Abstract
We investigate mechanisms of balancing selection by extending two deterministic models of selection in a one-locus two-allele genetic system to allow for frequency-dependent fitnesses. Specifically we extend models of constant selection to allow for general frequency-dependent fitness functions for sex-dependent viabilities and multiplicative fertilities, while non-multiplicative mating-dependent components remain constant. We compute protected polymorphism conditions that take the form of harmonic means involving both the frequency- and the mating-dependent parameters. This allows for a direct comparison of the equilibrium properties of the frequency-dependent models with those of the constant models and for an analysis of equilibrium of the general model of constant fertility. We then apply the theory to analyze the maintenance of inversion polymorphisms in Drosophila subobscura and D. pseudoobscura, for which data on empirical fitness component estimates are available in the literature. Regression on fitness estimates obtained at different starting frequencies enables us to implement explicit fitness functions in the models and therefore to perform complete studies of equilibrium and stability for particular sets of data. The results point to frequency dependence of fitness components as the main mechanism responsible for the maintenance of the inversion polymorphisms considered, particularly in relation to heterosis, although we also discuss the contribution of other selection mechanisms.
BECAUSE selection is known to maintain genetic polymorphisms in natural populations, extensive theoretical and experimental research on mechanisms of balancing selection has been carried out from the first half of the last century (see, e.g., Dobzhansky 1970). Nevertheless the relative importance of these mechanisms in maintaining genetic variation remains an open question but, interestingly, theoretical approaches reveal that the extent to which balancing selection can be responsible for standing genetic variation may indeed depend on the specific balancing selection mechanism considered (see Turelli and Barton 2004 and references therein). Finding out which mechanisms of selection are actually affecting genetic polymorphisms in natural populations is therefore a central topic of population genetics. In this article we address this subject by developing and studying models involving, at the same time, several mechanisms susceptible to leading to balancing selection, such as stage-, sex-, frequency-, and mating-dependent fitnesses, hence enabling a subsequent analysis of the different selection mechanisms in maintaining genetic polymorphisms for which data on fitness component estimates are available.
Fertility differences as properties of the mating pairs can yield complex modes of selection, thus contributing to the maintenance of genetic polymorphisms. Equilibrium properties of models of one-locus, two-allele fertility selection were analytically investigated by assuming several particular cases. Penrose (1949) proved that additive fertility is analogous to the classical model of sex-independent viability. Bodmer (1965) obtained sufficient conditions for the maintenance of the polymorphism for the multiplicative case and found that multiplicative fertility is formally analogous to sex-dependent viability, also referred to as two-sex viability selection, which allowed him to apply previous results for sex-dependent viability to the multiplicative fertility model. Sex-dependent viability, first considered by Haldane (1924)(1926), was the first genetic system under selection found to have three simultaneous internal equilibria (Owen 1953). Further results concerning equilibrium and stability of these equivalent models were achieved by separately analyzing all possible patterns of selection for the two sexes (Mérat 1969; Mandel 1971; Kidwell et al. 1977).
The study of the model of one-locus fertility can be reduced without loss of generality to the case in which sex symmetry is assumed (Hadeler and Liberman 1975; Feldman et al. 1983). Hadeler and Liberman (1975) restricted their study to a particular case of the two-allele genetic system, with more symmetries in the fitness matrix, and they prove that up to five internal equilibria can exist. Feldman et al. (1983) were able to perform a complete study of equilibrium and stability by assuming further symmetries, considering in particular that the fitness of each mating pair depends only on the number of heterozygotes involved. For this case, the number of possible internal equilibria reduces to three, with up to two of them stable, and the parameter space divides into six regions with different equilibrium properties. The authors analyze the six cases in relation to analogous situations of the multiplicative-fertility model, although no equivalent multiplicative formulation is found for most of the cases. Several particular instances of fertility with dominance were also considered (Nagylaki 1992, pp. 112–116, and references therein).
Frequency-dependent selection is known to be an important balancing mechanism (see, e.g., Ayala and Campbell 1974; Clarke and Partridge 1988). Some equilibrium studies followed a phenomenological approach by focusing on situations involving explicit or more or less constrained types of fitness functions depending on gene or genotype frequencies (e.g., Wright 1955; Clarke and O'Donald 1964; Sacks 1967; Anderson 1969; Raveh and Ritte 1976; Anxolabéhèrre and Périquet 1981; Curtsinger 1984; Nagylaki 1992, pp. 19 and 20). In other cases variable selection appears as a consequence of situations in which constant parameters for specific mechanisms are initially considered. Perhaps the most general and best known of these situations is the pairwise-interaction model of intraspecific competition first considered by Schutz et al. (1968). Cockerham et al. (1972) performed a study of equilibrium of the general formulation of this model and inspected some particular cases. Mueller (1988) obtained conditions of protected polymorphism for a model with specific viability and fecundity fitness functions derived for competition for food. Asmussen and Basnayake (1990) highlight the potential of the frequency-dependent model of competition in the maintenance of variation. Variable selection caused by competition remains a focus of current research (see Bürger 2005 and references therein). Fertility selection is known to be equivalent to linear variable selection (Prout 1965, 1969, 1971; Feldman et al. 1969). Also constant parameters of multiple-niche selection (Levene 1953) and supergene selection (Alvarez and Zapata 1997) produce frequency-dependent total fitness values. Equilibrium studies for models including both density- and frequency-dependent selection have also been carried out (Nagylaki 1979; Slatkin 1979; Asmussen 1983a,b; Mueller 1988).
The importance of frequency-dependent selection as a balancing selection mechanism becomes more evident in models considering drift (Robertson 1962) and also when contextualized in the multiallele framework. Superiority of the heterozygote is neither necessary (Kimura 1956) nor sufficient (Mandel 1959) for the maintenance of more than two alleles in a population, and constant viability selection itself does not seem to be very likely to explain multiple-allele polymorphisms commonly found in natural populations (Lewontin et al. 1978; Mandel 1980; Karlin 1981). Although fertility selection is more efficient than viability selection in maintaining two-allele genetic polymorphisms, numerical analyses show that this result does not extend to the multiallele framework (Clark and Feldman 1986). Rare-genotype frequency-dependent selection on the other hand can lead to maintenance of multiallele polymorphisms (see, e.g., Anderson 1969). Our present study is, however, restricted to the one-locus, two-allele framework.
After their discovery, Drosophila inversion polymorphisms attracted scientific attention as likely neutral markers (see, e.g., Powell 1997, p. 73), but their frequencies were found to be subjected to strong selective forces (Dobzhansky 1943, 1948; Dubinin and Tiniakov 1946; Dobzhansky et al. 1966; Rodríguez-Trelles et al. 1996), and, moreover, epistasis among the loci involved was found to be an essential factor for new inversions to rise in frequency in natural populations (Charlesworth and Charlesworth 1973). Coadaptation of inversions within subpopulations first pointed to superiority of the heterokaryotype as a simple and reasonable explanation of the maintenance of polymorphism (see Dobzhansky 1970). However, several chromosomal arrangements coexist in Drosophila natural populations (see Sperlich and Pfriem 1986; Krimbas and Powell 1992; Powell 1997), whereas superiority of the heterozygote (or equivalently heterokaryotype) is not likely to explain the maintenance of multiple-allele genetic systems (see above). In addition, the absence of excess of heterokaryotypes at equilibrium, expected under heterokaryotype superiority, suggested that important selection forces act at different stages of the life cycle (Sperlich and Pfriem 1986). Different researchers carried out experimental work on other selective mechanisms such as sex-dependent viability (Druger 1966; Druger and Nickerson 1972), frequency-dependent viability (Kojima and Tobari 1969; Anderson et al. 1986), and frequency-dependent fertility (Anderson and Watanabe 1974) and more particularly rare-male advantage (e.g., Petit and Ehrman 1969; Anderson and Brown 1984; Santos et al. 1986; Salceda and Anderson 1988), multiple-niche selection (e.g., Fernández Iriarte and Hasson 2000), and supergene selection or the effect of recombination in homokaryotypes (Charlesworth and Charlesworth 1975; Wasserman and Koepfer 1975). The explanation of the maintenance of inversion polymorphisms in Drosophila was also attempted by using a demographic approach (Anderson and Watanabe 1997). Frequencies of Drosophila inversion polymorphisms have been carefully measured in nature for decades (Anderson et al. 1991; Sole et al. 2002) and molecular data have recently been used to study their role in adaptation (Hoffmann et al. 2004 and references therein) and speciation (e.g., Noor et al. 2001).
