Abstract
Wnt proteins are a family of secreted proteins that regulate many aspects of cell growth, differentiation, function, and death. Considerable progress has been made in our understanding of the molecular links between Wnt signaling and bone development and remodeling since initial reports that mutations in the Wnt coreceptor low-density lipoprotein receptor–related protein 5 (LRP5) are causally linked to alterations in human bone mass. Of the pathways activated by Wnts, it is signaling through the canonical (i.e., Wnt/β-catenin) pathway that increases bone mass through a number of mechanisms including renewal of stem cells, stimulation of preosteoblast replication, induction of osteoblastogenesis, and inhibition of osteoblast and osteocyte apoptosis. This pathway is an enticing target for developing drugs to battle skeletal diseases as Wnt/β-catenin signaling is composed of a series of molecular interactions that offer potential places for pharmacological intervention. In considering opportunities for anabolic drug discovery in this area, one must consider multiple factors, including (a) the roles of Wnt signaling for development, remodeling, and pathology of bone; (b) how pharmacological interventions that target this pathway may specifically treat osteoporosis and other aspects of skeletal health; and (c) whether the targets within this pathway are amenable to drug intervention. In this Review we discuss the current understanding of this pathway in terms of bone biology and assess whether targeting this pathway might yield novel therapeutics to treat typical bone disorders.
Wnt/β-catenin signaling
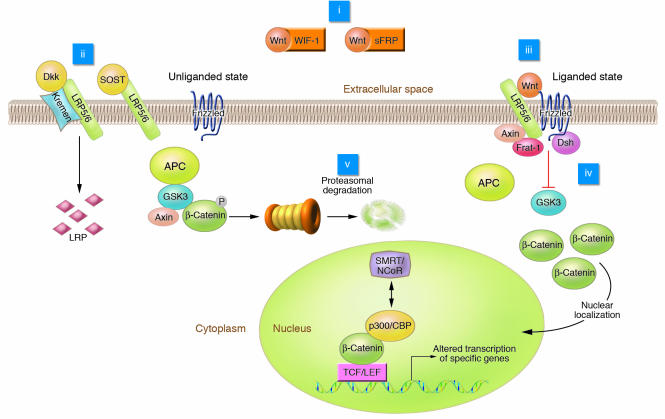
Wnt signaling plays an important role in development and maintenance of many organs and tissues, including bone (1). Although Wnt proteins signal through several pathways to regulate cell growth, differentiation, function, and death, the Wnt/β-catenin or canonical pathway appears to be particularly important for bone biology (reviewed in refs. 2, 3). The complexities of the Wnt/β-catenin signaling pathway in multiple cell types have been reviewed elsewhere (4, 5), and an outline of the pathway is shown in Figure 1. If Wnts are not expressed or if their binding to receptors is inhibited, degradation of β-catenin is facilitated via interactions with a protein complex consisting of adenomatous polyposis coli (APC), axin, and glycogen synthase kinase 3 (GSK3). APC and axin act as scaffold proteins allowing GSK3 to bind and phosphorylate β-catenin, identifying it for degradation by the β-TrCP–mediated ubiquitin/proteasome pathway.
Figure 1. Elements of Wnt/β-catenin signaling.
In the liganded state, binding of Wnt to the frizzled receptor inhibits GSK3 activity through mechanisms involving Axin, Frat-1, and Disheveled (Dsh). β-Catenin accumulates and is translocated to the nucleus, where it binds to TCF/LEF, causing displacement of transcriptional corepressors (e.g., silencing mediator of retinoid and thyroid receptors and nuclear receptor corepressor; SMRT/NCoR) with transcriptional coactivators (e.g., p300 and cAMP response element–binding protein; p300/CBP). Wnt signaling can be blocked by interactions of Wnt with inhibitory factors including WIF-1 and sFRP or the interaction of LRP5/6 with the Dkk/Kremen complex or sclerostin (SOST gene product). Phosphorylation of β-catenin by GSK3 stimulates β-catenin degradation. Potential intervention points for drug therapy (i–v) are indicated.
Activation of Wnt/β-catenin signaling occurs upon binding of Wnt to the 7-transmembrane domain–spanning frizzled receptor and low-density lipoprotein receptor–related protein 5 and 6 (LRP5/6) coreceptors (Figure 1). Signals are generated through the proteins Disheveled, Axin, and Frat-1, which disrupt the protein complex and inhibit the activity of GSK3, thus causing hypophosphorylation of its substrate, β-catenin (6). Stabilized β-catenin then accumulates in the cytosol and translocates to the nucleus, where this transcriptional coactivator interacts with T cell factor/lymphoid enhancer binding factor (TCF/LEF) transcription factors to mediate many of the effects of Wnts on gene transcription. Binding of β-catenin displaces transcriptional corepressors (e.g., silencing mediator of retinoid and thyroid receptors and nuclear receptor corepressor [SMRT/NCoR]) bound to TCF/LEF and recruits transcriptional coactivators (e.g., p300 and cAMP response element–binding protein [p300/CBP]) (7).
Wnt signaling is tightly regulated by members of several families of secreted antagonists. Interactions between Wnts and frizzled receptors are inhibited by members of the secreted frizzled-related protein (sFRP) family and Wnt inhibitory factor 1 (WIF-1; Figure 1). LRP5/6 coreceptor activity is inhibited by members of the sclerostin (SOST gene product) and Dickkopf (Dkk) families, all of which bind LRP5/6. Dkk1, -2, and -4 bind with various affinities to LRP5 and LRP6. Interaction of the Dkk/LRP complex with kremen internalizes the complex for degradation, thus diminishing the number of Wnt coreceptors available for signaling (8).
