Abstract
Odor aversion learning is often potentiated in the presence of flavor stimuli. Establishment of an aversion to an odor is greater when an odor + flavor compound is paired with illness than when the odor alone is paired with illness. Holland (1983) showed that under some circumstances auditory or olfactory stimuli previously paired with flavors may also potentiate odor aversion learning. The present experiments examined limitations on this representation-mediated potentiation of aversion learning. The results indicated that CSs that activate representations of potentiating cues are themselves immune to potentiation by other CS-activated representations, but remain susceptible to potentiation by their real stimulus associates.
As a result of associative learning, signals for important events may acquire the ability to substitute for their referents in the control of behavior. For example, Pavlovian conditioned stimuli (CSs) often come to control conditioned responses (CRs) similar in form to those originally elicited by the unconditioned stimuli (USs) with which they were paired (e.g., Mackintosh, 1974). Similarly, CSs often acquire reinforcement properties appropriate to their USs, serving as conditioned reinforcers or punishers (e.g., Fantino, 1977; Heth, 1976). In many cases this substitution can be quite specific to particular US properties. For example, Holland and Forbes (1982a) trained rats to respond to a tone when it was preceded by a peppermint-flavored sucrose solution but not when it was preceded by a wintergreen-flavored sucrose solution. In a test session, two distinct visual stimuli that previously had been paired with the flavored solutions successfully substituted for those flavors in the control of responding to the tone.
A common account for such findings is that associative learning endows a CS with the ability to evoke a memorial representation of the US (e.g., Rescorla, 1988). This associatively-activated US representation (Hall, 1996) may then substitute for the US itself in a variety of functions. Of special interest are observations that a CS-activated representation of a food US can substitute for that food in the acquisition or extinction of an aversion to that food (Hall, 1996; Holland, 1981). For example, Holland (1981, 1990) paired one CS with one flavored food and another CS with another flavored food. Later, one of the CSs was paired with the toxin lithium chloride (LiCl), in the absence of the foods themselves. Subsequent food consumption tests in the absence of the CSs showed that the rats had acquired an aversion specific to the food whose CS had been paired with toxin. Likewise, Holland and Forbes (1982b) first separately paired two auditory CSs with two distinct flavored solutions. After aversions were established to each of those solutions by pairing them with LiCl, one of the auditory CSs was repeatedly presented in the absence of the solutions or toxin. Consumption tests showed that these presentations of the CS alone partially extinguished the aversion to the solution originally signaled by that event. Thus, in both cases, an associatively-activated event representation substituted for a real event in the acquisition of new learning about that event.
The experiments reported here explored a limitation in the ability of associatively-activated event representations to substitute for their referents. Extending the observations just described, Holland (1983) reported that auditory or olfactory CSs paired with flavored solutions could substitute for those flavors in the potentiation of odor aversion learning. Many investigators have noted that the presence of a gustatory stimulus can potentiate the establishment of an odor aversion (e.g., Durlach & Rescorla, 1980; Palermino et al., 1980; Rusiniak et al., 1979). That is, pairings of an odor + taste compound with illness are more effective in establishing an aversion to a solution containing that odor than simple odor-illness pairings. Holland (1983, Exp. 3) found that a tone previously paired with a flavor similarly potentiated the conditioning of an aversion to an odor paired with LiCl, whereas a tone that had not been paired with a flavor overshadowed such odor aversion learning. However, some earlier studies (e.g., Durlach & Rescorla, 1980) yielded results that appeared to conflict with Holland’s data. In those studies, although rats that received pairings of an odor + taste compound with illness showed potentiated odor aversion learning, rats that received odor + taste compound presentations prior to simple odor-illness pairings failed to exhibit such potentiation. By Holland’s (1983) logic, the initial odor + taste pairings should have given the odor the ability to activate a representation of the taste, which might then serve to potentiate aversion learning to the odor when it was subsequently paired with illness.
To deal with this discrepancy in the ability of an associatively-activated flavor representation to potentiate odor aversion learning, Holland (1983) suggested that a representation activated by a CS may not be able to modulate conditioning to that CS itself. To test this limitation, Holland (1983, Exp. 4) paired a compound of two odors (O1O2) with toxin, after prior pairings of one of those odors, O1, with a flavor. A subsequent test of consumption of the two individual odors showed potentiation of aversion learning to O2, but not to O1. These rats consumed significantly less O2 than O1. Similarly, relative to control rats that received separate O1-toxin and O2-toxin pairings, these rats showed less consumption of O2, but similar consumption of O1. Thus, although prior O1-flavor pairings allowed a flavor representation activated by O1 to potentiate aversion learning to O2, that representation failed to potentiate learning about the odor cue that activated it.
The experiments described here evaluated this hypothesis further, and considered alternatives. For example, in Holland’s (1983, Exp. 4) study, the failure of potentiation of learning about O1 may indicate a more general limitation, that while a CS is activating a representation, it is immune to potentiation by any such representation. From this perspective, O1’s inability to potentiate conditioning of itself is secondary to its inability to be potentiated at all. Furthermore, this immunity might extend not only to potentiation by associatively-activated flavor representations, but also to potentiation by real flavors themselves. Characterizing the interactions of associatively-activated event representations with real events is an important step in understanding the mechanisms by which internally-generated events can influence learning and behavior.
Experiment 1 replicated Holland’s (1983, Exp. 4) observations of the selective potentiation of odor aversion learning by odor-activated flavor representations and compared such potentiation with that produced by the real flavors associated with those odors. Experiments 2 and 3 examined odor aversion learning to each element of a two-odor compound after prior pairings of one, both, or neither of those odors with flavors. Experiment 4 extended the observations of Experiments 1–3 to a three-odor compound.