In the first part of this article we develop and study two deterministic models of general variable selection by following a phenomenological approach that can involve different fitness functions for the early and late stages, without a priori restricting the recurrence equations to particular types of functions. These models are extensions of the selection models of Bodmer (1965) and Prout (1969) to allow for variable selection. In the next section, after some brief technical comments, we apply the first model to study mechanisms of balancing selection in two Drosophila inversion polymorphisms for which data on fitness component estimates are available in the literature. Finally we discuss the implications of both the theoretical results and the analysis of the inversion polymorphisms. The theoretical framework considered in this article, however, is not at all restricted to the inversion polymorphism scenario.
MODELS AND THEORETICAL RESULTS
Model of early and late variable selection:
Here we consider a model with frequency-dependent viabilities and sex- and frequency-dependent multiplicative fertilities ruling the frequencies, X, Y, and Z, of the three genotypes, A1A1, A1A2, and A2A2, of a one-locus two-allele genetic system. Mating is at random and generations do not overlap. The notation for this model is set forth in Table 1. The female and male fertility fitness functions for the three possible genotypes, fi(X, Z) and mi(X, Z), i = 1, 2, 3, depend directly on independent frequencies of the homozygous genotypes, X and Z, in the adults, and therefore they also depend implicitly on the heterozygous frequency Y = 1 − X − Z. The scope of the model includes almost any kind of fertility fitness functions (with only a minor restriction, which is mentioned below). This framework automatically encompasses variable fitness functions depending on the gene frequencies p = X + 1/2Y and q = Z + 1/2Y since they depend indirectly on the genotype frequencies. The same logic holds for the sex-independent viability fitness functions, vi(Xzy, Zzy), i = 1, 2, 3, except for the fact that they depend in a natural way on zygote frequencies (denoted by the subscript zy) instead of those of the adults. To take this fact into account when building the recurrence equations of the model, we need to consider the expressions providing the zygote frequencies of the three genotypes at one generation, X′zy, Y′zy, and Z′zy, from the adult frequencies at the previous generation, X, Y, and Z (where the primes denote “one generation after”). These expressions are
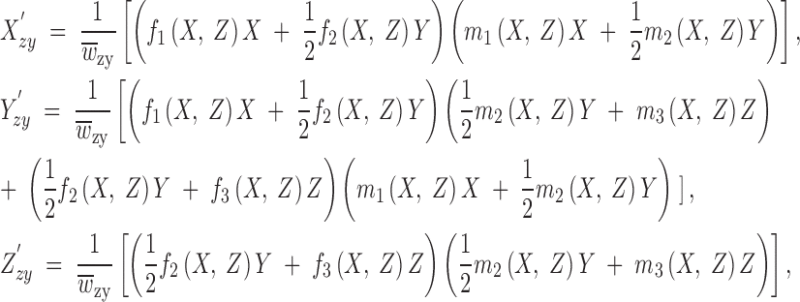 |
1 |
where  such that the frequencies X′zy, Y′zy, and Z′zy add to unity. Now we are in a position to express the recurrence equations of the model as
such that the frequencies X′zy, Y′zy, and Z′zy add to unity. Now we are in a position to express the recurrence equations of the model as
 |
2 |
where the zygote frequencies X′zy and Z′zy are given by (1), and  is such that X′ + Y′ + Z′ = 1. Note that in these recurrences the frequency-dependent viability functions v1X′zy, Z′zy, v2X′zy, Z′zy, and v3X′zy, Z′zy depend themselves on the frequency-dependent multiplicative fertilities by means of (1).
is such that X′ + Y′ + Z′ = 1. Note that in these recurrences the frequency-dependent viability functions v1X′zy, Z′zy, v2X′zy, Z′zy, and v3X′zy, Z′zy depend themselves on the frequency-dependent multiplicative fertilities by means of (1).
TABLE 1 .
Variables and fitnesses of the frequency-dependent multiplicative-fertility model for the three genotypes
| A1A1 | A1A2 | A2A2 | |
|---|---|---|---|
| Adult frequencies at generation t | X | Y | Z |
| Female fertilities | f1(X, Z) | f2(X, Z) | f3(X, Z) |
| Male fertilities | m1(X, Z) | m2(X, Z) | m3(X, Z) |
| Zygote frequencies at generation t + 1 | X′zy | Y′zy | Z′zy |
| Viabilities | v1X′zy, Z′zy | v2X′zy, Z′zy | v3X′zy, Z′zy |
| Adult frequencies at generation t + 1 | X′ | Y′ | Z′ |
Since Y = 1 − X − Z, (2) may be expressed in terms of the frequencies X and Z alone, and we can refer to them as X′(X, Z), Y′(X, Z), and Z′(X, Z). These expressions entail an extension of the constant-multiplicative fertility selection model analyzed by Bodmer (1965) to the general variable-fitness framework. Even for the constant-fitness multiplicative-fertility model it is not possible to obtain a general expression for the equilibrium points (Bodmer 1965; Mérat 1969; Mandel 1971; Kidwell et al. 1977). Therefore, to gain some insight into the equilibrium properties of our model of variable selection, we focused on the analysis of the stability of the trivial equilibria, (X, Z) = (1, 0) and (X, Z) = (0, 1). To this end we must assume that the fitness functions are differentiable at the fixation points. In regard to generality this is the only restriction we impose on the frequency-dependent fitness functions to be considered. The capacity of some computer packages to deal with abstract expressions (e.g., Mathematica, Wolfram 1996) allows us to build the Jacobian matrix for recurrence equations (2)—the matrix of the partial derivatives of the independent functions X′(X, Z) and Z′(X, Z). Since these recurrence equations involve nonspecified viability functions depending on nonspecified fertility functions, the Jacobian matrix is cumbersome, but it becomes much more treatable when evaluated at the trivial equilibria. The nonzero eigenvalues of the two resulting matrices at the two trivial equilibria are
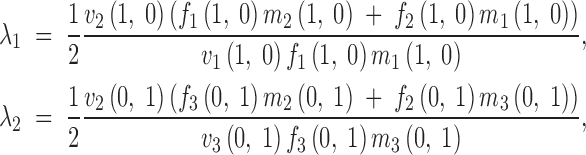 |
3 |
for the fixation points of A1 and A2, respectively.