Wnt signaling regulates bone mass
Bone mass is influenced by the balance achieved between bone-forming cells (osteoblasts) and bone-resorbing cells (osteoclasts). Loss-of-function mutations in human LRP5 are associated with osteoporosis-pseudoglioma syndrome, which is characterized by low bone mineral density and skeletal fragility (9). In contrast, mutations in the N terminus of human LRP5 (e.g., G171V) that reduce affinity of LRP5 for Dkk1 are associated with high bone mass (10–12). These human bone phenotypes are largely supported by animal models with altered expression of LRP5. For example, Lrp5–/– mice have a low bone mass phenotype due to reduced proliferation of precursor cells (13). Furthermore, mice that overexpress the G171V LRP5 mutant in osteoblasts have enhanced osteoblast activity, reduced osteoblast apoptosis (Figure 2), and a high bone mass phenotype reminiscent of that observed in humans with this mutation (14). Interestingly, overexpression of wild-type Lrp5 leads to a more subtle bone phenotype, suggesting that the G171V mutant has a gain-in-function phenotype suggestive of a dominant-positive mechanism. Loss of bone mineral density in Lrp5–/– mice is further exacerbated by loss of an Lrp6 allele, suggesting that Wnts signal through both the LRP5 and LRP6 coreceptors to influence bone mass (15). Finally, disruption of endogenous LRP inhibitors such as Dkk1 (11) or sclerostin (16) increases the ability of Wnts to stabilize β-catenin and stimulate osteogenesis, further cementing the evidence that signaling from LRP coreceptors is important for bone development.
Figure 2. Wnt/β-catenin signaling regulates osteogenesis through multiple mechanisms.
Wnts repress alternative mesenchymal differentiation pathways such as adipocyte and chondrocyte differentiation and promote osteoblast differentiation, proliferation, and mineralization activity while blocking osteoblast apoptosis. By increasing the ratio of osteoprotegerin (OPG) to RANKL, β-catenin represses osteoclastogenesis. Green plus signs indicate positive effects of Wnt; red minus signs indicate inhibitory effects of Wnt. Dlx5, distal-less homeobox 5; MSC, mesenchymal stem cell; Msx2, msh homeobox homolog 2; Osx, osterix; Runx2, runt-related transcription factor 2.
Direct roles for Wnt signaling in the regulation of trabecular bone formation and bone mass were further supported by studies of mice lacking the soluble Wnt inhibitor sFRP1 (17). These mice show reduced osteoblast and osteocyte apoptosis in vivo, and results from work with marrow-derived cells from Sfrp1–/– mice suggest that in addition to preventing apoptosis, Wnt signaling may also increase bone by stimulating differentiation and replication of osteoblasts (Figure 2). While bone phenotypes observed in mice with altered expression or activity of Wnt coreceptors or inhibitors support a simple and direct relationship between Wnt signaling and bone mass, the relationship between members of the Wnt signaling pathway and bone biology will undoubtedly be more complex. For example, a recent report indicates that, as expected, activation of Wnt/β-catenin signaling induces osteoblastogenesis and that these effects are blocked by Dkk1 (18). However, during osteoblastogenesis Wnt/β-catenin signaling — presumably initiated by Wnt7b — induces expression of Dkk2, which is then surprisingly required for subsequent mineralization. Thus Dkk2–/– mice have increased secreted matrix (osteoid) but impaired mineralization, culminating in an osteopenic phenotype (18). While mineralization is partially rescued in vitro by Dkk2, another inhibitor of Wnt signaling, sFRP3, fails to stimulate mineralization, suggesting that Dkk2 may act through a mechanism distinct from Wnt antagonizing activity. This concept is not unprecedented as sFRP1 inhibits osteoclastogenesis through binding of receptor activator of NF-κB ligand (RANKL; ref. 19) and reorients axonal growth through interactions with frizzled 2 (20). Although the functional significance is unknown, other Wnt inhibitors such as WIF-1 and sFRP2 may also be induced in osteoblasts (21).
Taken together, these studies suggest that endogenous Wnt signaling plays an important role in osteoblastogenesis and bone formation (10–18); however, which of the 19 Wnts are involved has yet to be delineated. It is likely that Wnt activity in bone marrow varies throughout stages of development and has important contributions from several Wnts. One of these may be Wnt7b, which is induced during osteoblastogenesis (18, 22). Another is Wnt10b, which is expressed in bone marrow (23, 24), and deficiency of which leads to reduced trabecular bone mass, bone mineral density, and serum osteocalcin level (24). In addition, Wnt1, Wnt4, and Wnt14 are expressed in calvarial tissue and osteoblast cultures (13), and Wnt1 and Wnt3a are induced by bone morphogenic protein 2 (BMP-2) in a mesenchymal precursor cell line (25). However, because Wnts function through autocrine and paracrine mechanisms, analysis of those Wnts that specifically contribute to bone formation, as well as the frizzled receptors mediating their effects, will require in situ analysis of gene expression within bone and marrow and confirmation by genetic approaches.
Wnt regulates osteoblastogenesis through the canonical pathway
One of the mechanisms whereby Wnt signaling increases bone formation is via stimulation of the development of osteoblasts, and there is considerable in vitro evidence supporting a role for Wnt/β-catenin (i.e., canonical) signaling in this process (Figure 2). For example, inhibition of GSK3 enzymatic activity with lithium chloride (LiCl; ref. 26) or small molecules (e.g., Chir99021 and LY603281-31-8) stimulates mesenchymal precursors to differentiate into osteoblasts (24, 27, 28). This concept is supported by observations with Wnt3a, Wnt1, Wnt10b, and constitutively active β-catenin, all of which activate signaling through β-catenin and stimulate osteoblastogenesis, while Dkk1, which inhibits this pathway, reduces osteoblastogenesis (24, 28, 29). Importantly, activation of Wnt/β-catenin signaling also inhibits adipogenesis of mesenchymal precursors (30, 31), which may have clinical importance given the positive correlation reported between marrow adipose content and bone fractures (32).
Further evidence that Wnt signaling increases bone mass through the Wnt/β-catenin pathway comes from the results of in vivo studies using pharmacological inhibitors of GSK3β. For example, administration of LiCl for 4 weeks dramatically increased bone formation rate and number of osteoblasts in C57BL/6 mice (33). Similar results were obtained in osteopenic Lrp5–/– mice, indicating that LiCl acts downstream of LRP5. Consistent with the in vitro results described above, inhibition of GSK3 reduced the number of marrow adipocytes over this period. LiCl influences other signaling pathways besides Wnt, and GSK3 regulates many proteins besides β-catenin. However, the fact that LiCl stabilizes β-catenin and increases TCF-based reporter gene activity and expression of Wnt-responsive genes strongly supports a mechanism mediated by the Wnt/β-catenin signaling pathway (33).