Experiment 1
The primary purpose of Experiment 1 was to extend Holland’s (1983, Exp. 4) demonstration of selectivity in representation-mediated potentiation of odor aversion learning. Notably, we compared the potentiation produced by a flavor representation with that produced by the flavor itself. It is important to determine whether the selectivity observed by Holland (1983, Exp. 4) is a unique property of odor-activated flavor representations, or is shared with the flavor itself. Prior association of an odor with a flavor may prevent the potentiation of odor aversion learning to that odor by either a flavor or its CS-activated representation. It is possible that while a CS is activating a representation of another event, it is immune to modulation by other CSs (real or associatively-activated). In addition, Experiment 1 provided a control procedure different from the one used by Holland (1983, Exp. 4). In that earlier study, the occurrence of potentiation in the experimental rats was evaluated by comparison with the performance of rats that received the same preexposure contingencies as the experimental rats (O1-flavor pairings and separate O2 presentations), but separate pairings of each odor with toxin in the aversion learning phase. In Experiment 1 of the present series, the comparison group received pairings of the O1O2 odor compound with toxin but no prior pairings of either odor with flavor. This procedure was intended to insure that the asymmetry in potentiation observed by Holland (1983, Exp. 4) was not due solely to interactions occurring within odor compounds at the time of aversion learning, regardless of the history of their elements.
Methods
Subjects and apparatus
The subjects were 28 male Sprague-Dawley rats (Charles River Laboratories) about 100 days old at the start of the experiment. They were housed in individual 17.8 × 25.4 × 17.8 cm stainless steel suspended cages, in a vivarium with the lights on from 6:00 a.m. to 8:00 p.m. Food was available ad lib, but access to fluids was restricted to daily 10 min experimental flavored liquid presentations at 8:00 a.m. (usually limited to 10 ml, see below) and 10 min access to unlimited amounts of unflavored tap water at 3:00 p.m.
Experiment 1 was conducted entirely in the home cages over 18 consecutive days. Fluids were presented in standard water bottles with drinking tubes that extended 3 cm into the cage, about 4 cm above the cage floor.
Procedure
An outline of the procedures of Experiment 1 is presented in Table 1. During their morning trials, the rats in Groups Con (n = 8) and Real2 (n = 6) received four presentations each of a flavored (F) sucrose (0.1 M) solution, and the odor solutions almond (1.0% v/v Durkee brand flavoring) and vanilla (1.0% v/v Durkee brand flavoring), randomly intermingled across the 12 preexposure days. On these days, the rats in Groups Real1 (n = 6) and Rep (n = 8) received 4 presentations of a compound (FO1) of the Flavor and one of those odors (mixed to preserve the concentrations of each), 4 presentations of the other odor (O2) alone, and 4 presentations of unflavored tap water. The identities of odors O1 and O2 were counterbalanced (almond or vanilla). In all groups, each of these presentations was limited to 10 ml to insure equal exposure to all of the fluids. Unpublished data from our laboratory indicate that rats initially prefer the sucrose + odor compounds to the odors alone, and prefer the odors alone to the O1O2 odor compound used in the next phase.
Table 1.
Outline of Experimental Procedures
| Experiment 1 | |||
|---|---|---|---|
| Pre-exposure | Conditioning | Test | |
| Group Real1: | O1F, O2 | FO1O2→toxin | O1, O2 |
| Group Real2: | O1, F, O2 | FO1O2→toxin | O1, O2 |
| Group Rep: | O1F, O2 | O1O2→toxin | O1, O2 |
| Group Con: | O1, F, O2 | O1O2→toxin | O1, O2 |
| Experiment 2 | |||
| Pre-exposure | Conditioning | Test | |
| Group Con: | O1, (F1), O2, (F2) | O1O2→toxin | O1, O2 |
| Group One: | O1F1, O2, (F2) | O1O2→toxin | O1, O2 |
| Group Two: | O1F1, O2F2 | O1O2→toxin | O1, O2 |
| Experiment 3 | |||
| Pre-exposure | Conditioning | Test | |
| Group Con: | (O1), F1, (O2), F2 | F1F2→toxin | F1, F2 |
| Group One: | O1F1, (O2), F2 | F1F2→toxin | F1, F2 |
| Group Two: | O1F1, O2F2 | F1F2→toxin | F1, F2 |
| Experiment 4 | |||
| Pre-exposure | Conditioning | Test | |
| Group Con: | O1, F1, O2, F2 | O1O2O3→toxin | O3, O2, O1 |
| Group One: | O1F1, O2, F2 | O1O2O3→toxin | O3, O2, O1 |
| Group Two: | O1F1, O2F2 | O1O2O3→toxin | O3, O2, O1 |
Notes. F = sucrose flavor, F1 and F2 = sucrose and saline flavors, counterbalanced, O1 and O2 = almond and vanilla odors, counterbalanced; O3 = lemon odor. In Experiments 2 and 3, half of the rats in Groups Con and One also received the trials noted parenthetically. In Experiments 1–3, test order was counterbalanced, whereas in Experiment 4 the test order was that shown in the table.
Although it is not critical to the present studies, other unpublished data from this laboratory support the claim that the almond and vanilla solutions are treated as odors and the sucrose primarily as a taste. Presentation of almond or vanilla solutions by saturating a disk around the drinking tube but outside the rat’s cage (and hence orally inaccessible) results in similar aversions, but no such transfer was observed with sucrose-saturated disks (but see Capaldi, Hunter, & Privitera, 2004).
On the next morning, all rats received a single aversion conditioning trial, in which 10 min access to 10 ml of either an FO1O2 compound of the flavor and both odors (Groups Real1 and Real2) or an O1O2 compound of both odors only (Groups Rep and Con) was followed by an injection of 5 ml/kg of 0.6-M LiCl. All solutions were mixed to preserve the concentrations of the individual elements. In the next day, all rats received 10 min access to unlimited amounts of unflavored tap water on both the morning and afternoon fluid presentations, to ensure that all rats had recovered from any dehydrating effects of the toxin injections.
Finally, over the next four mornings, each rat received two consumption test presentations of each of the two odors, O1 and O2. Each test comprised 10 min unlimited access to one of the odor solutions, which were presented in the order 1221 in half of the rats in each group and 2112 in the other half.
Data analysis
The amounts of consumption were determined by weighing the solution bottles before and after testing periods. The consumption data were subjected to standard analysis of variance (ANOVA), followed by post-hoc multiple comparisons, using the Tukey honest significant difference procedure. The level of statistical significance adopted was p <.05.