The protected polymorphism conditions are now given by the inequalities that the absolute values of these eigenvalues are larger than one (Prout 1968). For fitness values fulfilling these conditions, the maintenance of the polymorphism in the genetic system is guaranteed. Assuming, without loss of generality—other than that the heterozygotes are not lethal at any frequency—that every heterozygous fitness component equals one in (3), the protected polymorphism conditions can be expressed in terms of harmonic means of products of fitnesses affecting females and males as
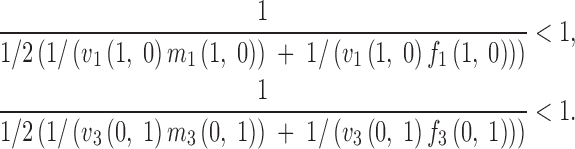 |
4 |
These expressions are influenced by the fitness functions only by means of the values of these functions at the fixation point of the genotype they affect, which allows for direct biological interpretation. Indeed, these values play the same role as the constant fitnesses do under protected polymorphism conditions of the constant multiplicative fertility model (Bodmer 1965; Kidwell et al. 1977). Hence for every possible fitness function, we can consider the value it takes at the fixation point of the genotype it affects. This leads to a parameter space for the variable model in which the parameter values are equivalent to the fitnesses of the constant case, and thus the regions of protected polymorphism are the same in both situations. In the regions of no protected polymorphism the variable selection can nevertheless lead to internal equilibria in more cases than the constant models because of the flexibility of the fitness functions to allow for complex situations.
From inequalities (4) it is straightforward to derive protected polymorphism conditions for particular models in which specific fitness functions are involved. One of the cases with real fitness estimates analyzed in the next section of this article involves constant viabilities, constant female fecundities, and gene-frequency-dependent linear and quadratic fitness functions for male sexual selection, m1(X, Z) = m1(p) = a − bp for the genotype A1A1 and m3(X, Z) = m3(p) = d + eq + gq2 for A2A2. By just substituting these assumptions in (4), the protected polymorphism conditions for this particular case are
 |
5 |
Since multiplicative fertility and two-sex viability are formally analogous selective forces (Bodmer 1965; Kidwell et al. 1977), recurrences and inequalities given in (2) and (4) can be applied to a frequency-dependent two-sex viability framework. In this different context X, Y, and Z are zygote frequencies, fi(X, Z) and mi(X, Z), i = 1, 2, 3 are female and male viability fitness functions, and the former sex-independent viabilities vi(Xzy, Zzy), i = 1, 2, 3, must be just ignored. We nonetheless directly consider sex- and frequency-dependent viabilities in a more general model below. The protected polymorphism conditions for the simplest case of sex-independent viability variable selection can be obtained as v1(1, 0) < 1, v3(0, 1) < 1, by just ignoring the sex-dependent components fi(X, Z) and mi(X, Z), i = 1, 3, in (4). This case was already addressed by Asmussen and Basnayake (1990), and we resume it in the next section of this article.
Model of variable selection and mating-interaction effects:
Now we analyze a more general model with sex-, frequency-, and mating-dependent selection. We consider both viabilities and multiplicative fertilities to be sex and frequency dependent and also include the contribution of constant mating-dependent effects—nonmultiplicative fertility and nonrandom mating. This model can be considered as the extension of the general model of selection described by Prout (1969) to a variable-fitness framework insofar as the mating-independent parameters are concerned. The notation for these parameters is very similar to the one set forth in Table 1, although setting new genotype labels (see Table 2) allows simplification of the formulas. The mating-interaction matrix A = (aij), on the other hand, gathers together the (nonvariable) parameters accounting for nonmultiplicative fertility of the mating pairs and nonrandom mating. Each parameter in this matrix is the product of these two effects on one particular mating pair. Assuming nonoverlapping generations the three recurrence equations describing the changes in zygote frequencies over generations are, for l = 1, 2, 3,
 |
6 |
where Gzy1 = Xzy, Gzy2 = Yzy, and Gzy3 = Zzy are the zygote frequencies at generation t. The primes denote generation t + 1, and the subscripts f and m denote female and male adults (in contrast to zy, zygote).  , l = 1, 2, 3, with
, l = 1, 2, 3, with 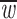 f such that
f such that 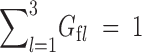 , provides the female adult frequencies at generation t (for the multiplicative fertilities to depend on) and analogous expressions hold for males. K = (kijl) is the so-called Mendelian operator, given by
, provides the female adult frequencies at generation t (for the multiplicative fertilities to depend on) and analogous expressions hold for males. K = (kijl) is the so-called Mendelian operator, given by
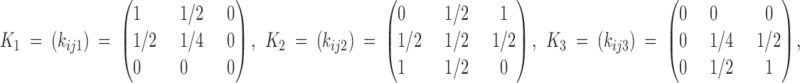 |
and 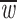 is such that
is such that  .
.
TABLE 2 .
Variables and fitnesses of the frequency-dependent and mating-interaction model for the three genotypes
| A1A1 | A1A2 | A2A2 | |
|---|---|---|---|
| Zygote frequencies at generation t | Xzy = Gzy1 | Yzy = Gzy2 | Zzy = Gzy3 |
| Female viabilities | vf1(Gzy1, Gzy3) | vf2(Gzy1, Gzy3) | vf3(Gzy1, Gzy3) |
| Male viabilities | vm1(Gzy1, Gzy3) | vm2(Gzy1, Gzy3) | vm3(Gzy1, Gzy3) |
| Adult female frequencies at generation t | Xf = Gf1 | Yf = Gf2 | Zf = Gf3 |
| Adult male frequencies at generation t | Xm = Gm1 | Ym = Gm2 | Zm = Gm3 |
| Female multiplicative fertilities | f1(Gf1, Gf3) | f2(Gf1, Gf3) | f3(Gf1, Gf3) |
| Male multiplicative fertilities | m1(Gm1, Gm3) | m2(Gm1, Gm3) | m3(Gm1, Gm3) |
| Mating-interaction parameters | |||
| Males |
|||
| Females |
A1A1 |
A1A2 |
A2A2 |
| A1A1 | a11 | a12 | a13 |
| A1A2 | a21 | a22 | a23 |
| A2A2 | a31 | a32 | a33 |
| Zygote frequencies at generation t + 1 | 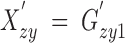 |
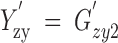 |
 |
We assume, like we did in the previous model, that the fitness functions are differentiable at the fixation points. This enables us to perform a study of stability of the fixation points for recurrence equations (6) by means of the Jacobian matrix and therefore to obtain sufficient conditions for the maintenance of the genetic polymorphism in the genetic system. Letting vf2(Gzy1, Gzy3) = vm2(Gzy1, Gzy3) = f2(Gf1, Gf3) = m2(Gm1, Gm3) = 1, these protected polymorphism conditions can be drawn in terms of harmonic means as
 |
7 |
The protected polymorphism conditions of the model analyzed in the previous section [inequalities (4)] may be considered as a particular case of these expressions, in spite of the fact that the two models focus on different stages of the life cycle. As in the previous model, the fitness functions now affect the protected polymorphism conditions (7) only by means of the values they take at the fixation point of the genotype they affect. Likewise, not every mating pair's fertility parameter plays a role in these inequalities. The fertility factor affecting one sex at one fixation point is in particular the fertility parameter of the only mating pair that is present at this fixation point, relative to the fertility of the mating pair that differs from the previous one in one allele substitution made in the sex in question.
Within inequalities (7) we also provide the protected polymorphism conditions for the constant fitness case considered by Prout (1969)—in the context of the estimation of fitness component values—in which constant fitness values substitute the fitness functions. More particularly, the general model of constant fertility is another interesting case we can analyze from our model of variable selection. This case considers the effect of the matrix A alone and, if random mating is assumed, the parameters aij account for only fertility. From inequalities (7), the protected polymorphism conditions for the general model of constant fertility turn out to be simply
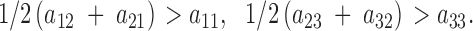 |
8 |
It is known that only the means of the reciprocal matings, like the ones in the left-hand sides of these expressions, are important to describe the dynamics of the model, and therefore the study of equilibrium reduces to assuming that the matrix A is symmetric (Hadeler and Liberman 1975; Feldman et al. 1983). Thus, assuming sex symmetry, as this case is biologically referred to, the number of variables of the parameter space reduces from nine to six and inequalities (8) may be written as
 |
9 |
These expressions are satisfied by one-quarter of the parameter space. Hadeler and Liberman (1975) obtained the condition for the instability of the fixation points for the four parameters in the particular case in which a11 = a33 and a12 = a23. Given their assumptions this condition can be obtained from inequalities (9) as only the first of them, since both become equivalent. Thus the protected polymorphism now covers one-half of the parameter space. Feldman et al. (1983) were able to perform a complete study of equilibrium by further assuming that a11 = a13. In this case the parameter space reduces to three dimensions, the protected polymorphism conditions are still the same as in the previous case, and they still cover one-half of the parameter space. The authors describe the equilibrium and stability properties of this model by dividing the parameter space into six equally probable situations, one of which shows one internal stable equilibrium without fulfilling the protected polymorphism conditions. For this case, thus, there is no protected polymorphism in one-quarter of the situations in which internal stable equilibrium points exist.
