Abstract
IL-23 induces the differentiation of naive CD4+ T cells into highly pathogenic helper T cells (Th17/ThIL-17) that produce IL-17, IL-17F, IL-6, and TNF-α, but not IFN-γ and IL-4. Two studies in this issue of the JCI demonstrate that blocking IL-23 or its downstream factors IL-17 and IL-6, but not the IL-12/IFN-γ pathways, can significantly suppress disease development in animal models of inflammatory bowel disease and MS (see the related articles beginning on pages 1310 and 1317). These studies suggest that the IL-23/IL-17 pathway may be a novel therapeutic target for the treatment of chronic inflammatory diseases.
Th17/ThIL-17 is a new CD4+ helper T cell subset that produces IL-17
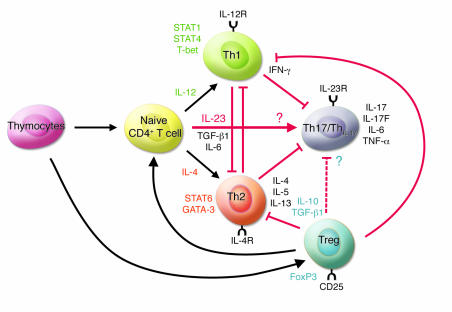
Upon antigenic stimulation, naive CD4+ T cells differentiate into 2 subsets, Th1 and Th2 cells, characterized by different cytokine production profiles and effector functions (Figure 1). Th1 cells produce large quantities of IFN-γ and mediate cellular immunity while Th2 cells, which are involved in humoral immunity, primarily produce IL-4, IL-5, and IL-13. IL-12, a heterodimer of the p40 and p35 subunits, induces the differentiation of naive CD4+ T cells into IFN-γ–producing Th1 cells through activation of STAT4. IFN-γ signals are transduced by STAT1, which activates a downstream transcription factor, T-bet, that enhances the expression of genes specific to Th1 cells. In contrast, IL-4 induces STAT6 activation, promoting the expression of GATA-3, a transcriptional factor essential for both IL-4 production and Th2 cell differentiation. Recently, it was reported that CD4+ T cells isolated from the inflamed joints of patients with Lyme disease contain a subset of IL-17–producing CD4+ T cells that are distinct from those producing either IL-4 or IFN-γ (Figure 1) (1). These IL-17–producing CD4+ T cells were dubbed Th17 or ThIL-17 cells (2–4).
Figure 1. IL-23 promotes the development of an IL-17–producing CD4+ helper T cell subset.
IL-23 induces the differentiation of naive CD4+ T cells into IL-17–producing helper T cells (Th17/ThIL-17) via mechanisms that are distinct from the Th1 and Th2 differentiation pathways. The transcriptional factors critical for the development of Th1 (STAT1, STAT4, and T-bet) and Th2 (STAT6) cells are not required for the induction of Th17/ThIL-17 cells. The transcriptional factor(s) essential for the development of Th17/ThIL-17 cells remain unknown. IFN-γ and IL-4 antagonize each other in the differentiation of Th1 and Th2 cells and the promotion of their function. IFN-γ also suppresses the differentiation of Th17/ThIL-17 cells by reducing IL-23R expression on CD4+ T cells. IL-4 also inhibits the development of Th17/ThIL-17 cells. It is not known, however, whether Th17/ThIL-17 cells inhibit the development of Th1 and Th2 cells. Tregs, an immune-modulating subset of CD4+ T cells, suppress the differentiation and effector function of Th1 and Th2 cells. Recent studies suggest that Treg-derived TGF-β induces the differentiation of Th17/ThIL-17 cells from naive CD4+ T cells in the presence of IL-6 in vitro (26). However, the precise effect(s) of Tregs on Th17/ThIL-17 cells are as yet unknown.
IL-17, a proinflammatory cytokine predominantly produced by activated T cells, enhances T cell priming and stimulates fibroblasts, endothelial cells, macrophages, and epithelial cells to produce multiple proinflammatory mediators, including IL-1, IL-6, TNF-α, NOS-2, metalloproteases, and chemokines, resulting in the induction of inflammation (5, 6). IL-17 expression is increased in patients with a variety of allergic and autoimmune diseases, such as RA, MS, inflammatory bowel disease (IBD), and asthma, suggesting the contribution of IL-17 to the induction and/or development of such diseases. Supporting this, the involvement of this cytokine in such responses is demonstrated in animal models; autoimmune disorders such as collagen-induced arthritis (CIA) and EAE, animal models for RA and MS, respectively, as well as allergic responses such as contact hypersensitivity, delayed-type hypersensitivity, and airway hypersensitivity were suppressed in IL-17–deficient (IL-17–/–) mice (7, 8) (Y. Komiyama et al., University of Tokyo, Tokyo, Japan, unpublished observations). Therefore, Th17/ThIL-17 cells are likely to play critical roles in the development of autoimmunity and allergic reactions.