Results and discussion
Rats consumed all of the available fluids (limited to 10 ml) in the preexposure and conditioning phases of Experiment 1. The data from the consumption tests are shown in Figure 1. First, sucrose itself potentiated aversion learning to both elements of the odor compound, regardless of prior pairings of O1 with F. The rats in Groups Real1 and Real2 (combined to form Group Real in Figure 1), in which a flavor accompanied the O1O2 odor compound on the conditioning trial, showed less consumption of both O1 and O2 than did the rats in Group Con, which received only the odor compound paired with toxin. The performance of rats in Groups Real1 (which received O1F compound presentations prior to aversion training) and Real2 (which received separate O1 and F presentations prior to aversion training) did not differ significantly (6.7 ± 1.0 and 7.4 ± 2.4 ml of O1 and O2, respectively, in Group Real1 and 8.2 ± 1.5 and 10.3 ±1.9 ml in Group Real2). Second, a representation of sucrose activated by O1 potentiated aversion learning to O2, but not learning to O1. The rats in Group Rep, in which O1 had been preexposed in compound with sucrose prior to the O1O2→toxin trial, consumed less O2 than O1, and less O2 than the rats in Group Con.
Figure 1.
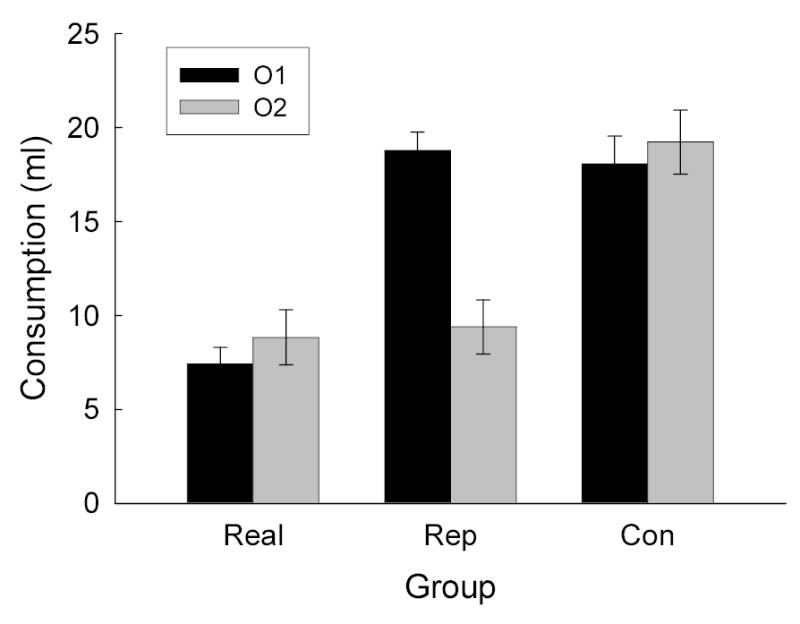
Consumption of the two odor solutions (O1 and O2) in the test sessions of Experiment 1. See Table 1 for experimental treatments of the groups and stimuli.
A preliminary 4 X 2 X 2 X 2 X2 groups X odor counterbalancing condition X test stimulus (O1 or O2) X test order (1221 or 2112) X test (first or second presentation of each odor) ANOVA showed no significant effects or interactions of odor counterbalancing, test order or test, Fs < 1. Consequently, for subsequent analyses, the consumption scores of both tests were averaged, and the counterbalancing and test order variables were dropped. Next, a groups X test stimulus ANOVA was conducted for Groups Real1 and Real2 alone. This analysis revealed no effects of group or group X test stimulus interaction, Fs < 1, so Groups Real1 and Real2 were combined to form Group Real.
A groups (Real, Rep or Con) X test stimulus (O1 or O2) ANOVA was then performed. Both the effect of groups, F(2, 25) = 22.20, and of test stimulus, F(1, 25) = 8.84, were significant, as was their interaction, F(2, 25) = 20.68. Post-hoc tests showed that consumption of O1 and O2 in Group Real and consumption of O2 in Group Rep was each reliably lower than consumption in each of the other three conditions. No other comparisons were significantly different.
Several aspects of these results are notable. First, in Group Rep, odor aversion learning was potentiated by an associatively-activated flavor representation. Second, that representation-mediated potentiation was as large as the potentiation produced by the flavor itself. In this regard, is worth pointing out that Holland (1983, Exp. 3), found that potentiation of conditioning of an aversion to a single odor by a flavor representation activated by a tone CS was nearly as large as potentiation by the flavor itself. Third, consistent with the findings of Holland (1983, Exp. 4), although an odor previously paired with a flavor potentiated the establishment of an aversion to another odor, it did not potentiate aversion conditioning to itself. Fourth, the presence of a real flavor during conditioning of the odor compound potentiated conditioning to both odors, regardless of whether one of them had been paired with that flavor (Group Real1) or not (Group Real2). Thus, the associative activation of a flavor representation by an odor does not prevent that odor from benefiting from the presence of a real flavor during aversion conditioning. Instead, that activation only reduces the susceptibility of the odor to potentiation by an associatively activated flavor representation. It should be noted however that in Group Real1, the real flavor that successfully potentiated odor aversion learning to the two odors was itself an associate of one of those odors. Thus, it remains possible that the associative activation of a flavor representation by an odor might prevent that odor from benefiting from the presence of a different, nonassociated real flavor during aversion conditioning. We do not address that question in this article.
The observation that the consumption patterns of Groups Real1 and Real2 did not differ argues against an alternate account for the performance of Group Rep based on the conditioning of odor preferences in the preexposure period. A number of researchers have reported that rats increase their preference for flavors/odors that are paired with sucrose solutions (e.g., Harris, Shand, Carroll, & Westbrook, 2004). By this view, in the present study, the rats in Group Rep may have acquired a preference for O1 because it was paired with sucrose. That preference might compete with the acquisition or expression of an aversion to O1, resulting in greater consumption of the O1 solution than of the O2 solution. However, in that case, Group Real1, which also received O1+ sucrose pairings, would be also expected to show a preference for O1, which was not observed here.