ANALYSIS OF VARIABLE SELECTION PATTERNS
The OST and O3+4+7 arrangements of Drosophila subobscura and ST and CH arrangements of the third chromosome of D. pseudoobscura were extensively studied as models to analyze the maintenance of genetic polymorphisms by natural selection. Table 3 summarizes the fitness estimates we obtained from the literature to analyze patterns of frequency-dependent selection in both species. Since these data include neither sex-dependent viabilities nor mating-interaction parameters, the early and late variable selection model we first considered in this article is adequate for the analysis. Its recurrence equations (2) on adult frequencies allow us to focus directly on frequency-dependent fertilities—also depending on adult frequencies, which are known in both species. The notation in Table 1 holds in this analysis except for the labels of the arrangements that now replace the A1 and A2 alleles.
TABLE 3 .
Fitness components of karyotypes ofDrosophila subobscura andD. pseudoobscura for different starting frequencies,p, of the “standard” arrangement (OST andST, respectively)
|
D. subobscura |
D. pseudoobscura |
||||||
|---|---|---|---|---|---|---|---|
| Fitness component | p | OST/OST | OST/O3+4+7 | O3+4+7/O3+4+7 | ST/ST | ST/CH | CH/CH |
| Viability | 0.125 | 1 | 0.71 ± 0.09c | ||||
| 0.25 | 1.33 ± 0.30 | 1 | 0.81 ± 0.09 | ||||
| 0.375 | 1 | 1.01 ± 0.13 | |||||
| 0.50 | 1.10 ± 0.03 | 1 | 0.95 ± 0.03a | 1.58 ± 0.15 | 1 | 0.96 ± 0.10 | |
| 0.625 | 1.14 ± 0.16 | 1 | |||||
| 0.75 | 0.83 ± 0.10 | 1 | 0.75 ± 0.17 | ||||
| 0.875 | 0.78 ± 0.11 | 1 | |||||
| Female fecundity | 0.50 | 0.96 ± 0.08 | 1 | 0.90 ± 0.06a | 1.11 ± 0.06 | 1 | 0.98 ± 0.06d |
| Male sexual selection | 0.05 | 1 | 0.73 ± 0.12e | ||||
| 0.30 | 1.27 ± 0.18 | 1 | 0.92 ± 0.02b | 3.69 ± 0.55 | 1 | 0.21 ± 0.05 | |
| 0.50 | 1.02 ± 0.05 | 1 | 0.86 ± 0.08 | 1.57 ± 0.20 | 1 | 0.26 ± 0.06 | |
| 0.70 | 0.74 ± 0.13 | 1 | 1.11 ± 0.04 | 0.51 ± 0.07 | 1 | 0.88 ± 0.19 | |
| 0.95 | 2.57 ± 0.93 | 1 | |||||
To find out what patterns of selection best fit the raw frequency-dependent fitness estimates in Table 3, we performed regressions of the data on fitness functions dependent on gene or genotype frequencies as considered in the literature (Anderson 1969; Anxolabéhère and Périquet 1981; Nagylaki 1992, pp. 19 and 20). Specifically we regressed the homokaryotype fitnesses on linear, quadratic, and hyperbolic functions. All of these functions are linear in the parameters but have different numbers of parameters to estimate, and therefore we used the adjusted coefficient of determination (shown in Table 4) to compare the fit to the data (Sokal and Rohlf 1995, p. 654). Since the existence of replicates in D. subobscura sexual selection estimates (see Figures 1 and 2) allows us to test the fit of the functions by means of the lack of fit test, we also performed regressions on a nonlinear hyperbola considered by Anxolabéhère (1980) but it did not improve the fit to the data (results not shown).
TABLE 4 .
Adjusted coefficient of determination for different regressions on the frequency-dependent fitness estimates ofDrosophila subobscura andD. pseudoobscura summarized in Table 3
| Regression |
D. subobscura |
D. pseudoobscura |
||||
|---|---|---|---|---|---|---|
| Fitness component | Variables | Function | OST/OST | O3+4+7/O3+4+7 | ST/ST | CH/CH |
| Viability | Gene frequencies | Linear | — | — | 0.50 | Negative |
| Quadratic | — | — | 0.59 | 0.82 | ||
| Sexual selection | Gene frequencies | Linear | 0.66 | 0.29 | Negative | Negative |
| Quadratic | 0.55 | 0.71 | 0.94 | 0.99 | ||
| Genotype frequencies | Linear | 0.65 | 0.17 | Negative | Negative | |
| Hyperbolic | 0.55 | 0.59 | Negative | Negative | ||
Coefficients of determination for the regressions we have chosen for the study of equilibrium are shown in italics.
Figure 1.—
Sexual selection estimates of the Drosophila subobscura homokaryotypes relative to the heterokaryotype (Santos et al. 1986) and regressions on gene frequencies that best fit the data in Table 3. The fitness functions are m1(X, Z) = 1.67 − 1.31p and m3(X, Z) = 2.06 − 4.33q + 3.86q2, where p and q are the adult gene frequencies of OST and O3+4+7. The functions m1 and m3 depend indeed on X and Z, by substituting q = 1 − p, and p = X + (1 − X − Z).
Figure 2.—
Sexual selection estimates of the Drosophila subobscura homokaryotypes relative to the heterokaryotype (Santos et al. 1986) and regressions on genotype frequencies that best fit the data in Table 3. The fitness functions are m1(X, Z) = 1.37 − 1.30X and m3(X, Z) = 0.82 + (0.03/Z).
Once we assume the action of explicit fitness functions, inequalities (4) enable us to check if the protected polymorphism conditions of the system are fulfilled or not. To determine the weight that the different fitness components have in the maintenance of the polymorphism, we first apply the expressions to the separate action of the variable components and then to the model including every available fitness component. Because the protected polymorphism conditions are not necessary conditions for the maintenance of polymorphisms, and the stability of one or both fixation points does not completely impede the maintenance of the polymorphism, we carried out further analyses to search for internal stable equilibrium points. For the variable viability selection component with all fitnesses relative to the one of the heterozygote, the equilibrium points are given by the solutions of
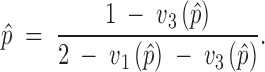 |
10 |
The stability of the equilibria can be inspected through the computation of the eigenvalue of the system—one equilibrium point is stable when the absolute value of the eigenvalue is smaller than one (e.g., Roughgarden 1979, p. 576)—which for this case is
 |
11 |
or equivalently by using the method described by Lewontin (1958). Such an analysis is presented in Asmussen and Basnayake (1990), where expressions for the stability of the internal equilibria and the fixation points are provided.