The IL-23/IL-17, but not IL-12/IFN-γ, axis is critical for the development of autoimmune inflammatory diseases
The development of autoimmune diseases, such as RA, MS, and IBD, is thought to be mediated by Th1 cells because high levels of IL-12 and IFN-γ are detected in inflammatory sites (9). In addition, treatment with mAbs against IL-12p40 suppresses such disease development in humans and animal disease models (9, 10). However, mice deficient in IL-12p35, IL-12 receptor β2 (IL-12Rβ2), IFN-γ, IFN-γR, or STAT1, which are critical molecules in IL-12/IFN-γ–mediated responses, exhibit an increased severity of diseases such as EAE and CIA (11–13). These observations are inconsistent with the notion that IL-12 is responsible for such disease development. As IL-23, an IL-12 family cytokine consisting of the p19 and p40 subunits, shares the p40 subunit with IL-12 and anti-p40 mAbs inhibit both cytokines, the involvement of IL-23 is suggested. Current evidence suggests that IL-23 is responsible for the differentiation and expansion of Th17/ThIL-17 cells from naive CD4+ T cells (2, 4, 14).
In this issue of the JCI, Yen et al. report on their use of IL-23p19–/– and IL-12p35–/– mice to demonstrate that IL-23, but not IL-12, is essential for the development of intestinal inflammation (15). They used IL-10–/– mice as a model of T cell–mediated IBD (16) and showed that the development of colitis was greatly suppressed by IL-23p19 deficiency but not IL-12p35 deficiency. Exogenous IL-23 administration accelerated the onset of colitis in Rag–/– mice engrafted with IL-10–/–CD4+ T cells. Notably, IL-17 production was abolished in IL-23p19–/– mice while IFN-γ and IL-4 production were unaffected. IL-17 and IL-6 expression by anti-CD3 mAb–stimulated memory CD4+ T cells were augmented by IL-23, but not by IL-12, indicating that IL-23 can simulate memory CD4+ T cells. This result contrasts with the ability of IL-12 to stimulate naive CD4+ T cells. Moreover, treatment with both anti–IL-6 and anti–IL-17 mAbs significantly ameliorated the severity of the intestinal inflammation induced by IL-23–treated Rag–/– mice engrafted with IL-10–/–CD4+CD45RBhi T cells. These observations suggest that IL-17 and IL-6 derived from memory T cells are responsible for the development of intestinal inflammation downstream of IL-23.
Also in this issue of the JCI, Chen et al. report on their use of newly developed anti–IL-23p19 mAbs to demonstrate the involvement of IL-23 in EAE (17). The authors previously demonstrated that IL-23p19–/– mice are resistant to EAE and CIA; production of IL-17, but not IFN-γ, is almost completely abolished in these mutant mice (3, 18, 19). In contrast, IL-23p35–/– mice exhibited decreased IFN-γ production and increased IL-17 production, suggesting that IFN-γ may suppress IL-17 production. Furthermore, IL-23–induced but not IL-12–induced proteolipid protein peptide–specific T cells are highly encephalogenic (3). Consistent with these reports, the development of EAE was efficiently suppressed by treatment with anti–IL-23p19 or anti–IL-12/IL-23p40 mAbs by inhibiting infiltration of IL-17–, IFN-γ–, and TNF-α–producing CD4+ T cells in the CNS. Disease severity correlated well with serum IL-17 levels; treatment with anti–IL-17 mAbs ameliorated the clinical disease score. Meanwhile, treatment with anti–IFN-γ mAbs exacerbated disease, consistent with previous observations that IFN-γ–/– and IFN-γR–/– mice are highly susceptible to EAE (12, 13). Thus, it is clearly shown that the IL-23/IL-17 pathway, rather than the IL-12/IFN-γ pathway, is critical for the development of autoimmune diseases.
