Abstract
In blood vessels, endothelia are submitted to constant shear effects and are, under normal conditions, capable of responding to any variation in hemodynamic forces. Caveolae — 50- to 100-nm plasma membrane invaginations present at the surface of terminally differentiated cells and particularly enriched in ECs — are composed of a high sphingolipid and cholesterol content and the protein caveolin-1 (Cav-1). Previous studies have suggested that caveolae and endothelial Cav-1 may regulate the vascular response to altered shear stress. In this issue of the JCI, Yu et al. have examined the role of Cav-1/caveolae in the regulation of flow-induced alterations (i.e., mechanotransduction) in vessels from wild-type mice, Cav-1–deficient mice, and Cav-1–deficient mice re-expressing Cav-1 only in ECs. Their data suggest that caveolae/Cav-1 may act as sensors of altered shear stress and that they also organize the signaling response in stimulated ECs (see the related article beginning on page 1284).
Shear stress: an important regulator of endothelial cell function
In the vasculature, blood vessels must respond rapidly to any external stimuli and especially to any physical change related to modifications in shear stress, which is a function of the blood viscosity and the velocity gradient at the arterial wall. In this context, blood vessels need to adapt and adjust their luminal diameters and their physical properties. ECs are the primary targets of these changes, as they are the first cell type exposed to these forces. One of the earliest findings that suggested an important role for ECs in this process was the observation of the ability of ECs to reorient and change shape during exposure to shear stress conditions. When submitted to steady laminar shear stress, ECs reorient in the direction of the flow and become remarkably elongated. These observations were made both in vitro (1) and in vivo (2, 3). These findings have suggested that ECs can respond and adapt to changes in blood flow. In fact, ECs act as sensors to transduce hydrodymanic forces. Not only does the morphology of ECs change, but other important signaling pathways have been shown to be regulated in response to altered shear stress. Several studies have shown that under laminar shear stress, the rate of EC proliferation is reduced compared with static conditions (4, 5). Oscillating and/or disturbed conditions have major effects on the pathology of the vasculature. In this regard, atherosclerotic lesions have been shown to develop primarily at sites of disturbed or altered blood flow, i.e., at bifurcations, branch ostia, and curved regions (6). Under oscillating and/or disturbed conditions, EC proliferation is increased compared with cells submitted to laminar shear stress and may allow ECs to repair injuries (7, 8). Other important regulatory pathways have now been shown to be activated under shear stress conditions (9–11). These pathways include those involved in changes in endothelial apoptotic, migratory, and permeability properties, among others. Many signaling molecules have also been shown to be involved, including PKC, focal adhesion kinase (FAK), s-Src, Rho family GTPases, PI3K, MAPKs, and the production of NO via eNOS (11).
Caveolae and caveolin-1: roles in vascular regulation of the shear stress–induced response
In order to properly respond to changes during conditions of shear stress, ECs rely on specific sensors such as integrins, ion channels, receptor tyrosine kinases, and caveolae. Caveolae are 50- to 100-nm plasma membrane invaginations that are characterized by the concentration of specific lipid components (sphingolipids/cholesterol) and the presence of the protein marker caveolin-1 (Cav-1) (12). In vivo, the EC cell type is highly enriched in caveolae and Cav-1. Caveolae have been shown to play an important role in the regulation of various signaling cascades as well as endothelial microvascular permeability (13). Consistent with these observations, important vascular abnormalities have been observed in Cav-1–deficient (Cav-1 KO) mice. Interestingly, caveolae numbers are remarkably increased in ECs that are cultured under laminar shear stress conditions compared with static conditions (14–17). In addition, it has been reported that caveolae and Cav-1 move toward the upstream edge of ECs and are associated with sites of Ca2+ wave initiation (14). It is important to note that Cav-1 has been shown to regulate several signaling pathways that are activated upon exposure of ECs to altered shear stress (11, 18, 19). Taken together, these observations are consistent with a role for Cav-1 in the regulation of the EC response to altered shear stress.