Since the multiplicative fertility model is formally analogous to the two-sex viability model (Bodmer 1965; Kidwell et al. 1977), and the one-sex viability selection model is known to have the same behavior as the simple viability model in terms of equilibrium (Cannings 1969), expressions (10) and (11) hold for sexual selection fitness functions by just substituting m1(p) and m3(p) for v1(p) and v3(p). When considering the joint effect of several fitness components, the internal equilibria can be computed numerically for particular cases by deterministic simulations that iteratively apply the recurrence equations (2), implemented with the specific fitness functions and constant parameters, from different starting points. To this end we have written specific programs in Mathematica (Wolfram 1996).
Drosophila subobscura:
As shown in Table 3, viability and female fecundity were estimated in this species for only one starting frequency of the OST and O3+4+7 chromosomal arrangements (Tarrío 1993), whereas male sexual selection was estimated for three starting frequencies and was found to be frequency dependent (Santos et al. 1986). Since no sexual selection estimates are available at frequencies close to the fixation points we analyzed two different patterns of variable selection–on gene and genotype frequencies. Both fit the data equally well (see coefficients of determination in Table 4) but show a different behavior at frequencies close to fixation.
Figure 1 shows the regressions on gene frequencies—on the frequencies of the arrangements—that best fit both homokaryotype fitness estimates. A linear regression fits the estimates well for OST, whereas a quadratic regression improves the fit for O3+4+7 by means of the adjusted coefficient of determination (Table 4), which penalizes the estimation of one extra parameter. Since there are only three starting frequencies, the quadratic regression is just the polynomial interpolation on the three means of the replicates of the fitness estimates. These means are the estimates given in Table 3, but it is the existence of the replicates shown in Figures 1 and 2—on which we actually performed the regressions—that allows us to compute the coefficient of determination for this case.
Figure 2 shows the regressions on genotype frequencies—the frequencies of the karyotypes—that best fit the data. A linear regression shows a good fit for OST (see Table 4 and Figure 2). Fitness estimates for O3+4+7 on the other hand fit best with the constraints of the hyperbolic function. This function is an equilateral hyperbola of the type m(Z) = a + b(1/Z), fulfilling that the axis of ordinates is one of the asymptotes, and hence (assuming a positive value for b) it decreases for increasing frequencies and has a lower limit of a + b in [0, 1] at frequency one, which gives some biological meaning to the regression parameters. The smaller the value of b the more pronounced the curvature of the graph is. Since the function necessarily goes to infinity when the homokaryotype frequency goes to zero, and it does not fulfill the constriction that it is differentiable at this fixation point, we actually have to consider a slightly different function that takes constant values in a small neighborhood of zero instead—say [0, 10−4].
The results of the study of equilibrium are summarized in Table 5. We focus first on the regressions on gene frequencies (Figure 1), and therefore inequalities (5) describe the protected polymorphism of the genetic system. When considering frequency-dependent sexual selection alone, O3+4+7 is a stable fixation point that can thus be achieved by iteration of the recurrence equations from polymorphic situations, whereas OST is unstable. By means of (10) and (11) we have found one stable and one unstable internal equilibrium points, with the unstable one being relatively close to the fixation point of O3+4+7. The interval between this unstable equilibrium and the fixation point is the set of starting frequencies from which the polymorphism would be lost. The same qualitative results hold when including the constant fitness estimates in the complete model, the only difference being slight changes in the values for the equilibrium points. Neither superiority of the heterokaryotype in fecundity nor directional selection favoring OST in viability is strong enough to switch the O3+4+7 fixation point to unstable for the regression on the gene-frequencies pattern. Nevertheless they reduce the percentage of starting frequencies leading to fixation to <10%, by bringing the unstable internal equilibrium closer to the fixation of O3+4+7.
TABLE 5 .
Equilibria and stability predicted by the different patterns of variable selection overDrosophila subobscura andD. pseudoobscuraon gene and genotype frequencies considered for the study (see Figures 1–4 and Table 4) and by the model including all fitness component estimates from Table 3 for both species
|
Drosophila subobscura |
Drosophila pseudoobscura |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fixation points |
Internal p̂ |
Fixation points |
Internal p̂ |
||||||
| Fitness component | Regression variables | OST | O3+4+7 | Stable | Unstable | ST | CH | Stable | Unstable |
| Viability | Gene frequencies | — | — | — | — | Unstable | Unstable | 0.785 | None |
| Sexual selection | Gene frequencies | Unstable | Stable | 0.565 | 0.183 | Stable | Unstable | 0.621 | 0.798 |
| Genotype frequencies | Unstable | Unstable | 0.562 | None | — | — | — | — | |
| All | Gene frequencies | Unstable | Stable | 0.668 | 0.082 | Unstable | Unstable | 0.799 | None |
| Genotype frequencies | Unstable | Unstable | 0.667 | None | — | — | — | — | |
We now focus on regressions on genotype frequencies (Figure 2). Protected polymorphism conditions given in inequalities (4) are satisfied when considering frequency-dependent sexual selection alone. The stable equilibrium for this pattern differs only in thousandths from the one we obtained when considering gene-frequency-dependent sexual selection alone (see Table 5). Superiority of the heterokaryotype in female fecundity reinforces the maintenance of the polymorphism if we consider both late components at the same time. When further including directional viability favoring OST the protected polymorphism still holds, and the internal stable equilibrium is again virtually the same as when considering the complete model with the gene-frequency-dependent pattern (Table 5). In general the two patterns behave almost identically at intermediate frequencies, where the regressions are strongly conditioned by the data, and differently close to the fixation points, where there are no available fitness estimates. Both regression patterns explain well the maintenance of the polymorphism when considered in the complete model, and for the genotype-frequency pattern, moreover, protected polymorphism exists, which more strongly prevents the loss of variability.
For both regression patterns, when considering frequency-dependent sexual selection alone, the internal stable equilibrium points display heterokaryotype superiority. (For the gene-frequencies pattern this can be inferred by just looking at Figure 1.) However, this is not the case at the internal stable equilibrium point of the complete model. The female total fitnesses of OST/OST and O3+4+7/O3+4+7 homokaryotypes, relative to the heterokaryotype, are respectively (for all frequencies) 1.06 and 0.86, thus showing directional selection favoring OST. We have computed the fitnesses for the same genotypes of males according to the gene-frequencies pattern at p̂ = 0.668 (Table 5) as respectively 0.87 and 0.99. This can be considered as directional selection with complete dominance of the favored karyotype, O3+4+7, or more precisely heterokaryotype superiority very biased toward the fixation of O3+4+7. According now to the genotype-frequencies pattern, at p̂ = 0.667 (Table 5) we found directional selection favoring O3+4+7 in males with homokaryotype fitnesses of 0.87 and 1.04. So for both patterns there is rather strong selection opposite in sexes at the internal stable equilibrium point of the complete model.
Drosophila pseudoobscura:
The right-hand side of Table 3 shows fitness estimates for the ST and CH inversion polymorphism of the third chromosome of D. pseudoobscura. Female fecundity was studied assuming constant fitnesses (Moos 1955) and both viability (Anderson et al. 1986) and male sexual selection (Anderson and Brown 1984) are known to be frequency dependent. To handle these data we actually exploit the fact that our model enables us to simultaneously consider early and late variable selection. Although the viability estimates of D. pseudoobscura were obtained in experiments mostly performed with only two karyotypes competing, we pooled data with the same starting frequencies together and computed standard errors by using delta methods (Weir 1990) when necessary.
Quadratic gene-frequency regressions show good fit to the viability estimates (Figure 3; see coefficients of determination in Table 4). Since these experiments were not performed at Hardy-Weinberg starting frequencies, no regressions on genotype frequencies can be performed on these data. The best regressions on gene frequencies for the sexual selection estimates are also quadratic on gene frequencies for both homokaryotypes (Figure 4), and the negative coefficients of determination show that the other regressions fit poorly.