Role of β-catenin at various stages of osteoblast development
During embryonic development, the level of β-catenin is increased in differentiating osteoblasts (34), and pharmacological and genetic approaches have indicated that Wnt signaling increases bone mass through a number of mechanisms including renewal of stem cells (35), stimulation of preosteoblast replication (13), induction of osteoblastogenesis (13), and inhibition of osteoblast and osteocyte apoptosis (Figure 2) (17). These variable results likely arise because Wnt/β-catenin signaling regulates bone development and accrual through different mechanisms at different stages of life. This concept is supported by the results of studies using mouse models in which targeted deletion of β-catenin occurs early or late in osteoblastogenesis. For example, dermo-Cre mice have a targeted deletion of β-catenin in mesenchymal precursors of chondrogenesis and osteogenesis (36, 37). These mice show a reduction in all relevant markers of osteogenesis and an absence of both endochondral and intramembranous bone at E18.5 in the developing embryo. Thus β-catenin is required for the early stages of osteoblastogenesis, and indeed its absence steers the fate of mesenchymal precursors toward chondrogenesis (34, 37).
To examine the importance of β-catenin later in osteoblast development, constitutively active β-catenin was overexpressed in osteoblasts expressing collagen αI-CRE (38). These mice manifest an osteopetrotic phenotype; however, no change in osteoblast activity or histomorphometric evidence of bone formation was observed. Instead, bone resorption and osteoclastogenesis were defective due to increased expression of osteoprotegerin, a decoy receptor for RANKL (38). On the other hand, targeted deletion of β-catenin in mature osteoblasts with collagen αI-Cre caused increased bone resorption and a marked increase in the number of tartrate-resistant acid phosphatase–positive (TRAP-positive) multinucleated osteoclasts due to reduced expression of osteoprotegerin (38). Consistent with these observations in mice, autosomal-dominant osteopetrosis type I patients with a gain-of-function T253I mutation in LRP5 have decreased numbers of small osteoclasts, although osteoclastogenesis in response to RANKL was normal in vitro (39). Finally, mice in which β-catenin has been deleted using osteocalcin-CRE and mice in which β-catenin has been activated with conditional Apc mutants provide further support for the finding that β-catenin regulates osteoblast differentiation. In addition, these mice also demonstrate that β-catenin regulates osteoclastogenesis through effects on expression of osteoprotegerin and RANKL (40).
A role for β-catenin in regulation of osteoclastogenesis has been clearly delineated through multiple genetic approaches; however, there is also considerable evidence that altering Wnt signaling upstream of β-catenin does not increase bone formation through altered resorption. For example, alterations in osteoclast variables were not observed in Lrp5–/–, Sfrp1–/–, or Wnt10b–/– mice or with LiCl treatment (13, 17, 24). One can speculate that complete loss or overexpression of β-catenin are more extreme perturbations of this signaling system than are normally observed through alterations of Wnt activity earlier in the pathway. In addition, constitutively active β-catenin may lack autoregulatory pathways triggered by the Wnt pathway.
Mechanisms whereby Wnt signaling regulates bone mass
As described above, Wnt signaling increases bone mass through diverse mechanisms. While effects on osteoblastogenesis and apoptosis have been studied in some mechanistic detail and will be elaborated upon here, this is not to diminish the potential importance of other mechanisms mentioned earlier that are less well studied, including renewal of stem cells (35), stimulation of preosteoblast replication (13), and enhancement of osteoblast activity (Figure 2) (13, 17).
Osteoblastogenesis versus adipogenesis.
There is considerable evidence for the existence of a mesenchymal stem cell that gives rise to both osteogenic and adipogenic cells, and in vitro and in vivo experimental models have provided compelling evidence for a reciprocal relationship between these cell lineages (41–43). For example, cultures of bone marrow stromal cells as well as immortalized clonal lines (e.g., ST2) are capable of both osteogenic and adipogenic differentiation, depending upon culture conditions. Furthermore, single cell clones from bone marrow can differentiate in vitro into either adipocytes or osteoblasts (44). In addition to signaling by Wnt/β-catenin, a number of factors influence the fate of these marrow-derived mesenchymal stem cells, including retinoic acid, BMPs, vitamin D3, glucocorticoids, notch, sonic hedgehog, parathyroid hormone, parathyroid hormone–related peptide, and PPARγ ligands (24, 43, 45–47). Indeed, Wnt signaling may be required for or even mediate a subset of effects of BMP, parathyroid hormone, and hedgehog on cell fate decisions toward osteoblastogenesis (25, 48).
Pharmacological and genetic treatments that activate Wnt/β-catenin signaling in mesenchymal precursors repress adipogenesis and stimulate osteoblastogenesis (Figure 2). In preadipocyte models expression of Wnt does not influence induction of the transcription factors CCAAT/enhancer binding protein β (C/EBPβ) and C/EBPδ, but Wnt signaling blocks induction of master adipogenic transcription factors C/EBPα and PPARγ (30). Suppression of Wnt/β-catenin signaling with dominant-negative TCFs or sFRPs stimulates spontaneous adipogenesis, indicating that endogenous Wnts inhibit preadipocyte differentiation (30, 31). Wnt signaling is initiated in part by Wnt10b. Its expression is high in dividing and confluent preadipocytes, and Wnt10b is rapidly suppressed upon induction of differentiation (30, 31). In addition, ectopic expression of Wnt10b stabilizes free cytosolic β-catenin and is a potent inhibitor of adipogenesis. Most conclusively, Wnt10b antisera promotes adipogenesis when added to media of 3T3-L1 preadipocytes. Interestingly, expression of Wnt5b is transiently induced during differentiation of 3T3-L1 cells, and adenoviral expression of Wnt5b causes a slight increase in adipogenesis, presumably due to destabilization of β-catenin (49, 50). Wnt5b may activate noncanonical Wnt signaling, which has been reported to antagonize Wnt/β-catenin signaling (51), or Wnt5b may compete with other Wnts for binding to frizzled receptors. Further work is required to assess whether Wnt5b inhibits osteoblastogenesis.