Experiment 2
In Experiment 1, although an odor-activated flavor representation potentiated aversion conditioning to another simultaneously-present odor, it did not potentiate the establishment of an aversion to the odor that activated that representation. Experiments 2 and 3 considered whether that reduction is confined to the effects of representations activated by the target CS itself, or extends to representations activated by other CSs. The associative strength of a CS may only be immune to modification by an event representation activated by that CS itself. This limitation would, for example, prevent “self-reinforcement” of a CS by its own associatively-activated representation of the US during extinction, but would permit the formation of associations between another stimulus added during extinction and the US, a mechanism suggested for second-order conditioning (Davey & McKenna, 1983; Konorski, 1948). Alternately, the associative strength of CSs that are themselves activating event representations may be immune to modulation by associatively-activated event representations, regardless of their source.
Experiment 2 used procedures similar to those of Experiment 1 to distinguish between these alternatives. The treatments of Groups One and Con were similar to those of Groups Rep and Con of Experiment 1, respectively. In Group Two, both odors were paired with flavors (O1F1, O2F2) prior to compound odor aversion conditioning (O1O2→toxin). If O1 were only immune to potentiation by F1, and O2 only immune to potentiation by F2 (the representations each activates), then aversion learning to O1 could still be potentiated by the F2 representation activated by O2, and aversion learning to O2 could be potentiated by the F1 representation activated by F1. Thus, both O1 and O2 would show potentiated learning relative to learning in Group Con, which received O1O2→toxin pairings without odor-flavor pairings. By contrast, if CSs that are themselves activating event representations are immune to modulation by any associatively-activated event representations, regardless of their source, then neither O1 not O2 would show potentiated learning relative to controls.
Method
Subjects and apparatus
The subjects were 23 male Sprague-Dawley rats (Charles River Laboratories) about 120 days old at the start of the experiment. They had served as subjects in a previous Pavlovian conditioning experiment that involved food deprivation and visual and auditory CSs paired with food pellet delivery in standard conditioning chambers. In both that study and in Experiment 2, they were housed in individual 48 × 27 × 21 cm clear polycarbonate tub cages with wood chip bedding, in a vivarium with the lights on from 6:00 a.m. to 8:00 p.m. As in Experiment 1, food was available ad lib, but access to fluids was restricted to daily 10 min experimental flavored liquid presentations at 8:00 a.m. (usually limited to 10 ml, see below) and 10 min access to unlimited amounts of unflavored tap water at 3:00 p.m.
Experiment 2 was conducted entirely in the home cages over 12 consecutive days. Fluids were presented in standard water bottles with drinking tubes that extended 5 cm into the cage, about 10 cm above the cage floor.
Procedure
An outline of the procedures of Experiment 2 is presented in Table 1. During their morning trials, the rats in Group Two (n = 7) received four 10-min, 10-ml presentations each of two odor-flavor compounds (O1F1 and O2F2) randomly intermingled over the 8 preexposure days. The two flavors were 0.1-M sucrose (S) and 0.15-M NaCl (N), and the two odors were the almond and vanilla solutions used in Experiment 1, except McCormick brand flavors (1% v/v) were used as the odorants. On these days, the rats in Group One received 4 presentations of O1F1 and either 4 presentations of O2 (n = 4) or 2 presentations of O2 and 2 presentations of F2 (n = 4). The rats in Group Con received either 4 presentations each of O1 and O2 (n = 4), or 2 presentations each of O1, O2, F1, and F2 (n = 4). The purpose of subdividing Groups One and Con was to provide information about the effects of differential preexposure to the odors among the groups. Many investigators (e.g., Lubow, Wagner, & Weiner, 1982) have found greater preexposure effects (latent inhibition or perceptual learning) to a stimulus if that stimulus is presented alone than if it is presented in compound with another stimulus during preexposure. Differences in consumption of O1 relative to O2 in testing of rats in Group One, for example, may be influenced by reduced effectiveness of preexposures of O1 within a compound, relative to equal numbers of O2-alone preexposures. To the extent that such preexposure effects contribute to the results of these studies, reduction in the numbers of O2-alone presentations should reduce the difference between O1 and O2 consumption. The compounds of N and S with almond and vanilla (in Groups One and Two), and the roles of each odor as O1 or O2 were completely counterbalanced, except that in Group Two (which included only 7 rats), one of the four possible flavor-odor combinations was only represented once.
On the next morning, all rats received a single aversion conditioning trial, in which 10 min access to 10 ml of an O1O2 compound solution was followed by an injection of 5 ml/kg of 0.6-M LiCl. On the next day, all rats received 10 min access to unlimited amounts of unflavored tap water on both the morning and afternoon fluid presentations, to ensure that all rats had recovered from any dehydrating effects of the toxin injections.
Finally, over the next two mornings the rats received a consumption test presentation of each of the two odors, O1 and O2, in counterbalanced order. Each test comprised 10 min unlimited access to one of the odor solutions.
Results
The rats consumed all of the available fluids in the preexposure and conditioning phases of Experiment 2. The data from the consumption tests are shown in Figure 2. As in Experiment 1, in Group One a representation of flavor, activated by O1, potentiated aversion learning to another odor, O2, but not learning to O1 itself. The rats in Group One, in which O1 had been preexposed in compound with a flavor prior to the O1O2→toxin trial, consumed less O2 than the rats in Group Con, but similar amounts of O1. Furthermore, the rats in Group One consumed less O2 than O1. By contrast, the rats in Group Two showed no evidence of potentiated aversion learning to either O1 or O2, despite having been preexposed to each of those odors in compound with a flavor. Consumption of O1 and O2 in Group Two did not differ from that in Group Con. Thus, in Group Two, odor-activated flavor representations failed to potentiate odor aversion learning to odors that themselves activated flavor representations.
Figure 2.