Figure 3.—
Viability estimates of the Drosophila pseudoobscura homokaryotypes relative to the heterokaryotype (Anderson et al. 1986) and regressions that best fit the data in Table 3. The fitness functions are 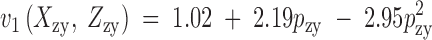 and
and 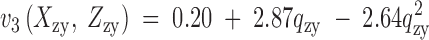 , where pzy and qzy are the zygotic gene frequencies of ST and CH. The functions v1 and v3 may be expressed directly in terms of the frequencies of the genotypes in an analogous way to m1 and m3 in Figure 1.
, where pzy and qzy are the zygotic gene frequencies of ST and CH. The functions v1 and v3 may be expressed directly in terms of the frequencies of the genotypes in an analogous way to m1 and m3 in Figure 1.
Figure 4.—
Sexual selection estimates of the Drosophila pseudoobscura homokaryotypes relative to the heterokaryotype (Anderson and Brown 1984) and regressions that best fit the data in Table 3. The fitness functions are m1(X, Z) = 10.84 − 30.38p + 22.76p2 and m3(X, Z) = 2.65 − 7.74q + 6.03q2, where p and q are as in Figure 1.
As shown in Table 5 together with the rest of the results of the study of equilibrium, viability fitness functions (Figure 3) fulfill the protected polymorphism conditions given in inequalities (4). Anderson et al. (1986) previously inferred a protected polymorphism in their discussion of these data on variable viabilities. By means of (10) and (11) we have found only one internal stable equilibrium point. Regarding now sexual selection fitness functions (Figure 4), they also lead to one internal stable equilibrium point, although they do not prevent the fixation of ST. The location of one unstable internal equilibrium point at (roughly) p = 0.8 reveals that sexual selection would allow the loss of CH arrangement when its frequency is <0.2. Since fecundity selection directionally favors ST, it reinforces both the instability of CH and the stability of ST. Considering, last, the joint effect of all the fitness estimates in the complete model, inequalities (4) hold and hence there is a protected polymorphism, preventing the system from the loss of variability. By applying the recurrence equations we have found only one internal equilibrium point, which is stable, so that we get to the same qualitative equilibrium results as when considering viability alone. Thus our frequency-dependent model applied to the fitness component estimates explains maintenance of the ST/CH polymorphism in D. pseudoobscura by means of protected polymorphism.
As was also the case for D. subobscura, the internal stable equilibrium point when considering sexual selection alone displays heterokaryotype superiority. The same result holds for frequency-dependent viability. However, at the internal stable equilibrium point of the complete model, p̂ = 0.799 (Table 5), the total fitnesses of the homokaryotypes ST/ST and CH/CH relative to the heterokaryotype are 1.22 and 1.31 for females and 0.97 and 0.90 for males. There is, thus, heterokaryotype disadvantage with an unstable internal equilibrium point at p̂f = 0.585 in females and heterokaryotype superiority with a stable internal equilibrium point at p̂m = 0.769 in males. At p̂ = 0.799, therefore, selection acts in favor of ST (toward p = 1) in females and against ST (toward p = 0.769) in males.
DISCUSSION
Theoretical results:
Protected polymorphism conditions, as sufficient conditions for the maintenance of polymorphisms, provide information about whether variability can be lost in a genetic system or not. When protected polymorphism conditions are fulfilled, there is no starting polymorphic frequency from which deterministic loss of variability can occur. This prevents the fixation of alleles after an occasional switch in frequency caused by, for instance, temporary drift effects such as bottlenecks—as long as they do not cause the fixation of one allele themselves. When a population is at a stable internal equilibrium but the protected polymorphism conditions do not hold, such occasional phenomena could eventually bring the system to a point from which selection alone can lead to the fixation of one allele.
Protected polymorphism conditions constitute an ideal tool to inspect the maintenance of polymorphism in genetic systems in which frequency-dependent parameters need to be considered. The main advantage of this approach is that these conditions involve only the values that the variable parameters take at the fixation points. This holds when considering general fitness functions that are different for the two sexes and also for the early and late stages, which causes the input of some fitness functions to depend on the output of the others. We highlight two main convenient consequences of this fact. First, it allows for notable simplicity and biological interpretation of the formulas [see inequalities (4)]. For the variable models we consider in this article, the protected polymorphism conditions take the same form of harmonic means of the selective parameters as in models with constant selection, enabling a direct comparison of the parameter spaces of the variable and constant models. Second, it demonstrates that, for each variable fitness component, only estimates obtained for two genotypes competing in the vicinities of the fixation points are necessary to inspect the protected polymorphism conditions of the variable genetic systems, independent of the pattern of frequency dependence of the variable parameters. Protected polymorphism conditions can still be obtained when further including fertility as a general mating-interaction selection force and nonrandom mating in the variable models [inequalities (7–9)]. Similar to variable selection, not every mating pair's fitness is involved in these expressions. The results for the constant fertility model are interesting in themselves because the equilibrium studies for this model were previously performed for particular cases (see Introduction).
However, it is noteworthy that protected polymorphism conditions are limited in three aspects. First, since they are not necessary conditions for the maintenance of the polymorphism, internal stable equilibria can still occur when the inequalities are not fulfilled. Second, they do not indicate the existence of multiple internal equilibria. Finally, although protected polymorphism conditions are sufficient conditions for the maintenance of the polymorphisms, they do not completely guarantee the existence of internal stable equilibrium points, because awkward instances can lead to limit cycles instead (Nagylaki 1992, p. 65).
Drosophila inversion polymorphism:
Our frequency-dependent models enable us to accomplish an analysis of the maintenance of Drosophila inversion polymorphisms, for which data on several fitness component estimates were found to vary for different starting frequencies (see Table 3). A complete study of equilibrium is possible since regressing on the data allows us to obtain explicit fitness functions to enter into the model (Figures 1–4). Regarding the type of fitness functions to be considered for the regressions, polynomials up to second order—linear and quadratic functions—seem to be adaptable enough to describe the data well. When using other functions, such as hyperbolas, that can be suitable for depicting rare genotype advantage, it is important to pay detailed attention to the constraints these types of functions cause in the regression curves, to distinguish them from the particularities of the curves actually caused by the data.
The importance of superiority of the heterozygote (or heterokaryotype) and frequency-dependent selection as selective mechanisms in the maintenance of genetic polymorphisms in Drosophila has been extensively discussed without conclusively tipping the scales in favor of any of them (see Tobari 1993; Powell 1997, pp. 109–114). We have found frequency-dependent selection to be much more important for the Drosophila inversion polymorphisms we have analyzed. In D. subobscura, frequency-dependent male sexual selection rules the qualitative behavior of the genetic system, in terms of both the stability of the fixation points and the number of internal stable and unstable equilibria. Directional viability and superiority of the heterokaryotype in female fecundity could, however, contribute to prevent the fixation of the O3+4+7 arrangement. In D. pseudoobscura frequency-dependent viability plays a more influential role in determining the maintenance of the polymorphism. Kojima and Tobari (1969)(see also Tobari 1993; Powell 1997, p. 112) studied frequency-dependent viability in inversion polymorphisms of D. ananassae and found heterokaryotype superiority at the internal equilibrium points. We have obtained the same result for D. subobscura and D. pseudoobscura inversion polymorphisms when considering one frequency-dependent selection component at a time. For the models considering all the selection components available, however, we have shown that the total fitnesses of females and males at the stable equilibrium points display more complex modes of selection involving total selection acting in considerably strong opposite sense in the two sexes. This suggests that sex-dependent selection, more than heterokaryotype superiority, contributes with frequency-dependent selection to the maintenance of the inversion polymorphisms we have analyzed. More generally we have shown that very different frequency-dependent data-based patterns of selection—beyond mathematical coincidences in the type of functions—in which superiority of the heterozygote is not the rule at all along the frequencies (see Figures 1–4) generate internal stable equilibria and often fulfill the protected polymorphism conditions (Table 5). Some of the cases considered also show how the flexibility of variable fitness functions can lead to internal stable equilibria when the protected polymorphism conditions are not fulfilled.