Mesenchymal precursors such as ST2 cells express low but biologically relevant levels of adipogenic transcription factors C/EBPα and PPARγ and osteoblast transcription factors such as runt-related transcription factor 2 (Runx2), msh homeobox homolog 2 (Msx2), distal-less homeobox 5 (Dlx5), and osterix (24). Expression of these 2 classes of transcription factors is maintained at low levels due to negative feedback, and imbalance leads to differentiation. For example, Msx2 binds to C/EBPα and inhibits its ability to transactivate the PPARγ promoter, and Msx2 represses adipogenesis (52, 53). Similarly, PPARγ binds to Runx2 and inhibits transactivation of the osteocalcin promoter, and activation of PPARγ represses osteoblastogenesis (54). Constitutive Wnt/β-catenin signaling favors expression of osteoblast genes at the expense of adipocyte genes (24). Wnt signaling could regulate the fate of mesenchymal precursors by repressing adipocyte transcription factors, stimulating osteoblast transcription factors, or both (Figure 2). Increased bone mass in Pparg+/– mice, increased osteogenesis in precursor cells from PPARγ-null mice, and decreased bone density following treatment of mice with a PPARγ agonist make this factor an attractive target (55–57). Indeed, suppression of PPARγ is required for Wnt10b to stimulate osteoblastogenesis (24). A recent report indicated that a transcriptional regulator, transcription coactivator with PDZ domain (TAZ), mediates the effects of BMP-2 on mesenchymal cell fate by inhibiting PPARγ activity while stimulating that of Runx2; however, the potential role of TAZ-mediated effects of Wnt/β-catenin signaling has not been reported (58).
Apoptosis.
Induction of bone accrual in mouse models with increased Wnt signaling is due in part to reduced apoptosis of osteoblasts and osteocytes (14, 17, 59). Wnt signaling inhibits apoptosis in response to a wide variety of cellular insults, including chemotherapeutic agents and serum deprivation (60–62). Prevention of apoptosis occurs in a wide variety of cell models, including mesenchymal precursors, preosteoblasts, and osteoblasts. While signaling by canonical Wnts appears to universally protect against apoptosis through mechanisms involving β-catenin and activation of PI3K/Akt, other mechanistic aspects are dependent on cell type. For example, in rat intestinal epithelial cells, induction of cyclooxygenase-2 and Wnt-induced secreted protein 1 (WISP-1), but not Bcl-2, are critical for repression of apoptosis caused by c-myc (60). In preadipocytes, increased production of insulin-like growth factors feeds back through an autocrine/paracrine mechanism to block apoptosis due to serum deprivation (61). Finally, in preosteoblasts, activation of Src, ERK, and Akt by Wnt3a is required for prevention of apoptosis. In this cell model, Wnt signaling induces expression of Bcl-2 through a process requiring active ERK (62). The mechanism or mechanisms by which Wnt/β-catenin signaling brings about an increase in the number of osteoblasts and osteocytes in vivo have yet to be determined.
Wnt signaling as cause and treatment for bone diseases
Historically, diseases of bone loss have been treated with agents that block bone resorption; however, this type of therapy stimulates only a modest increase in bone mineral density, and osteoporotic patients retain an elevated risk for fracture. With the recent introduction of teriparatide (human parathyroid hormone 1–34) into clinical practice, the potential to treat patients with an anabolic therapy was introduced (63). This drug is proven to decrease risk of vertebral and nonvertebral fractures in patients with postmenopausal osteoporosis (64). Pharmaceuticals that specifically activate the Wnt/β-catenin pathway in bone also hold tremendous promise as anabolic agents that may add to or complement treatment with teriparatide (3). Potential patient populations may include those with osteopenia or osteoporosis due to (a) causes unrelated to Wnt signaling and causes that do not impair effects of Wnt signaling on bone formation and (b) defects in Wnt signaling, as long as the drug acts downstream of the defect.
In any drug discovery program, issues of safety are paramount, especially for treatment of chronic disease of bone that will likely involve long-term therapy. This is particularly true for activators of Wnt/β-catenin signaling, since Wnts were first identified as insertion sites for mouse mammary tumor virus (64) and since mutations in APC and β-catenin that increase Wnt signaling are associated with colon and other cancers (65). When considering how best to target drug discovery in the Wnt/β-catenin pathway, identification and screening upstream in the pathway is more promising than targeting β-catenin and downstream events. For example, humans and mice with altered expression of LRP5, sFRP1, and Wnt10b all have alterations in bone mass with relatively few effects elsewhere (9, 17, 24). Side effects of drug therapy targeting the Wnt/β-catenin pathway are unknown. Functional haploinsufficiency for LRP5 may cause familial exudative vitreoretinopathy (66), and activation of Wnt10b signaling in fat decreases adiposity and increases skin thickness (67). In contrast, altering expression of β-catenin causes profound developmental effects (68, 69) and in bone regulates osteoblastogenesis, osteoclastogenesis, and probability of benign osteomatas (34, 38, 40). This underscores also that drugs should be selected for moderate effects on the pathway, as strong activators will have a much higher probability of effects in nontarget tissues. Despite the risks, the paucity of anabolic drugs for regulating bone mass and the compelling evidence demonstrating that Wnt/β-catenin signaling stimulates bone formation justify the considerable effort being put forth by the pharmaceutical industry to target this pathway.
Osteoporosis.
Osteoporosis is a prevalent skeletal disorder characterized by compromised bone strength and consequent increased risk of fractures. Postmenopausal women are at higher risk for developing osteoporosis and osteoporosis-related fractures. There are multiple etiologies for this complex metabolic bone disease, and, with the exception of osteoporosis-pseudoglioma syndrome due to mutations in LRP5 (9), it is unknown whether Wnt signaling plays a role. Interestingly, dexamethasone increases expression of Dkk1 and sFRP1 and represses Wnt/β-catenin signaling in human osteoblasts, suggesting a role for this pathway in glucocorticoid-induced osteoporosis (70–72). Further mechanistic work in human osteoporosis will be important to fully understand the relevance of Wnt signaling pathways in this disease.