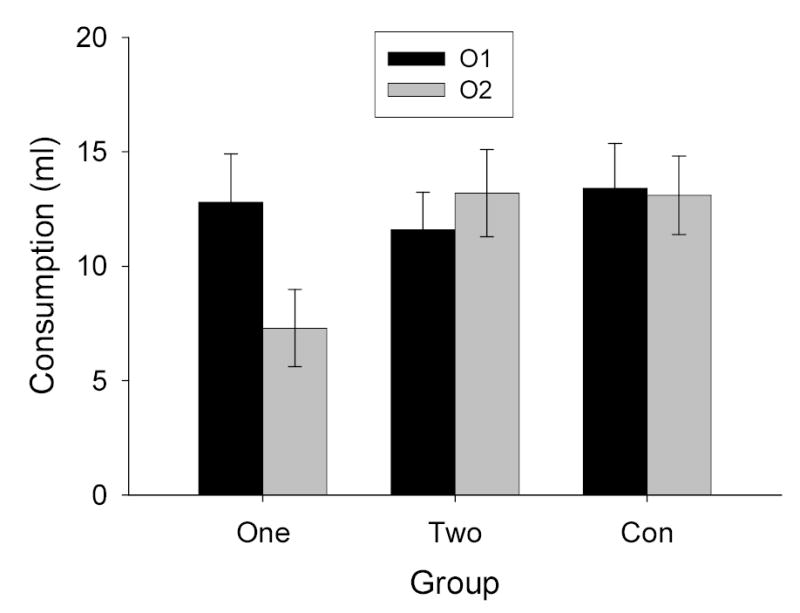
Consumption of the two odor solutions (O1 and O2) in the test sessions of Experiment 2. See Table 1 for experimental treatments of the groups and stimuli.
Preliminary ANOVAs, like those conducted in Experiment 1, showed that neither test order nor counterbalancing of the two flavors had significant effects or interactions, Fs < 1, so those factors were eliminated from the analysis. Likewise, the subdivision of Groups One and Con had no effects, either in each group individually or pooled across groups, Fs <1; thus the amount of simple preexposure to the events apparently did not contribute to the potentiation effects observed here. By contrast, consumption of vanilla was greater than that of almond, so this factor was retained in the final Group X test cue (O1 or O2) X odor (Almond or Vanilla) ANOVA. In that analysis, the main effect of odor was significant, F(1, 17) = 37.65, but this variable did not interact significantly with any other effect or interaction, Fs < 1.94. Of the remaining main effects and interactions, only the group X test cue interaction was significant, F(2, 17) = 3.49. Post-hoc comparisons showed that O2 consumption of Group One differed significantly from consumption in all other conditions. No other comparisons were significant.
The results of Group One replicated those of Experiment 1 and of Holland (1983, Exp. 4): an odor that activated a flavor representation was immune to potentiation by that representation. The results of Group Two show that this immunity was not limited to representations activated by that odor, but extended to flavor representations activated by other odors as well. At the same time, although odors that activate flavor representations were immune to potentiation by other associatively-activated event representations, the performance of Group Real in Experiment 1 shows that they remained normally susceptible to potentiation by the real flavor associates of those odors.
Finally, it is notable that potentiation effects observed in this experiment were similar regardless of whether an odor was paired with sucrose or saline flavors. Although the limited-quantity procedures of this experiment did not permit an assessment of preferences for the various solutions and compounds, unpublished preference tests in other rats show that under these deprivation conditions, the sucrose solution used is highly preferred to water, which in turn is slightly (but consistently) preferred to the saline solution. Thus, the representation-mediated potentiation effects observed here would not seem to involve odor preference learning, as described in the discussion of Experiment 1.
Experiment 3
Experiment 3 addressed the same experimental questions as were posed in Experiment 2, except it considered how flavor-activated odor representations affect conditioning of aversions to flavors. The experimental procedures and materials were identical to those of Experiment 2 except that the two flavors were used in the conditioning and test phases, rather than the two odors. Thus, Experiment 3 examined the effects of prior odor-flavor pairings on the conditioning of aversions to the flavors when a flavor + flavor compound is paired with toxin.
Previous studies of the modulation of flavor aversion learning by other tastes or by odors have yielded mixed results, including both cases of overshadowing (i.e., less conditioning to an element after compound conditioning than after element conditioning) and potentiation (e.g. Batsel, Paschalli, Gleason, & Batson, 2001; Bouton, Dunlap, & Swartzentruber, 1987; Bouton & Whiting, 1982; Slotnick, Westbrook, & Darling, 1997). Although outcomes in these experiments have varied considerably (see Batsell & Blankenship, 2003, and LoLordo & Droungas, 1989, for reviews), common to several of them (e.g., Bouton, Jones, McPhillips, & Swartzentruber, 1986; Slotnick et al., 1997) is the observation that the relative salience of the compound elements may determine whether overshadowing or potentiation is obtained. From this perspective, if the relative salience of the odors and tastes of Experiment 2 favored the observation of potentiation of odor learning by flavors, then in Experiment 3 those same stimuli might favor the overshadowing of flavor learning by the odors (see also Kucharski & Spear, 1985). If this were the case, the procedures of Experiment 3 would provide an opportunity to examine limitations of the function of associatively-activated event representations in the case of representation-mediated overshadowing (Holland, 1983, Exps. 1–2). Nevertheless, Experiment 3 was designed to extend the generality of the results of Experiment 2, regardless of whether that extension was to representation-mediated overshadowing, or to representation-mediated potentiation of aversion learning in another stimulus modality.
Method
Subjects and apparatus
The subjects were 32 male Sprague-Dawley rats (Charles River Laboratories) about 120 days old at the start of the experiment. They had served as subjects in a previous Pavlovian conditioning experiment that involved food deprivation and visual and auditory CSs paired with food pellet delivery in standard conditioning chambers. They were housed and maintained as in Experiment 2.
Experiment 3 was conducted entirely in the home cages over 12 consecutive days. Fluids were presented in standard water bottles with drinking tubes as in Experiment 2.