The equilibrium point at roughly p̂ = 0.8 we predict for ST/CH arrangements of D. pseudoobscura (Table 5) is in very good agreement with the equilibrium frequencies reported for the experimental populations (see Dobzhansky and Pavlovsky 1953; Anderson and Brown 1984). For OST/O3+4+7 arrangements of D. subobscura, however, this does not seem to be the case, since Zapata et al. (1986) observed that experimental populations achieve equilibrium points at (roughly) p̂ = 0.9, in contrast with our predictions from the fitness estimates, in which p̂ < 0.7 (Table 5). This difference could likely be due to the fact that variable viability selection, which plays a major role in the maintenance of the ST/CH polymorphism in D. pseudoobscura, has not been studied for D. subobscura. However, it must also be kept in mind that two other general selection mechanisms, frequency-dependent female fecundity (Anderson and Watanabe 1974) and sex-dependent viability (Druger 1966; Druger and Nickerson 1972), were found in some experiments on chromosomal arrangements of D. pseudoobscura. As a final point, supergene selection is an inversion-related balancing selective force to take into account for a precise approximation of the equilibrium frequencies of chromosomal arrangements of Drosophila to be achieved (Wasserman 1968; Alvarez and Zapata 1997).
Acknowledgments
The authors thank Joachim Hermisson for valuable comments on an earlier version of the article and kind support during part of the investigation and Pleuni Pennings, John Parsch, and one anonymous reviewer for discussion toward a better presentation of the work. This survey was partially supported by a fellowship from the Secretaría Xeral de Investigación e Desenvolvemento da Xunta de Galicia to J.M.A.C.
References
- Alvarez, G., and C. Zapata, 1997. Conditions for protected polymorphism under supergene selection. Genetics 146 717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, W. W., 1969. Polymorphism resulting from the mating advantage of the rare male genotypes. Proc. Natl. Acad. Sci. USA 64 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, W. W., and C. J. Brown, 1984. A test for rare male mating advantage with Drosophila pseudoobscura karyotypes. Genetics 107 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, W. W., and T. K. Watanabe, 1974. Selection by fertility in Drosophila pseudoobscura. Genetics 77 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, W. W., and T. K. Watanabe, 1997. A demographic approach to selection. Proc. Natl. Acad. Sci. USA 94 7742–7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, W. W., J. Arnold, S. A. Sammons and D. G. Yardley, 1986. Frequency-dependent viabilities of Drosophila pseudoobscura karyotypes. Heredity 56 7–17. [Google Scholar]
- Anderson, W. W., J. Arnold, D. G. Baldwin, A. T. Beckenbach, C. J. Brown et al., 1991. Four decades of inversion polymorphism in Drosophila pseudoobscura. Proc. Natl. Acad. Sci. USA 88 10367–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anxolabéhère, D., 1980. The influence of sexual and larval selection on the maintenance of polymorphism at the sepia locus in Drosophila melanogaster. Genetics 95 743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anxolabéhère, D., and G. Périquet, 1981. The role of frequency-dependent selection in the outcome of experimental populations in evolution. Genetica 55 3–9. [Google Scholar]
- Asmussen, M. A., 1983. a Density dependent selection incorporating intraspecific competition. I. A haploid model. J. Theor. Biol. 101 113–127. [DOI] [PubMed] [Google Scholar]
- Asmussen, M. A., 1983. b Density dependent selection incorporating intraspecific competition. II. A diploid model. Genetics 103 335–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmussen, M. A., and E. Basnayake, 1990. Frequency-dependent selection: the high potential for permanent genetic variation in the dialelic, pairwise interaction model. Genetics 125 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala, J., and C. A. Campbell, 1974. Frequency-dependent selection. Annu. Rev. Ecol. Syst. 5 115–138. [Google Scholar]
- Bodmer, W. F., 1965. Differential fertility in population genetics models. Genetics 51 411–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürger, R., 2005. A multilocus analysis of intraspecific competition and stabilizing selection on a quantitative trait. J. Math. Biol. 50 355–396. [DOI] [PubMed] [Google Scholar]
- Cannings, C., 1969. Unisexual selection at one autosomal locus. Genetics 62 225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, B., and D. Charlesworth, 1973. Selection of new inversions in multilocus genetic systems. Genet. Res. 21 167–183. [Google Scholar]
- Charlesworth, B., and D. Charlesworth, 1975. An experiment on recombination load in Drosophila melanogaster. Genet. Res. 25 267–274. [DOI] [PubMed] [Google Scholar]
- Clark, A. G., and M. W. Feldman, 1986. A numerical simulation of the one-locus, multiple-allele fertility model. Genetics 113 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, B., and P. O'Donald, 1964. Frequency-dependent selection. Heredity 19 201–206. [Google Scholar]
- Clarke, B., and L. Partridge (Editors), 1988 Frequency-Dependent Selection. Royal Society, London.
- Cockerham, C. C., P. M. Burrows, S. S. Young and T. Prout, 1972. Frequency-dependent selection in randomly mating populations. Am. Nat. 106 493–515. [Google Scholar]
- Curtsinger, J. W., 1984. Evolutionary principles for polynomial models of frequency-dependent selection. Proc. Natl. Acad. Sci. USA 81 2840–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky, Th., 1943. Genetics of natural populations. IX. Temporal changes in the composition of populations of Drosophila pseudoobscura. Genetics 28 162–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky, Th., 1948. Genetics of natural populations. XVI. Altitudinal and seasonal changes produced by natural selection in certain populations of Drosophila pseudoobscura. Genetics 33 158–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky, Th., 1970 Genetics of the Evolutionary Process. Columbia University Press, New York.
- Dobzhansky, Th., and O. Pavlovsky, 1953. Indeterminate outcome of certain experiments of Drosophila populations. Evolution 7 198–210. [Google Scholar]
- Dobzhansky, Th., W. W. Anderson and O. Pavlovsky, 1966. Genetics of natural populations. XXVIII. Continuity and change in populations of Drosophila pseudoobscura in western United States. Evolution 20 418–427. [DOI] [PubMed] [Google Scholar]
- Druger, M., 1966. Stability of chromosomal polymorphism in populations of Drosophila pseudoobscura. Heredity 21 317–321. [DOI] [PubMed] [Google Scholar]
- Druger, M., and R. P. Nickerson, 1972. Maintenance of chromosomal polymorphism in a population of Drosophila pseudoobscura: viability under crowded and uncrowded conditions. Evolution 26 322–325. [DOI] [PubMed] [Google Scholar]
- Dubinin, N. P., and G. G. Tiniakov, 1946. Inversion gradients and natural selection in ecological races of Drosophila funebris. Genetics 31 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, M. W., M. Nabholz and W. F. Bedmer, 1969. Evolution of the Rh polymorphism: a model for the interaction of incompatibility, reproductive compensation, and heterozygote advantage. Am. J. Hum. Genet. 21 171–193. [PMC free article] [PubMed] [Google Scholar]
- Feldman, M. W., F. B. Christiansen and U. Liberman, 1983. On some models of fertility selection. Genetics 105 1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández Iriarte, P., and E. Hasson, 2000. The role of the use of different host plants in the maintenance of the inversion polymorphism in the cactophilic Drosophila buzzatii. Evolution 54 1295–1302. [DOI] [PubMed] [Google Scholar]
- Hadeler, K. P., and U. Liberman, 1975. Selection models with fertility differences. J. Math. Biol. 2 19–32. [Google Scholar]
- Haldane, J. B. S., 1924. A mathematical theory of natural and artificial selection. I. Trans. Camb. Philos. Soc. 23 19–41. [Google Scholar]
- Haldane, J. B. S., 1926. A mathematical theory of natural and artificial selection. III. Proc. Camb. Philos. Soc. 23 363–372. [Google Scholar]
- Hoffmann, A. A., C. M. Sgrò and A. R. Weeks, 2004. Chromosomal inversion polymorphisms and adaptation. TREE 19 482–488. [DOI] [PubMed] [Google Scholar]
- Karlin, S., 1981. Some natural viability systems for a multiallelic locus: a theoretical study. Genetics 97 457–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell, J. F., M. T. Clegg, F. M. Stewart and T. Prout, 1977. Regions of stable equilibria for models of differential selection in the two sexes under random mating. Genetics 85 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, M., 1956. Rules for testing stability of a selective polymorphism. Proc. Natl. Acad. Sci. USA 42 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima, K., and Y. N. Tobari, 1969. Selective modes associated with karyotypes in Drosophila ananassae. II. Heterosis and frequency-dependent selection. Genetics 63 639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimbas, C. B., and J. R. Powell (Editors), 1992 Drosophila Inversion Polymorphism. CRC Press, Boca Raton, FL.