To address the effects of increasing Wnt signaling on bone mass under normal and osteoporosis conditions, expression of Wnt10b was directed to bone marrow using the fatty acid–binding protein 4 (FABP4) promoter (24, 67). Wnt10b increased bone mineral density throughout the weight-bearing skeleton. Increased trabecular bone was observed throughout the endocortical compartment, with a 4-fold increase in bone volume fraction in the femoral distal metaphysis and improved material properties including strength. Although there was a trend toward decreased bone volume fraction and mineral density in ovariectomized FABP4-Wnt10b mice, these mice were protected due to their higher initial bone mass. Thus the potential health benefit from increasing Wnt/β-catenin signaling by Wnt10b is underscored by resistance to bone loss associated with estrogen depletion as well as aging (24).
Transgenic models such as FABP4-Wnt10b mice provide supporting evidence that Wnt signaling can impair development of osteoporosis; however, expression of Wnt10b in marrow of these transgenic mice is not inducible and may have altered bone development (24). Thus it is more desirable to evaluate approaches in skeletally mature animals with pharmacological activators of Wnt/β-catenin signaling. Recent work indicates that inhibition of GSK3 increases bone formation, density, and strength in an ovariectomized rat model (27). Ovariectomy of rats at 6 months of age leads to significant trabecular bone loss within 4 weeks, with a high turnover signature that resembles bone loss observed in postmenopausal women (73). Oral administration of LY603281-31-8, a GSK3α and -3β dual inhibitor, to ovariectomized rats for 2 months resulted in an increase in trabecular area, thickness, and number that was accompanied by improved trabecular connectivity as evidenced by decreased trabecular separation (27). Accordingly, biomechanical analysis found that LY603218-31-8 significantly improved vertebral strength, stiffness, and work to failure relative to ovariectomized controls. In addition, bone mineral density at both cancellous and cortical sites was significantly improved (27). The magnitude of responses to GSK3 inhibition was comparable to that observed with once-daily administration of teriparatide. In addition, genes reflecting enhanced osteoblast activity such as Runx2, collagen αI, collagen αV, bone sialoprotein, and biglycan were induced in trabecular bone obtained from distal femur. Increased bone mass was also observed with LiCl treatment of SAMP6 mice, which have premature osteoporosis due to impaired osteoblastogenesis (33). Taken together, these observations offer strong evidence for an increase in bone formation in response to inhibitors of GSK3 and suggest that activators of Wnt/β-catenin signaling show promise as therapeutic agents for osteoporosis.
Bone-related cancers.
Given the important role of Wnt signaling for bone development, it is possible that agents modifying this pathway could be of value to skeletal disorders other than osteoporosis. For example, Tian and coworkers recently analyzed the bone marrow of patients with newly diagnosed multiple myeloma and identified an increase in Dkk1 in the serum of these patients (74). Notably, the severity of the bone lesion was correlated with increased Dkk1 levels in these patients. The authors indicate that not all newly diagnosed patients show elevated levels of Dkk1 in their serum and that this finding may be restricted to a subset of end-stage severe multiple myeloma patients. The authors propose that Dkk1 produced by myeloma cells blocks differentiation of osteoblasts and promotes the early proliferation leading to reduced viability of pluripotent stem cells, later shifting the balance between osteoblasts and osteoclasts in favor of osteoclasts (74). This then facilitates the lytic lesions in bone that are a hallmark of this painful disease. Although expression of Dkk1 is limited to a subset of severe multiple myeloma patients, it is conceivable that early intervention with activators of Wnt/β-catenin signaling could slow development of bone lesions in these patients. Again, while activation of Wnt signaling may decrease some of the painful symptoms caused by excessive secretion of Dkk1, great care will need to be taken in targeting this pathway in patients because of the potential to increase progression of cancer.
Potential for Wnt signaling as a pharmacological target: “druggable” interventions?
Wnt/β-catenin signaling offers multiple steps that may be considered for pharmacological intervention, and some of these are highlighted in Figure 1 (i–v). Important features to consider in selecting drug discovery targets include the type of target (i.e., G protein–coupled receptors, enzymes, protein-protein interactions, or transcriptional factors), cellular location, role of the target in the pathway (central regulator versus fine tuning), and selectivity of the target for the pathway of interest. Historically, the best targets for small molecules are receptors or enzymes, particularly those at extracellular sites. Protein or antibody strategies can be useful to target protein-protein interactions extracellularly. Obviously, selectivity of the target for bone in this case is an important consideration to limit off-target tissue toxicities.