In a study in this issue of the JCI, Yu et al. (20) have examined the role of caveolae and Cav-1 in the regulation of flow-induced alterations using Cav-1 KO mice and Cav-1 KO mice overexpressing Cav-1 specifically in ECs. Overexpression of Cav-1 in ECs in Cav-1 KO mice offers an elegant approach for examining the specific role of endothelial Cav-1 in this process. To alter shear stress conditions in vivo, they ligated the left carotid artery for 14 days, thereby resulting in modified blood flow in the common carotid artery. This led to reduced lumen diameter in control animals but not in Cav-1 KO mice. In contrast, increased wall thickness, associated with enhanced cellular proliferation, was observed in Cav-1 KO mice (Figure 1). But the most important finding was that re-expression of Cav-1 in ECs was sufficient to prevent these changes in Cav-1 KO mice. As previously described (21, 22), acetylcholine-mediated arterial relaxation was enhanced and tended to increase vasodilation in Cav-1 KO arteries. Endothelium-derived NO has a profound effect on vessel tonicity and permeability. Within the endothelial plasma membrane, eNOS is highly enriched in caveolae (23). Optimal eNOS activity occurs when the interaction between eNOS and Cav-1 is competitively disrupted by calcium-calmodulin binding to eNOS (24, 25). In addition, eNOS may be activated by phosphorylation of serine 1176 (mouse eNOS) via activation of the Akt pathway (26, 27) during vascular remodeling using the same model (28). In the current study, Yu et al. (20) provide compelling evidence that Cav-1 KO carotids show reduced flow-dependant signaling and coupling of eNOS activity. In agreement with this hypothesis, activation of eNOS via phosphorylation of Ser1176 was reduced in Cav-1 KO arteries. This deficient activation appeared to be due, at least in part, to the mislocalization of eNOS in a perinuclear compartment. Importantly, re-expression of endothelial Cav-1 in Cav-1 KO ECs was sufficient to reverse these effects.
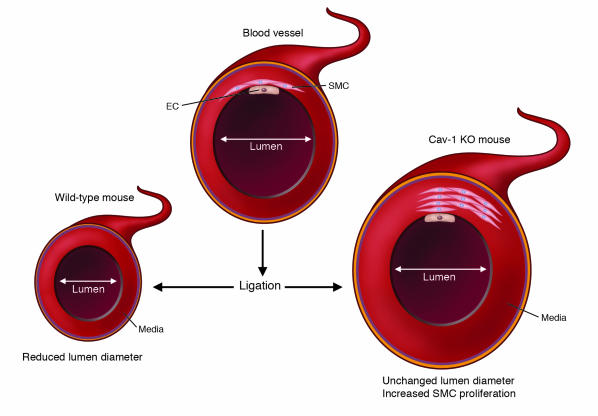
Figure 1. Cav-1 deficiency is associated with an altered response to modified shear stress conditions.
In this issue of the JCI, Yu et al. (20) have demonstrated that Cav-1 deficiency is associated with a reduced vascular response upon the alteration of shear stress conditions. Accordingly, reduced blood flow in the common carotid artery (due to ligation) was associated with reduced vessel lumen diameter in the case of wild-type animals. However, in Cav-1 KO mice, increased SMC proliferation was detected in the media but no change in the vessel lumen diameter was observed. Re-expression of Cav-1 in ECs alone was sufficient to eliminate this phenotype in Cav-1 KO mice.
This paper highlights the importance of the proper integration and coordination of the different signal transduction pathways within caveolae (Figure 2). While under basal conditions, Cav-1 can repress eNOS activity (Figure 2), it is now becoming clear that Cav-1 allows for the proper activation of eNOS activity upon stimulation of ECs (29). As previously suggested (19, 30), caveolae may act as platforms that hold signaling molecules associated with various signaling pathways in an inactive state. Upon stimulation (e.g., altered shear stress), signals may be rapidly transferred to downstream effectors to allow cells to respond quickly and efficiently. However, eNOS may not be the only protein affected in Cav-1 KO mice. Other important regulators of EC function, including VEGFR2, FAK, and integrins, may also play important roles in the phenotypes observed in Cav-1 KO mice. Additionally, several molecules involved in calcium translocation have been localized to caveolae where they are active (31). Given the rapid changes in intracellular calcium concentrations in ECs under altered shear stress (32), caveolae and Cav-1 may be involved in the very early sensing events leading to the endothelial response.