- Levene, H., 1953. Genetic equilibrium when more than one ecological niche is available. Am. Nat. 87 331–333. [Google Scholar]
- Lewontin, R. C., 1958. A general method for investigating the equilibrium of gene frequency in a population. Genetics 43 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewontin, R. C., L. R. Ginzburg and S. D. Turjapurkar, 1978. Heterosis as an explanation for large amounts of genetic polymorphism. Genetics 88 149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel, S. P. H., 1959. The stability of a multiple allelic system. Heredity 13 289–302. [Google Scholar]
- Mandel, S. P. H., 1971. Owen's model of a genetical system with differential viability between sexes. Heredity 26 49–63. [DOI] [PubMed] [Google Scholar]
- Mandel, S. P. H., 1980. The triangle inequality in balanced genetical polymorphisms. Genetics 96 557–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mérat, P., 1969. Sélection différente dans les deux sexes. Discussion générale des possibilitès d'équilibre pour un locus autosomal a deux allèles. Ann. Genet. Sel. Anim. 1 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos, J. R., 1955. Comparative physiology of some chromosomal types in Drosophila pseudoobscura. Evolution 9 141–151. [Google Scholar]
- Mueller, L. D., 1988. Density-dependent population growth and natural selection in food-limited environments: the Drosophila model. Am. Nat. 132 786–809. [Google Scholar]
- Nagylaki, T., 1979. Dynamics of density- and frequency-dependent selection. Proc. Natl. Acad. Sci. USA 76 438–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagylaki, T., 1992 Introduction to Theoretical Population Biology. Springer-Verlag, Berlin.
- Noor, M. A., K. L. Grams, L. A. Bertucci and J. Reiland, 2001. Chromosomal inversions and the reproductive isolation of species. Proc. Natl. Acad. Sci. USA 98 12084–12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen, A. R. G., 1953. A genetical system admitting of two distinct stable equilibria under natural selection. Heredity 7 97–102. [Google Scholar]
- Penrose, L. S., 1949. The meaning of “fitness” in human populations. Ann. Eugen. 14 301–304. [DOI] [PubMed] [Google Scholar]
- Petit, C., and L. Ehrman, 1969. Sexual selection in Drosophila. Evol. Biol. 3 177–223. [Google Scholar]
- Powell, J. R., 1997 Progress and Prospects in Evolutionary Biology: The Drosophila Model. Oxford University Press, Oxford.
- Prout, T., 1965. The estimation of fitness from genotypic frequencies. Evolution 19 546–551. [Google Scholar]
- Prout, T., 1968. Sufficient conditions for multiple niche polymorphism. Am. Nat. 102 493–496. [Google Scholar]
- Prout, T., 1969. The estimation of fitness from population data. Genetics 63 949–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prout, T., 1971. The relation between fitness components and population prediction in Drosophila. I. The estimation of fitness components. Genetics 68 127–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveh, A., and U. Ritte, 1976. Frequency-dependence and stability. Math. Biosci. 30 371–374. [Google Scholar]
- Robertson, A., 1962. Selection for heterozygotes in small populations. Genetics 47 1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Trelles, F., G. Alvarez and C. Zapata, 1996. Time-series analysis of seasonal changes of the O inversion polymorphism of D. subobscura. Genetics 142 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughgarden, J., 1979 Theory of Population Genetics and Evolutionary Ecology: An Introduction. Macmillan Publishing, New York.
- Sacks, J. M., 1967. A stable equilibrium with minimum average fitness. Genetics 56 705–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salceda, V. M., and W. W. Anderson, 1988. Rare male mating advantage in a natural population of Drosophila subobscura. Proc. Natl. Acad. Sci. USA 85 9870–9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, M., R. Tarrío, C. Zapata and G. Alvarez, 1986. Sexual selection on chromosomal polymorphism in Drosophila subobscura. Heredity 57 161–169. [Google Scholar]
- Schutz, W. M., C. A. Brim and S. A. Usanis, 1968. Intergenotypic competition in plant populations. I Feedback systems with stable equilibria in populations of autogamous homozygous lines. Crop Sci. 8 61–66. [Google Scholar]
- Slatkin, M., 1979. The evolutionary response to frequency and density dependent interactions. Am. Nat. 114 384–398. [Google Scholar]
- Sokal, R. R., and F. J. Rohlf, 1995 Biometry: The Principles and Practice of Statistics in Biological Research, Ed. 3. W. H. Freeman, New York.
- Sole, E., J. Balanya, D. Sperlich and L. Serra, 2002. Long-term changes in the chromosomal inversion polymorphism of Drosophila subobscura. Evolution 56 830–835. [DOI] [PubMed] [Google Scholar]
- Sperlich, D., and P. Pfriem, 1986 Chromosomal polymorphism in natural and experimental populations, pp. 257–309 in The Genetics and Biology of Drosophila, edited by M. Ashburner, H. L. Carson and J. N. Thompson. Academy Press, London.
- Tarrío, R., 1993 Estudio de la acción de la selección natural sobre el polimorfismo de inversiones del cromosoma O de D. subobscura mediante la estimación de componentes selectivos. Ph.D. Thesis, Universidade de Santiago de Compostela, Santiago de Compostela, Spain.
- Tobari, Y. N., 1993 Heterosis and frequency dependent selection, pp. 151–160 in Drosophila ananassae: Genetical and Biological Aspects, edited by Y. N. Tobari. Japan Scientific Societies Press, Tokyo/Krager, Baden, Switzerland.
- Turelli, M., and N. Barton, 2004. Polygenic variation maintained by balancing selection: pleiotropy, sex-dependent allelic effects and G × E interactions. Genetics 166 1053–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman, M., 1968. Recombination-induced chromosomal heterosis. Genetics 72 691–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman, M., and H. R. Koepfer, 1975. Fitness of karyotypes in Drosophila pseudoobscura. Genetics 79 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir, B. S., 1990 Genetic Data Analysis. Sinauer Associates, Sunderland, MA.
- Wolfram, S., 1996 Mathematica, Ed. 3. Cambridge University Press, Cambridge, UK.
- Wright, S., 1955. Classification of the factors of evolution. Cold Spring Harbor Symp. Quant. Biol. 20 16–24. [DOI] [PubMed] [Google Scholar]
- Zapata, C., G. Alvarez, M. Dosil and A. Fondevila, 1986. Gametic coadaptation in chromosomal polymorphism of Drosophila subobscura. II. Changes of gametic disequilibrium in experimental populations. Genetica 72 149–160. [Google Scholar]