IL-23 causes inflammation through IL-17–dependent and IL-17–independent pathways
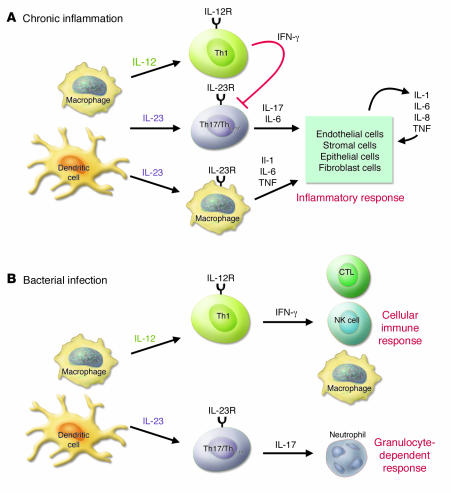
Anti–IL-17 treatment did not prevent the onset and relapse of EAE with the same efficiency as anti–IL-23p19 or anti–IL-12/IL-23p40 treatment, suggesting that the effects of IL-23 cannot be explained by the action of IL-17 alone. We also observed that EAE progression is only partially suppressed even in IL-17–/– mice, suggesting the involvement of additional factors (Komiyama et al., unpublished observations). The transcription factor T-bet is responsible for enhancing IFN-γ production and increasing IL-12Rβ2 expression (20). In contrast to IFN-γ–/– mice, T-bet–/– mice are highly resistant to EAE development (11). As Th17/ThIL-17 cells were present in T-bet–/– mice, Chen et al. suggested that Th17/ThIL-17 cells are not sufficient to induce disease and that additional T-bet–dependent factors and/or cell populations play significant roles in disease pathogenesis (17). With respect to this, T-bet expression on dendritic cells, but not on T cells, is required for IL-1 and chemokine production, contributing to the development of collagen antibody–induced arthritis (21). In the current studies, Chen et al. (17) suggested that IL-23 may directly activate a subset of macrophages and dendritic cells expressing IL-23R, resulting in the production of inflammatory mediators, such as TNF-α and IL-1 (18, 22). However, as progression of diseases such as EAE and CIA is only minimally affected in TNF-α–/– mice and anti–TNF-α treatment of wild-type mice results in more severe MS (23), TNF-α is not critical for the development of this disease. In contrast, IL-6–/– and IL-1–/– mice are significantly resistant, and IL-1 receptor antagonist–deficient (IL-1Ra–/–) mice are more susceptible to EAE (24, 25). Thus, IL-6 and IL-1 likely play important roles in the development of this disease. These observations suggest that IL-23 can induce chronic inflammation through 2 independent pathways: (a) activation of Th17/ThIL-17 cells; and (b) induction of IL-1 and IL-6 production via myeloid cell activation (Figure 2).
Figure 2. The role of the IL-23/IL-17 axis in inflammation and infection.
The IL-23/IL-17 axis plays an important role in the development of chronic inflammation and in host defenses against bacterial infection. (A) In chronic inflammation, antigen-stimulated dendritic cells and macrophages produce IL-23, which promotes the development of Th17/ThIL-17 cells. Th17/ThIL-17 cells produce IL-17, which enhances T cell priming and triggers potent inflammatory responses by inducing the production of a variety of inflammatory mediators. IL-23 also acts on dendritic cells and macrophages in an autocrine/paracrine manner to stimulate the generation of proinflammatory cytokines, such as IL-1, IL-6, and TNF-α. IL-12–stimulated Th1 cells produce IFN-γ and suppress the differentiation of Th17/ThIL-17 cells. Th1 cells may play an immunoregulatory, not a pathogenic, role in the development of chronic inflammation. (B) Upon bacterial infection, IL-23 is rapidly produced by activated macrophages and dendritic cells at the site of infection. IL-23 then activates local resident Th17/ThIL-17 cells and other IL-17–producing cells, such as CD8+ T cells and γδ T cells. Production of IL-17 by these cells induces G-CSF production from stromal cells. The IL-23/IL-17/G-CSF pathway augments neutrophil recruitment to the infection site, contributing to extracellular bacterial clearance. IL-23 also increases the production of IL-1, IL-6, and TNF-α in an autocrine/paracrine manner. In contrast, Th1 cells produce IFN-γ and stimulate CD8+ cytotoxic T lympocytes, NK cells, and macrophages. IFN-γ enhances antigen presentation by inducing expression of MHC molecules and activates cells to produce cytolytic molecules, including perforin and granzyme, which promote the elimination of intracellular bacteria.