A review of the canonical Wnt signaling pathway suggests several interesting potential intervention points (Figure 1). (i) Availability of Wnt for binding to frizzled receptors is regulated by binding to Wnt inhibitory proteins such as sFRPs and WIF-1, and it is conceivable that small molecules or peptides could inhibit these interactions. Support for this approach comes from results of studies of Sfrp1–/– mice, which have increased bone formation without other obvious phenotypes (17). (ii) Availability of the LRP5 complex for Wnt/β-catenin signaling is also regulated by proteins from the Dkk and sclerostin families. Dkk1 interferes with canonical Wnt signaling in vertebrates by binding directly to LRP5. Simultaneously, Dkk interacts with a transmembrane protein, kremen, which causes internalization of the Dkk/LRP complex and a loss of Wnt signaling. Thus if interactions with Dkk1 were inhibited, more LRP5 would be available for activation of the Wnt pathway. Mutations in LRP5 that decrease affinity for Dkk and increase bone mass in humans suggest that this approach might be successful (10). In addition to Dkk, recent evidence suggests that sclerostin may also bind and inhibit signaling by LRP5/6 (75). Thus disruption of these interactions may also yield an increase in bone formation as evidenced by individuals with van Buchem disease (76). Protein therapeutic strategies offer the greatest chance of success at disrupting interactions between LRP and binding proteins, as there has been limited success at building small molecule inhibitors of protein-protein interactions. (iii) Since the frizzled receptor is a member of the G protein–coupled receptor family, which has been a highly successful family for generation of small molecule pharmacologic agents, it may be possible to foresee small molecule screening strategies having some degree of success. However, identification of small molecule mimics for type II G protein–coupled receptors has only been marginally successful (77). In addition, frizzled receptors are atypical members of the 7-transmembrane–spanning domain family of G protein–coupled receptors, and little is known about how to identify molecular agonists for this type of receptor. In addition, identities of those frizzled receptors that influence bone mass are unknown. (iv) Wnt/β-catenin signaling stabilizes β-catenin by inhibiting GSK3, and a variety of small molecule inhibitors increase osteoblastogenesis in vitro and bone formation in vivo. Although characterization of small molecule inhibitors of GSK3 is still underway, safety issues have not been reported for LiCl (78), which is widely used by adult patients to treat bipolar disorder. (v) In looking at targets further downstream of GSK3 (Figure 1), the degradation of β-catenin is mediated by the ubiquitin/proteasome pathway, and inhibiting these enzymes increases bone formation (79). However, specificity of these protease inhibitors remains a challenge in the area of pharmaceutical intervention. Although speculative, interaction of β-catenin/TCF with transcriptional coactivators is increased by acetylation of β-catenin. Thus histone deacetylase inhibitors could conceivably be used to increase expression of specific genes pertinent to bone cells, although specificity is likely to be an issue (7).
Safety considerations in targeting the Wnt pathway.
Treatment of chronic disorders such as osteoporosis require heightened awareness of safety considerations, and given the important role of the Wnt pathways in development, the toxicologic potential of molecules modulating the Wnt pathway should be given thorough consideration. One area of speculative concern with regard to targeting of drugs to the Wnt pathway has been induction of cancer. While to date no reports connecting human tumors to mutation or dysregulation of genes encoding Wnt ligands or receptors have been made, certain components within the Wnt pathway have been implicated. For example, nuclear β-catenin functions to maintain the proliferative potential of keratinocytes in culture (80). A more direct relationship with human tumors is suggested by elevation of β-catenin levels in various cancers (81), including some types of skin cancer, as a moderate increase of β-catenin nuclear staining was observed with basal cell carcinomas (82). Mutations in APC or AXIN2 leading to accumulation of β-catenin have also been associated with colorectal cancer, as have activating mutations in β-catenin (83–87).
In addition to β-catenin, other players in the canonical Wnt signaling cascade have been linked to tumorigenesis. Inhibition of GSK3 results in increased cyclin D1, cyclin E, and c-Myc, and overexpression of these cell cycle regulators has been linked with tumor cell formation (88, 89), leading to concern that long-term inhibition of GSK3 may increase the risk of carcinogenesis. This of course will be an important safety consideration for development of GSK3 inhibitors. However, it should be possible to generate inhibitors of this enzyme without a significant cancer risk, as long-term use of the nonspecific GSK3 inhibitor lithium is not known to be associated with increased risk of cancer in bipolar patients (78). Furthermore, activation of GSK3 by histone deacetylase inhibitors has been associated with targeting tumor cells for elimination by natural killer cells (90). As molecules emerge from ongoing drug discovery efforts that target aspects of the Wnt signaling pathway, attention to tumor potential and other toxicities will be of paramount importance. Hopefully the worldwide efforts currently underway to target Wnt/β-catenin signaling will be successful and generate therapeutics that positively impact human skeletal health.
Footnotes
Nonstandard abbreviations used: APC, adenomatous polyposis coli; BMP, bone morphogenic protein; C/EBP, CCAAT/enhancer binding protein; Dkk, Dickkopf; GSK, glycogen synthase kinase; LEF, lymphoid enhancer binding factor; LiCl, lithium chloride; LRP, low-density lipoprotein receptor–related protein; RANKL, receptor activator of NF-κB ligand; Runx2, runt-related transcription factor 2; sFRP, secreted frizzled-related protein; TCF, T cell factor; WIF-1, Wnt inhibitory factor 1; WISP, Wnt-induced secreted protein.
Conflict of interest: V. Krishnan and H.U. Bryant own stock in Eli Lilly & Co. O.A. MacDougald has declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 116:1202–1209 (2006). doi:10.1172/JCI28551.