Procedure
An outline of the procedures of Experiment 3 is presented in Table 1. During their morning trials, the rats in Group Two (n = 11) received four 10-min, 10-ml presentations each of two odor-flavor compounds (O1F1 and O2F2) randomly intermingled over the 8 preexposure days. The two flavors were 0.1-M sucrose (S) and 0.15-M NaCl (N), and the two odors were the almond and vanilla solutions used in Experiment 2. On these days, the rats in Group One received 4 presentations of O1F1 and either 4 presentations of F2 (n = 6) or 2 presentations of O2 and 2 presentations of F2 (n = 5). The rats in Group Con received either 4 presentations each of F1 and F2 (n = 5), or 2 presentations each of O1, O2, F1, and F2 (n = 5). These subdivisions of the latter groups were intended to assess the role of the amount of simple preexposure, as in Experiment 2. The compounds of N and S with almond and vanilla (in Groups One and Two), and the roles of each flavor as F1 and F2 were counterbalanced as much as possible.
On the next morning, all rats received a single aversion conditioning trial, in which 10 min access to 10 ml of an F1F2 (SN) compound solution was followed by an injection of 5 ml/kg of 0.6-M LiCl. On the next day, all rats received 10 min access to unlimited amounts of unflavored tap water on both the morning and afternoon fluid presentations, to ensure that all rats had recovered from any dehydrating effects of the toxin injections.
Finally, over the next two mornings the rats received a consumption test presentation of each of the two flavors, F1 and F2, in counterbalanced order. Each test comprised 10 min unlimited access to one of the solutions.
Results
The rats consumed all of the available fluids (limited to 10 ml) in the preexposure and conditioning phases of Experiment 3. The data from the consumption tests are shown in Figure 3. The results were similar to those of Experiment 2. In Group One, a representation of an odor, activated by F1, potentiated aversion learning to another flavor, F2, but not learning to F1 itself. The rats in Group One, in which F1 had been preexposed in compound with an odor prior to the F1F2→toxin trial, consumed less F2 than F1, and less F2 than the rats in Group Con. In addition, consumption of F1 was greater in Group One than in Group Con. By contrast, the rats in Group Two showed no evidence of potentiated aversion learning to either F1 or F2, despite having been preexposed to each of those flavors in compound with an odor. Consumption of F1 and F2 in Group Two did not differ from that in Group Con. Thus, flavor-activated odor representations failed to potentiate aversion learning to flavors that themselves activated odor representations. As in the previous study, the potentiation effects observed in Experiment 3 were similar regardless of whether the target taste CS was sucrose (strongly preferred to water) or saline (less preferred than water).
Figure 3.
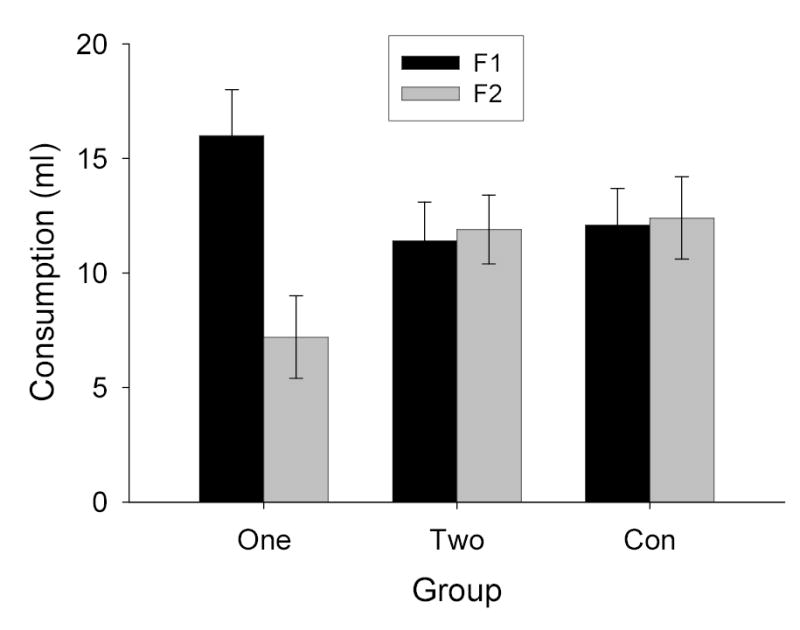
Consumption of the two flavor solutions (F1 and F2) in the test sessions of Experiment 3. See Table 1 for experimental treatments of the groups and stimuli.
Preliminary ANOVAs like those described in Experiment 1 showed that neither test order nor counterbalancing of the two odors had significant effects or interactions, Fs < 1. Likewise, subdivision of Groups One and Con had no effects, either in each group individually or pooled across groups, Fs < 1. Thus, these factors were eliminated from subsequent analysis. By contrast, test consumption of sucrose was greater than that of saline, so this factor was retained in the final Group X test (F1 or F2) X flavor (sucrose or saline) ANOVA. In that analysis, the main effect of flavor was significant, F(1, 26) = 126.54, but this effect did not interact significantly with any other effect or interaction, Fs < 1.92. Of the remaining main effects and interactions, only the main effect of test, F(1, 26) = 8.84, and group X test interaction was significant, F(2, 26) = 17.32. Post-hoc comparisons showed that both F1 and F2 consumption in Group One differed significantly from consumption in each of the other conditions. No other comparisons were significant.
The results of Experiment 3 replicate those of Experiment 2, extending them to the case of potentiation of a taste aversion by an odor. As in Experiment 2, in Group One, a CS that activated an event representation was immune to potentiation by that representation. The results of Group Two show that this immunity was not limited to representations that were activated by that CS, but extended to representations activated by other CSs as well. It remains to be seen whether flavors that activate odor representations are susceptible to potentiation by real odors, as was the case with the opposite modality combination in Experiment 1.
The observation of greater F1 consumption (less aversion learning) in Group One than in Groups Con and Two suggests another potential interpretation of the absence of potentiation in Group Two. It is possible that an odor representation overshadows conditioning of the flavor that activates it, in addition to potentiating conditioning to other flavors. In this case, each of the flavors in Group Two would be subject to both overshadowing and potentiation, and thus might show no greater net learning than the rats in Group Con. It is notable however, that there was no evidence for such overshadowing of O1 learning in Group Rep of Experiment 1 or Group One of Experiment 2, despite similar levels of consumption in testing. Those data are more consistent with a variation of this account, in which a CS-activated event representation uniquely overshadows aversion learning to the CS that activates it, but potentiates aversion learning to all CSs present, including the activating CS. In this manner, the activating CS might then show neither net overshadowing nor net potentiation. In the General Discussion I will consider a more formal version of this account, framed in the context of Wagner’s (1981) SOP theory.