Additional IL-17 family molecules may also play an important role in the development of inflammatory diseases. The IL-17 family currently consists of 6 family members; some of these family members, such as IL-17F, share significant amino acid homology with IL-17 (also known as IL-17A), are induced by IL-23, bind the same receptor as IL-17, and are produced by Th17/ThIL-17 cells. Thus, it is possible that these additional IL-17 members may be involved in inflammatory responses. It is important to elucidate the functions of these IL-17 family molecules in normal physiology and in disease pathogenesis.
The Th17/ThIL-17 differentiation mechanism is not yet known
Naive CD4+ T cells isolated from mice deficient in STAT1, STAT4, or T-bet retained the ability to differentiate into Th17/ThIL-17 cells in vitro following TCR stimulation in the presence of IL-23 (2). The generation of Th17/ThIL-17 cells following immunization with antigen stimulation was also normal in mice deficient for STAT4, STAT6, or T-bet (4). Thus, these Th1- and Th2-specific transcriptional factors are not involved in the differentiation of Th17/ThIL-17 cells, indicating that the Th17/ThIL-17 lineage is independent from these classical Th cell lineages. In this issue, Chen et al. (17) demonstrate that T-bet–/– lymph node cells produced IL-17 upon stimulation with anti-CD3 mAbs, consistent with previous reports (2, 4). Exogenous IL-23, however, did not further enhance IL-17 production in T-bet–/– lymph node cells, suggesting that T-bet may influence IL-23 responsiveness during early Th17/ThIL-17 development (17). Thus, the transcriptional factors involved in Th17/ThIL-17 cell development still remain to be elucidated (Figure 1). With regard to this, STAT3 was recently implicated in the IL-23R signaling pathway (22).
As IL-12Rβ1, the common subunit of IL-12R and IL-23R, is constitutively expressed in naive CD4+ T cells, IL-12Rβ2 and IL-23R expression are critical for the responsiveness to IL-12 and IL-23 and development of Th1 and Th17/ThIL-17 cell lineages. Only memory and/or activated T cells express IL-23R; naive Th17/ThIL-17 progenitor cells are devoid of this molecule (22). However, when naive CD4+ T cells were stimulated with IL-23 in the presence of anti–IL-4 and anti–IFN-γ mAbs, a large IL-17–producing population was observed, indicating that IFN-γ and IL-4 inhibit the differentiation of Th17/ThIL-17 cells from naive CD4+ T cells. IFN-γ and STAT1 signaling inhibit the differentiation by downregulating the expression of IL-23R (2). Although IL-4 also inhibits Th17 cell expansion, the mechanism governing this suppression is not known (2, 4). Thus, the identification of the signals that induce IL-23R expression on naive CD4+ T cells is crucial in elucidating the mechanisms of Th17/ThIL-17 cell lineage differentiation.
Recently, Veldhoen et al. reported that Treg-derived TGF-β induces the differentiation of Th17/ThIL-17 cells from naive CD4+ T cells in the presence of IL-6 in vitro (Figure 1) (26). TGF-β–mediated Th17/ThIL-17 cell differentiation is promoted by dendritic cell–derived IL-1β and TNF-α. They showed that IL-23 is not essential for the development of Th17/ThIL-17 cells, but required for their survival and expansion through the positive feedback loop that upregulates IL-6, IL-1β, and TNF-α. Thus, current evidence provides us with 2 Th17/ThIL-17 cell differentiation pathways; one is IL-23 dependent and the other is IL-23 independent. Further studies are definitely required to address the precise roles of IL-23 and other factors in the development of Th17/ThIL-17 cells in vivo.
Concluding remarks
While the importance of IL-12 in host defense against bacteria is widely accepted, the role of IL-23 in host defense is not well understood (Figure 2). Recent studies have revealed that IL-12 and IL-23 have different roles in host defense. Mice deficient in IFN-γ, IFN-γR, or STAT1 are highly susceptible to many different pathogens, including Leishmania major, Listeria monocytogenes, and Mycobacterium tuberculosis (27). IL-23 and IL-17 are also important in host defenses against infection. It should be noted that IL-12/IFN-γ are primarily involved in host defenses against intracellular pathogens while IL-23/IL-17 are important for defenses against extracellular pathogens, including Klebsiella pneumoniae (28). This is because IFN-γ stimulates the immune system to kill intracellular bacteria and infected host cells while IL-17 recruits and activates neutrophils. The detailed host defense mechanisms involving IL-23 and IL-17, however, still remain to be elucidated.