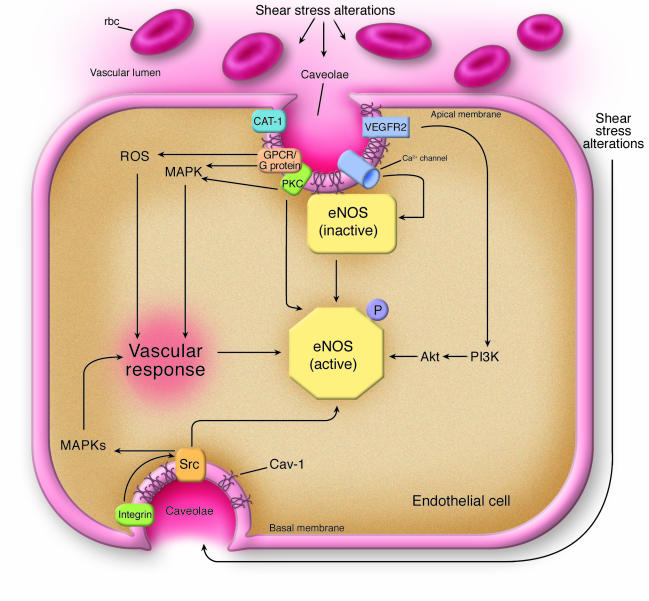
Figure 2. Cav-1–regulated signaling pathways in endothelial cells.
According to Yu et al. (20), caveolae and Cav-1 appear to be involved in some of the earliest steps associated with the detection of altered shear stress conditions in blood vessels. In addition, the vascular response (see Figure 1) is induced via caveolae and Cav-1, as demonstrated for several signaling pathways. Cav-1 plays important roles in regulating eNOS function. In unstimulated cells, eNOS is maintained in an inactive state through its association with Cav-1. Upon stimulation (e.g., shear stress), Cav-1 and caveolae may allow for the proper organization of various signal transduction pathways or organize the different regulatory proteins necessary for rapid and efficient eNOS activation. Calcium channels and the arginine transporter CAT-1 are localized to caveolae and may therefore allow for efficient eNOS activation (Ca2+ entry and dissociation from Cav-1 in the presence of calmodulin) and availability of substrate. Further activation is also possible via the PI3K and Akt signaling pathways, which enhance NO production following eNOS phosphorylation. Activation of the VEGFR2 signaling pathway is critical for PI3K/Akt activation, but other pathways appear to also mediate MAPK activation (ERK1/2 in particular). These pathways also involve G protein–coupled receptors (GPCRs) and G proteins. Shear stress is associated with increased oxidative stress conditions that lead to the production of ROS. Additionally, shear stress may affect EC interactions with extracellular matrix proteins. This may in turn activate integrin-mediated signaling pathways via caveolae. Src may also directly alter eNOS activity by inducing tyrosine phosphorylation (33). Note that only the major affected signal transduction pathways are shown.
These data suggest that endothelial caveolae and Cav-1 allow arteries to sense, organize, and mediate signal transduction, thereby giving arteries the ability to change their physical properties and to maintain/regulate normal blood flow in the face of altered shear stress conditions.
Footnotes
Nonstandard abbreviations used: Cav-1, caveolin-1.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 116:1222–1225 (2006). doi:10.1172/JCI28509.
See the related article beginning on page 1284.
References
- 1.Dewey C.F., Jr., Bussolari S.R., Gimbrone M.A., Jr., Davies P.F. The dynamic response of vascular endothelial cells to fluid shear stress. . J. Biomech. Eng. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 2.Flaherty J.T., et al. Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ. Res. 1972;30:23–33. doi: 10.1161/01.res.30.1.23. [DOI] [PubMed] [Google Scholar]
- 3.Langille B.L., Adamson S.L. Relationship between blood flow direction and endothelial cell orientation at arterial branch sites in rabbits and mice. Circ. Res. 1981;48:481–488. doi: 10.1161/01.res.48.4.481. [DOI] [PubMed] [Google Scholar]
- 4.Levesque M.J., Nerem R.M., Sprague E.A. Vascular endothelial cell proliferation in culture and the influence of flow. Biomaterials. 1990;11:702–707. doi: 10.1016/0142-9612(90)90031-k. [DOI] [PubMed] [Google Scholar]
- 5.Akimoto S., Mitsumata M., Sasaguri T., Yoshida Y. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21(Sdi1/Cip1/Waf1). . Circ. Res. 2000;86:185–190. doi: 10.1161/01.res.86.2.185. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham K.S., Gotlieb A.I. The role of shear stress in the pathogenesis of atherosclerosis. . Lab. Invest. 2005;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 7.Davies P.F., Remuzzi A., Gordon E.J., Dewey C.F., Jr., Gimbrone M.A., Jr. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc. Natl. Acad. Sci. U. S. A. 1986;83:2114–2117. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langille B.L., Reidy M.A., Kline R.L. Injury and repair of endothelium at sites of flow disturbances near abdominal aortic coarctations in rabbits. . Arteriosclerosis. 1986;6:146–154. doi: 10.1161/01.atv.6.2.146. [DOI] [PubMed] [Google Scholar]
- 9.Fisher A.B., Chien S., Barakat A.I., Nerem R.M. Endothelial cellular response to altered shear stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L529–L533. doi: 10.1152/ajplung.2001.281.3.L529. [DOI] [PubMed] [Google Scholar]