References
- 1.Cadigan K.M., Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 2.Westendorf J.J., Kahler R.A., Schroeder T.M. Wnt signaling in osteoblasts and bone diseases. . Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 3.Rawadi G., Roman-Roman S. Wnt signalling pathway: a new target for the treatment of osteoporosis. Expert Opin. Ther. Targets. 2005;9:1063–1077. doi: 10.1517/14728222.9.5.1063. [DOI] [PubMed] [Google Scholar]
- 4.Logan C.Y., Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 5.Moon R.T., Kohn A.D., De Ferrari G.V., Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat. Rev. Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 6.Hay E., et al. Interaction between LRP5 and Frat1 mediates the activation of the Wnt canonical pathway. J. Biol. Chem. 2005;280:13616–13623. doi: 10.1074/jbc.M411999200. [DOI] [PubMed] [Google Scholar]
- 7.Levy L., et al. Acetylation of beta-catenin by p300 regulates beta-catenin-Tcf4 interaction. Mol. Cell. Biol. 2004;24:3404–3414. doi: 10.1128/MCB.24.8.3404-3414.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao B., et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. . Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 9.Gong Y., et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 10.Ai M., Holmen S.L., Van Hul W., Williams B.O., Warman M.L. Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol. Cell. Biol. 2005;25:4946–4955. doi: 10.1128/MCB.25.12.4946-4955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyden L.M., et al. High bone density due to a mutation in LDL-receptor-related protein 5. . N. Engl. J. Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 12.Van Wesenbeeck L., et al. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am. J. Hum. Genet. 2003;72:763–771. doi: 10.1086/368277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato M., et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babij P., et al. High bone mass in mice expressing a mutant LRP5 gene. J. Bone Miner. Res. 2003;18:960–974. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- 15.Holmen S.L., et al. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J. Bone Miner. Res. 2004;19:2033–2040. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- 16.Li X., et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 17.Bodine P.V., et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol. Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 18.Li X., et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat. Genet. 2005;37:945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- 19.Hausler K.D., et al. Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation. J. Bone Miner. Res. 2004;19:1873–1881. doi: 10.1359/JBMR.040807. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez J., et al. SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat. Neurosci. 2005;8:1301–1309. doi: 10.1038/nn1547. [DOI] [PubMed] [Google Scholar]
- 21.Vaes B.L., et al. Comprehensive microarray analysis of bone morphogenetic protein 2-induced osteoblast differentiation resulting in the identification of novel markers for bone development. . J. Bone Miner. Res. 2002;17:2106–2118. doi: 10.1359/jbmr.2002.17.12.2106. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y., et al. The LRP5 high-bone-mass G171V mutation disrupts LRP5 interaction with Mesd. Mol. Cell. Biol. 2004;24:4677–4684. doi: 10.1128/MCB.24.11.4677-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reya T., et al. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity. 2000;13:15–24. doi: 10.1016/s1074-7613(00)00004-2. [DOI] [PubMed] [Google Scholar]
- 24.Bennett C.N., et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rawadi G., Vayssiere B., Dunn F., Baron R., Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J. Bone Miner. Res. 2003;18:1842–1853. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 26.Stambolic V., Ruel L., Woodgett J.R. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 27.Kulkarni N.E., et al. Orally bioavailable GSK-3alpha/beta dual inhibitor increases markers of cellular differentiation in vitro and bone mass in vivo. . J. Bone Miner. Res. 2006 doi: 10.1359/jbmr.060316. In press. [DOI] [PubMed] [Google Scholar]
- 28.Jackson A., et al. Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone. 2005;36:585–598. doi: 10.1016/j.bone.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Gregory C.A., et al. How wnt signaling affects bone repair by mesenchymal stem cells from the bone marrow. Ann. N. Y. Acad. Sci. 2005;1049:97–106. doi: 10.1196/annals.1334.010. [DOI] [PubMed] [Google Scholar]
- 30.Ross S.E., et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 31.Bennett C.N., et al. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 32.Nuttall M.E., Gimble J.M. Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone. 2000;27:177–184. doi: 10.1016/s8756-3282(00)00317-3. [DOI] [PubMed] [Google Scholar]
- 33.Clement-Lacroix P., et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17406–17411. doi: 10.1073/pnas.0505259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Day T.F., Guo X., Garrett-Beal L., Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Reya T., Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 36.Hu H., et al. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- 37.Hill T.P., Spater D., Taketo M.M., Birchmeier W., Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev. Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Glass D.A., 2nd, et al. 8:751–764. [Google Scholar]
- 39.Henriksen K., et al. Osteoclasts from patients with autosomal dominant osteopetrosis type I caused by a T253I mutation in low-density lipoprotein receptor-related protein 5 are normal in vitro, but have decreased resorption capacity in vivo. . Am. J. Pathol. 2005;167:1341–1348. doi: 10.1016/S0002-9440(10)61221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holmen S.L., et al. Essential role of beta-catenin in postnatal bone acquisition. J. Biol. Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 41.Sottile V., Halleux C., Bassilana F., Keller H., Seuwen K. Stem cell characteristics of human trabecular bone-derived cells. Bone. 2002;30:699–704. doi: 10.1016/s8756-3282(02)00674-9. [DOI] [PubMed] [Google Scholar]
- 42.Pereira R.C., Delany A.M., Canalis E. Effects of cortisol and bone morphogenetic protein-2 on stromal cell differentiation: correlation with CCAAT-enhancer binding protein expression. Bone. 2002;30:685–691. doi: 10.1016/s8756-3282(02)00687-7. [DOI] [PubMed] [Google Scholar]
- 43.Lecka-Czernik B., et al. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143:2376–2384. doi: 10.1210/endo.143.6.8834. [DOI] [PubMed] [Google Scholar]
- 44.Park S.R., Oreffo R.O., Triffitt J.T. Interconversion potential of cloned human marrow adipocytes in vitro. Bone. 1999;24:549–554. doi: 10.1016/s8756-3282(99)00084-8. [DOI] [PubMed] [Google Scholar]
- 45.Canalis E., Delany A.M. Mechanisms of glucocorticoid action in bone. Ann. N. Y. Acad. Sci. 2002;966:73–81. doi: 10.1111/j.1749-6632.2002.tb04204.x. [DOI] [PubMed] [Google Scholar]
- 46.Sciaudone M., Gazzerro E., Priest L., Delany A.M., Canalis E. Notch 1 impairs osteoblastic cell differentiation. Endocrinology. 2003;144:5631–5639. doi: 10.1210/en.2003-0463. [DOI] [PubMed] [Google Scholar]
- 47.Chan G.K., et al. Parathyroid hormone-related peptide interacts with bone morphogenetic protein 2 to increase osteoblastogenesis and decrease adipogenesis in pluripotent C3H10T 1/2 mesenchymal cells. Endocrinology. 2003;144:5511–5520. doi: 10.1210/en.2003-0273. [DOI] [PubMed] [Google Scholar]