IL-17 is produced not only by Th17/ThIL-17 cells, but also by activated CD8+ T cells, TCRγδ+ T cells, and neutrophils (5, 29). We have observed that CD4–CD8–TCRγδ+ T cells also produce IL-17 in IL-1Ra–/– mice, which spontaneously develop autoimmune arthritis (Komiyama et al., unpublished observations). Development of arthritis in these mice can be completely suppressed by IL-17 deficiency (6). Thus, IL-17 production by cells distinct from Th17/ThIL-17 cells may also be involved in inflammatory responses and host defense mechanisms. It remains unclear, however, which of these producer cells are involved in the different allergic and infectious diseases and how the differentiation pathways of these cell lineages are controlled.
Taken together, accumulating evidence suggests that 3 independent pathways are involved in inflammatory responses: IL-12/IFN-γ, IL-4/IL-5/IL-13, and IL-23/IL-17. These pathways are largely exclusive, although the effect of Th17/ThIL-17 cells on Th1 and Th2 cells is not well understood. Identification of the major immune pathways responsible for the development of each disease is important for its treatment because suppression of 1 pathway may accelerate the others. Therapeutic targeting of the newly discovered IL-23/IL-17 immune axis may prove effective for the treatment of autoimmune and allergic inflammatory responses.
Acknowledgments
We would like to thank Shigeru Kakuta and Susumu Nakae (Stanford University School of Medicine, Stanford, California, USA) for critical reading of the manuscript.
Footnotes
Nonstandard abbreviations used: CIA, collagen-induced arthritis; IBD, inflammatory bowel disease; IL-1Ra, IL-1 receptor antagonist; R, receptor.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 116:1218–1222 (2006). doi:10.1172/JCI28508.
See the related article beginning on page 1310.
References
- 1.Infante-Duarte C., Horton H.F., Byrne M.C., Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 2.Harrington L.E., et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 3.Langrish C.L., et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park H., et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolls J.K., Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Nakae S., et al. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakae S., et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 8.Nakae S., Nambu A., Sudo K., Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. . J. Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 9.Gately M.K., et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 10.Mannon P.J., et al. Anti-interleukin-12 antibody for active Crohn’s disease. N. Engl. J. Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 11.Bettelli E., et al. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J. Exp. Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter C.A. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 13.McKenzie B.S., Kastelein R.A., Cua D.J. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal S., Ghilardi N., Xie M.H., de Sauvage F.J., Gurney A.L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 15.Yen D., et al. IL-23 is essential for T cell–mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. . 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strober W., Fuss I.J., Blumberg R.S. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y., et al. Anti–IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cua D.J., et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 19.Murphy C.A., et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afkarian M., et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. . Nat. Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 21.Wang J., et al. Transcription factor T-bet regulates inflammatory arthritis through its function in dendritic cells. J. Clin. Invest. 2006;116:414–421. doi: 10.1172/JCI26631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parham C., et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. . J. Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 23.Kollias G., Kontoyiannis D. Role of TNF/TNFR in autoimmunity: specific TNF receptor blockade may be advantageous to anti-TNF treatments. Cytokine Growth Factor Rev. 2002;13:315–321. doi: 10.1016/s1359-6101(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 24.Matsuki T., Nakae S., Sudo K., Horai R., Iwakura Y. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. . Int. Immunol. 2006;18:399–407. doi: 10.1093/intimm/dxh379. [DOI] [PubMed] [Google Scholar]
- 25.Samoilova E.B., Horton J.L., Hilliard B., Liu T.S., Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J. Immunol. 1998;161:6480–6486. [PubMed] [Google Scholar]
- 26.Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Shtrichman R., Samuel C.E. The role of gamma interferon in antimicrobial immunity. Curr. Opin. Microbiol. 2001;4:251–259. doi: 10.1016/s1369-5274(00)00199-5. [DOI] [PubMed] [Google Scholar]
- 28.Happel K.I., et al. Divergent roles of IL-23 and IL-12 in host defense againstKlebsiella pneumoniae . . J. Exp. Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stark M.A., et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]