-Robertson for helpful comments on the manuscript. We are thankful for technical assistance from Sharon Haber. This work was supported by National Institutes of Health grants 5K12 GM000680 (T.-M.W.) and CA54050 (G.F.C.).
References
- Brachman, E. E., and E. B. Kmiec, 2003. Targeted nucleotide repair of cycl mutations in Saccharomyces cerevisiae directed by modified single-stranded DNA oligonucleotides. Genetics 163 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner, S. D., H. M. Nash, W. S. Lane and G. L. Verdine, 1998. Repair of oxidatively damaged guanine in Saccharomyces cerevisiae by an alternative pathway. Curr. Biol. 8 393–403. [DOI] [PubMed] [Google Scholar]
- Cupples, C. G., and J. H. Miller, 1989. A set of lacZ mutations in Escherichia coli that allow rapid detection of each of the six base substitutions. Proc. Natl. Acad. Sci. USA 86 5345–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande, A. M., and C. S. Newlon, 1992. The ARS consensus sequence is required for chromosomal origin function in Saccharomyces cerevisiae. Mol. Cell. Biol. 12 4305–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley, M. C., and G. F. Crouse, 1998. The role of mismatch repair in the prevention of base pair mutations in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 95 15487–15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg, E. C., G. C. Walker and W. Siede, 1995 DNA Repair and Mutagenesis. ASM Press, Washington, DC.
- Glaab, W. E., K. R. Tindall and T. R. Skopek, 1999. Specificity of mutations induced by methyl methanesulfonate in mismatch repair-deficient human cancer cell lines. Mutat. Res. 427 67–78. [DOI] [PubMed] [Google Scholar]
- Hampsey, M., 1991. A tester system for detecting each of the six base-pair substitutions in Saccharomyces cerevisiae by selecting for an essential cysteine in iso-1-cytochrome c. Genetics 128 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikov, V. V., I. L. Derkatch, V. N. Noskov, O. V. Tarunina, Y. O. Chernoff et al., 2001. Mutagenic specificity of the base analog 6-N-hydroxylaminopurine in the LYS2 gene of yeast Saccharomyces cerevisiae. Mutat. Res. 473 151–161. [DOI] [PubMed] [Google Scholar]
- Pavlov, Y. I., V. V. Suslov, P. V. Shcherbakova, T. A. Kunkel, A. Ono et al., 1996. Base analog N6-hydroxylaminopurine mutagenesis in Escherichia coli: genetic control and molecular specificity. Mutat. Res. 357 1–15. [DOI] [PubMed] [Google Scholar]
- Pavlov, Y. I., C. S. Newlon and T. A. Kunkel, 2002. Yeast origins establish a strand bias for replicational mutagenesis. Mol. Cell 10 207–213. [DOI] [PubMed] [Google Scholar]
- Poloumienko, A., A. Dershowitz, J. De and C. S. Newlon, 2001. Completion of replication map of Saccharomyces cerevisiae chromosome III. Mol. Biol. Cell 12 3317–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, A. D., M. Neishabury, D. H. Jones, S. H. Reed, S. Boiteux et al., 1999. Spontaneous mutation, oxidative DNA damage, and the roles of base and nucleotide excision repair in the yeast Saccharomyces cerevisiae. Yeast 15 205–218. [DOI] [PubMed] [Google Scholar]
- Shcherbakova, P. V., and Y. I. Pavlov, 1993. Mutagenic specificity of the base analog 6-N-hydroxylaminopurine in the URA3 gene of the yeast Saccharomyces cerevisiae. Mutagenesis 8 417–421. [DOI] [PubMed] [Google Scholar]
- Shcherbakova, P. V., V. N. Noskov, M. R. Pshenichnov and Y. I. Pavlov, 1996. Base analog 6-N-hydroxylaminopurine mutagenesis in the yeast Saccharomyces cerevisiae is controlled by replicative DNA polymerases. Mutat. Res. 369 33–44. [DOI] [PubMed] [Google Scholar]
- Storici, F., L. K. Lewis and M. A. Resnick, 2001. In vivo site-directed mutagenesis using oligonucleotides. Nat. Biotechnol. 19 773–776. [DOI] [PubMed] [Google Scholar]
- Theis, J. F., C. Yang, C. B. Schaefer and C. S. Newlon, 1999. DNA sequence and functional analysis of homologous ARS elements of Saccharomyces cerevisiae and S. carlsbergensis. Genetics 152 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, D., A. D. Scot, R. Barbey, M. Padula and S. Boiteux, 1997. Inactivation of OGG1 increases the incidence of G.C→T.A transversions in Saccharomyces cerevisiae: evidence for endogenous oxidative damage to DNA in eukaryotic cells. Mol. Gen. Genet. 254 171–178. [DOI] [PubMed] [Google Scholar]
- Thompson, J. D., D. G. Higgins and T. J. Gibson, 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, W., B. L. Chow, M. Hanna and P. W. Doetsch, 2001. Deletion of the MAG1 DNA glycosylase gene suppresses alkylation-induced killing and mutagenesis in yeast cells lacking AP endonucleases. Mutat. Res. 487 137–147. [DOI] [PubMed] [Google Scholar]
- Yutani, K., K. Ogasahara, T. Tsujita, K. Kanemoto, M. Matsumoto et al., 1987. Tryptophan synthase alpha subunit glutamic acid 49 is essential for activity. Studies with 19 mutants at position 49. J. Biol. Chem. 262 13429–13433. [PubMed] [Google Scholar]
- Zalkin, H., and C. Yanofsky, 1982. Yeast gene TRP5: structure, function, regulation. J. Biol. Chem. 257 1491–1500. [PubMed] [Google Scholar]
- Zhu, J., C. S. Newlon and J. A. Huberman, 1992. Localization of a DNA replication origin and termination zone on chromosome III of Saccharomyces cerevisiae. Mol. Cell. Biol. 12 4733–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]