- 48.Kulkarni N.H., et al. Effects of parathyroid hormone on Wnt signaling pathway in bone. J. Cell. Biochem. 2005;95:1178–1190. doi: 10.1002/jcb.20506. [DOI] [PubMed] [Google Scholar]
- 49.Kanazawa A., et al. Wnt5b partially inhibits canonical Wnt/beta-catenin signaling pathway and promotes adipogenesis in 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2005;330:505–510. doi: 10.1016/j.bbrc.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Kanazawa A., et al. Association of the gene encoding wingless-type mammary tumor virus integration-site family member 5B (WNT5B) with type 2 diabetes. Am. J. Hum. Genet. 2004;75:832–843. doi: 10.1086/425340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Topol L., et al. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J. Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng S.L., Shao J.S., Charlton-Kachigian N., Loewy A.P., Towler D.A. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. . J. Biol. Chem. 2003;278:45969–45977. doi: 10.1074/jbc.M306972200. [DOI] [PubMed] [Google Scholar]
- 53.Ichida F., et al. Reciprocal roles of MSX2 in regulation of osteoblast and adipocyte differentiation. . J. Biol. Chem. 2004;279:34015–34022. doi: 10.1074/jbc.M403621200. [DOI] [PubMed] [Google Scholar]
- 54.Jeon M.J., et al. Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J. Biol. Chem. 2003;278:23270–23277. doi: 10.1074/jbc.M211610200. [DOI] [PubMed] [Google Scholar]
- 55.Akune T., et al. PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Invest. 2004;113:846–855. doi:10.1172/JCI200419900. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ali A.A., et al. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146:1226–1235. doi: 10.1210/en.2004-0735. [DOI] [PubMed] [Google Scholar]
- 57.Kawaguchi H., et al. Distinct effects of PPARgamma insufficiency on bone marrow cells, osteoblasts, and osteoclastic cells. J. Bone Miner. Metab. 2005;23:275–279. doi: 10.1007/s00774-005-0599-2. [DOI] [PubMed] [Google Scholar]
- 58.Hong J.H., et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 59.Bodine P.V., et al. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J. Cell. Biochem. 2005;96:1212–1230. doi: 10.1002/jcb.20599. [DOI] [PubMed] [Google Scholar]
- 60.You Z., et al. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J. Cell Biol. 2002;157:429–440. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Longo K.A., et al. Wnt signaling protects 3T3-L1 preadipocytes from apoptosis through induction of insulin-like growth factors. J. Biol. Chem. 2002;277:38239–38244. doi: 10.1074/jbc.M206402200. [DOI] [PubMed] [Google Scholar]
- 62.Almeida M., Han L., Bellido T., Manolagas S.C., Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. . J. Biol. Chem. 2005;280:41342–41351. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- 63.Neer R.M., et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. . N. Engl. J. Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 64.Nusse R., Varmus H.E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 65.Bienz M., Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 66.Toomes C., et al. Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am. J. Hum. Genet. 2004;74:721–730. doi: 10.1086/383202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Longo K.A., et al. Wnt10b inhibits development of white and brown adipose tissues. J. Biol. Chem. 2004;279:35503–35509. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- 68.Haegel H., et al. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 69.Huelsken J., et al. Requirement for beta-catenin in anterior-posterior axis formation in mice. . J. Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohnaka K., Taniguchi H., Kawate H., Nawata H., Takayanagi R. Glucocorticoid enhances the expression of dickkopf-1 in human osteoblasts: novel mechanism of glucocorticoid-induced osteoporosis. Biochem. Biophys. Res. Commun. 2004;318:259–264. doi: 10.1016/j.bbrc.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 71.Ohnaka K., Tanabe M., Kawate H., Nawata H., Takayanagi R. Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem. Biophys. Res. Commun. 2005;329:177–181. doi: 10.1016/j.bbrc.2005.01.117. [DOI] [PubMed] [Google Scholar]
- 72.Wang F.S., et al. Secreted frizzled-related protein 1 modulates glucocorticoid attenuation of osteogenic activities and bone mass. Endocrinology. 2005;146:2415–2423. doi: 10.1210/en.2004-1050. [DOI] [PubMed] [Google Scholar]
- 73.Kalu D.N. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15:175–191. doi: 10.1016/0169-6009(91)90124-i. [DOI] [PubMed] [Google Scholar]
- 74.Tian E., et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 75.Semenov M., Tamai K., He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. . J. Biol. Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 76.Brunkow M.E., et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am. J. Hum. Genet. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bagger Y.Z., et al. Oral salmon calcitonin induced suppression of urinary collagen type II degradation in postmenopausal women: a new potential treatment of osteoarthritis. Bone. 2005;37:425–430. doi: 10.1016/j.bone.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 78.Cohen Y., Chetrit A., Cohen Y., Sirota P., Modan B. Cancer morbidity in psychiatric patients: influence of lithium carbonate treatment. Med. Oncol. 1998;15:32–36. doi: 10.1007/BF02787342. [DOI] [PubMed] [Google Scholar]
- 79.Garrett I.R., et al. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J. Clin. Invest. 2003;111:1771–1782. doi: 10.1172/JCI200316198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu A.J., Watt F.M. Beta-catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development. 1999;126:2285–2298. doi: 10.1242/dev.126.10.2285. [DOI] [PubMed] [Google Scholar]
- 81.Karim R., Tse G., Putti T., Scolyer R., Lee S. The significance of the Wnt pathway in the pathology of human cancers. Pathology. 2004;36:120–128. doi: 10.1080/00313020410001671957. [DOI] [PubMed] [Google Scholar]
- 82.Doglioni C., et al. Alterations of beta-catenin pathway in non-melanoma skin tumors: loss of alpha-ABC nuclear reactivity correlates with the presence of beta-catenin gene mutation. Am. J. Pathol. 2003;163:2277–2287. doi: 10.1016/s0002-9440(10)63585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lustig B., Behrens J. The Wnt signaling pathway and its role in tumor development. . J. Cancer Res. Clin. Oncol. 2003;129:199–221. doi: 10.1007/s00432-003-0431-0. [DOI] [PubMed] [Google Scholar]
- 84.Rubinfeld B., et al. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 85.Korinek V., et al. Constitutive transcriptional activation by a β-catenin-TCF complex in APC–/– colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 86.Liu W., et al. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat. Genet. 2000;26:146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- 87.Morin P.J., et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 88.Dong J., et al. Role of glycogen synthase kinase 3beta in rapamycin-mediated cell cycle regulation and chemosensitivity. Cancer Res. 2005;65:1961–1972. doi: 10.1158/0008-5472.CAN-04-2501. [DOI] [PubMed] [Google Scholar]
- 89.van Noort M., Meeldijk J., van der Zee R., Destree O., Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J. Biol. Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 90.Skov S., et al. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res. 2005;65:11136–11145. doi: 10.1158/0008-5472.CAN-05-0599. [DOI] [PubMed] [Google Scholar]