Finally, although the observation of potentiation of flavor aversion learning by an odor is consistent with many previous reports (e.g., Batsel et al., 2001; Slotnick, et al., 1997; see Batsel & Blankenship, 2003 for a recent review), the observation of potentiation of both the tastes by the odors in this experiment and of the odors by the tastes in Experiment 2 is inconsistent with previous suggestions that potentiation depends on the relatively lower salience of the potentiated stimulus. Thus, the critical conditions for the observation of potentiation or overshadowing remain to be determined.
Experiment 4
In Experiments 2 and 3, when both CSs activated event representations (Group Two), neither CS showed potentiated aversion learning, whereas when only one of the CSs activated a representation, only that CS failed to show potentiated aversion learning. We interpreted that outcome as indicating that CSs that activate representations are immune to potentiation by associatively-activated event representations. Another possible account is that the evocation of representations by two cues produced a compound representation (e.g. sucrose + saline or almond + vanilla) that was less effective in potentiating aversion learning than a more discrete, single representation would be. That is, these associatively-activated event representations may combine destructively, rather than additively. In Experiment 4, we evaluated this possibility by examining aversion learning with a three-odor compound. Three groups of rats were trained with procedures like those of Experiment 2, except that a novel odor, O3, was added to the O1O2 compound on the aversion training trial. If the activation of a sucrose + saline representation in Group Two was an inadequate potentiator, then neither O3 nor O1/O2 would show potentiated aversion learning. However, if only odor CSs that activate flavor representations are immune to potentiation by other flavor representations, then aversion learning to O3 would be anticipated.
Method
Subjects and apparatus
The subjects were 24 male Sprague-Dawley rats (Charles River Laboratories) about 120 days old at the start of the experiment. They had served as subjects in a previous conditioning experiment that involved food deprivation and visual and auditory CSs paired with food pellet delivery in standard conditioning chambers. The rats were housed in the same manner as in Experiments 2 and 3.
Procedure
An outline of the procedures of Experiment 4 is presented in Table 1. The procedures were identical to those of Experiment 2 except (1) Groups One and None were not subdivided; all rats in these groups received all appropriate trial types (2) a lemon odor O3 (0.5% v/v, McCormick brand) was added to the O1O2 compound on the aversion training trial and (3) testing comprised three test trials, the first with O3, the second with O2, and the final test with O1.
Results
The rats consumed all of the available fluids in the preexposure and conditioning phases of Experiment 4. The data from the consumption tests are shown in Figure 4. Although, as in Experiment 2, the rats in Group Two showed no evidence of potentiated aversion learning to either O1 or O2, those rats showed substantial potentiation of aversion learning to O3, the odor that did not evoke a flavor representation. Consumption of O3, the novel odor (dark bars in Figure 4) was lower in both Groups One and Two than in Group Con, consistent with the claim that aversion learning to O3 was potentiated by associatively-activated representations of either a single flavor or both flavors simultaneously. Also as in Experiment 2, aversion learning to O2 (light bars) was potentiated by a CS-activated flavor representation only if O2 did not also activate a flavor representation; consumption of O2 was lower in Group One than in either Group Two (in which both odors activated flavor representations) or Group None (in which no flavor representation was activated). Finally, also consistent with the results of Experiment 2, consumption of O1 (intermediate bars) did not differ across the groups.
Figure 4.
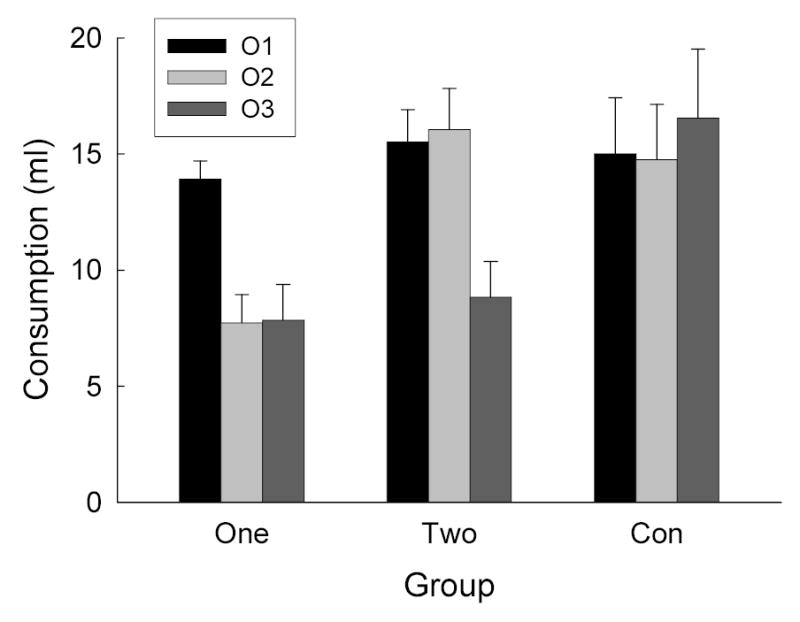
Consumption of the three odor solutions (O1, O2, and O3) in the test sessions of Experiment 4. See Table 1 for experimental treatments of the groups and stimuli.
Preliminary group X F1 identity X O1 identity X O2 identity ANOVAs showed that the counterbalancing of the flavors and odors had no significant effects or interactions, so these factors were eliminated from the analysis. Because test odor was confounded with test order, separate 1-way ANOVAs were conducted for each test. The effect of group was significant for O3, F(2, 21) = 5.67, and O2, F(2, 21) = 5.64, but not O1, F < 1. Post-hoc comparisons showed that O3 consumption was significantly higher in Group Con than in either of the other two groups, and that O2 consumption was reliably lower in Group One than in either of the other two groups.