- 10.Langille B.L. Morphologic responses of endothelium to shear stress: reorganization of the adherens junction. Microcirculation. 2001;8:195–206. doi: 10.1038/sj/mn/7800085. [DOI] [PubMed] [Google Scholar]
- 11.Li Y.S., Haga J.H., Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 2005;38:1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 12.Razani B., Lisanti M.P. Caveolin-deficient mice: insights into caveolar function and human disease. J. Clin. Invest. 2001;108:1553–1561. doi: 10.1172/JCI200114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassan G., Lisanti M., Frank P. InCaveolae and lipid rafts: roles in signal transduction and the pathogenesis of human diseases. M.P. Lisanti and P.G. Frank, editors. Elsevier.; 2005. Caveolae and caveolins in the vascular system: functional roles in endothelia, macrophages, and smooth muscle cells. pp. 187–209. [Google Scholar]
- 14.Isshiki M., et al. Sites of Ca(2+) wave initiation move with caveolae to the trailing edge of migrating cells. J. Cell Sci. 2002;115:475–484. doi: 10.1242/jcs.115.3.475. [DOI] [PubMed] [Google Scholar]
- 15.Sun R.J., Muller S., Stoltz J.F., Wang X. Shear stress induces caveolin-1 translocation in cultured endothelial cells. Eur. Biophys. J. 2002;30:605–611. doi: 10.1007/s00249-001-0195-x. [DOI] [PubMed] [Google Scholar]
- 16.Boyd N.L., et al. Chronic shear induces caveolae formation and alters ERK and Akt responses in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1113–H1122. doi: 10.1152/ajpheart.00302.2003. [DOI] [PubMed] [Google Scholar]
- 17.Rizzo V., Morton C., DePaola N., Schnitzer J.E., Davies P.F. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1720–H1729. doi: 10.1152/ajpheart.00344.2002. [DOI] [PubMed] [Google Scholar]
- 18.Rizzo V., Sung A., Oh P., Schnitzer J.E. Rapid mechanotransduction in situ at the luminal cell surface of vascular endothelium and its caveolae. . J. Biol. Chem. 1998;273:26323–26329. doi: 10.1074/jbc.273.41.26323. [DOI] [PubMed] [Google Scholar]
- 19.Frank P.G., Woodman S.E., Park D.S., Lisanti M.P. Caveolin, caveolae, and endothelial cell function. Arterioscler. Thromb. Vasc. Biol. 2003;23:1161–1168. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- 20.Yu J., et al. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J. Clin. Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drab M., et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 22.Razani B., et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J. Biol. Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 23.Shaul P.W., et al. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J. Biol. Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 24.Ju H., Zou R., Venema V.J., Venema R.C. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J. Biol. Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 25.Michel J.B., Feron O., Sacks D., Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+ -calmodulin and caveolin. . J. Biol. Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 26.Dimmeler S., et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 27.Fulton D., et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudic R.D., et al. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J. Clin. Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sbaa E., Frerart F., Feron O. The double regulation of endothelial nitric oxide synthase by caveolae and caveolin: a paradox solved through the study of angiogenesis. Trends Cardiovasc. Med. 2005;15:157–162. doi: 10.1016/j.tcm.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Lisanti M.P., Scherer P., Tang Z.-L., Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 1994;4:231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 31.Isshiki M., Ying Y.S., Fujita T., Anderson R.G. A molecular sensor detects signal transduction from caveolae in living cells. J. Biol. Chem. 2002;277:43389–43398. doi: 10.1074/jbc.M205411200. [DOI] [PubMed] [Google Scholar]
- 32.Ando J., Komatsuda T., Kamiya A. Cytoplasmic calcium response to fluid shear stress in cultured vascular endothelial cells. In Vitro Cell. Dev. Biol. 1988;24:871–877. doi: 10.1007/BF02623896. [DOI] [PubMed] [Google Scholar]
- 33.Fulton D., et al. Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr-83. J. Biol. Chem. 2005;280:35943–35952.. doi: 10.1074/jbc.M504606200. [DOI] [PubMed] [Google Scholar]