The results of Experiment 4 replicated those of Experiment 2 very closely, and in addition showed that aversion learning to a novel odor was potentiated as much by a compound of odor-activated flavor representations as by a single such representation. Thus, the failure of potentiation of aversion learning to O2 in Group Two was unlikely to be because a compound representation is a less effective potentiator than a single representation.
General Discussion
The results of these experiments extended those of Holland (1983, Exp. 3 and 4). First, associatively-activated representations of a flavor (Exp. 1, 2, and 4), or an odor (Experiment 3) potentiated the acquisition of an aversion to an odor or a flavor (respectively). For example, in Experiments 1 and 2, pairings of a compound O1O2 odor with LiCl resulted in a greater aversion to O2 alone if O1 was previously paired with a flavor. The magnitude of that representation-mediated potentiation was indistinguishable from that produced by presentation of the flavor itself. Second, potentiation of aversion learning by a CS-activated event representation was limited to CSs that were not themselves activating a representation of another event. In the preceding example, although the acquisition of an aversion to O2 was potentiated by O1’s activation of a flavor representation, aversion learning to O1 itself was not. Similarly, when both O1 and O2 were previously paired with flavors (Group Two), aversion learning was not potentiated to either of them. Comparable results were obtained with either odors (Exp. 1, 2, and 4) or flavors (Experiment 3) as the target stimuli in aversion learning. Third, although prior pairings of both O1 and O2 with flavors prevented potentiation of conditioning to either of those odor cues, they nevertheless produced potentiation of a third odor without such prior history (Experiment 4).
Finally, this immunity to potentiation of aversion learning to CSs that activated representations of other events was limited to representation-mediated potentiation. In Experiment 1, a flavor potentiated the establishment of odor aversions regardless of whether those odors activated flavor representations. Thus, despite the similarity in magnitude of potentiation by real flavors and by associatively-activated flavor representations in Experiment 1, those data show that flavors and associatively-activated flavor representations are not processed in identical manners. In accounting for data like those displayed by the rats in Group One in Experiments 2 and 3, Holland (1983) suggested that, in working memory systems involved in the formation of associations, animals distinguish between stimuli that are evoking representations and stimuli that are not doing so.
Before speculating on why events that activate representations of other events are treated differently from events that do not do so, a number of potentially simpler accounts for the present data should be considered. In these studies, the operation that presumably was responsible for the associative activation of event representations was simple pairing of an odor and a flavor during a preexposure phase, relative to either unpaired presentation of those events or presentations of only one of them. How else might this operation influence learning about the target cues in these studies? Apart from endowing some of the cues with the ability to activate representations of other stimuli, stimulus pairings during the preexposure phase may have altered the occurrence of three other learning phenomena that might contributed to the results observed: latent inhibition, perceptual learning, and the establishment of stimulus preferences.
Latent inhibition refers to a reduction in learning rate or associability as a result of prior exposure to a CS. Variations in aversion conditioning across the various training conditions may have been related to variations in the effectiveness of those procedures in establishing latent inhibition. However, latent inhibition seems an unlikely contributor to the present data. Typically, latent inhibition to a CS is greater if the CS is preexposed separately than if it is preexposed in compound with another stimulus (e.g., Holland & Forbes, 1980; Wagner et al., 1982). In Group One of Experiments 1 and 2, for example, preexposure to O2 alone should have slowed subsequent learning about O2 more than preexposure to O1 in compound with F would slow conditioning of O1. The opposite of that outcome was obtained. Likewise, in Group Two, preexposure to O1 and O2 within compounds should have slowed subsequent individual conditioning to those cues less than preexposure to those cues separately in the other groups. Again, the opposite result was observed.
At first glance, some form of perceptual learning may be a more plausible contributor to the present results. Operationally, perceptual learning refers to the opposite outcome as latent inhibition, a facilitation of subsequent learning about a cue as a result of its preexposure. A variety of theoretical accounts for this enhanced CS associability have been offered recently (e.g., Hall, 1991, 2003; McLaren & Mackintosh, 2000; Le Pelley, 2004), and many of its determinants are now established. Although not necessarily embraced by either Hall (2003) or McLaren and Mackintosh (2000), the simple assumption that preexposing a stimulus by itself might produce greater enhancements in its associability than preexposing it in compound with another event is consistent with the major features of the present data. First, in Group One, aversion learning to O2 was greater than that to O1 because prexposure to O2 alone enhanced its aassociability more than preexposure to O1 within a compound. Second, there was more aversion learning about O2 in Group One than in Group None after O1O2-LiCl pairings (despite equivalent preexposure to O2 in both groups) because there was less competition from O1 in Group One than in Group None. Note that in Group One, O1 was preexposed within a compound whereas in Group None O1 was preexposed alone. Third, aversion learning to both O1 and O2 was low in Group Two, because both cues were preexposed in compounds, reducing the likelihood of perceptual learning about either of them.
Nevertheless, other data make a perceptual learning account less likely. First, there was no evidence for such compound versus element preexposure effects when potentiation was induced by a real flavor rather than an associatively-activated flavor representation (Group Real of Experiment 1). Second, there was no evidence for differential effects of compound or element preexposure on conditioning to odors in the absence of potentiation. In Holland’s (1983, Exp. 4) study, two groups of rats received O1F and O2 preexposure. Group C then received pairings of an O1O2 compound with LiCl whereas Group E received separate pairings of each odor with LiCl. From the perceptual learning perspective just described, both groups should have shown greater aversion learning with O2 than with O1, but that effect was only observed in Group C. Finally, although the idea that preexposure to a CS may enhance its associability is consistent with observations of perceptual learning, previous studies using these same stimuli and similar training procedures have shown latent inhibition rather than perceptual learning effects (e.g., Holland & Forbes, 1980).
A third possible source of variation in responding due to preexposure effects was noted briefly in Experiment 2. Many investigators have noted that pairings of a neutral flavor or odor CS with sucrose can establish a preference for that CS (e.g., Harris, Shand, Carroll, & Westbrook, 2004). In principle, such a preference may have contributed to the results of both Group Rep in Experiment 1 of the present series, and Holland (1983, Exp. 4, Group C): O1-sucrose pairings may have enhanced consumption of O1 in testing, relative to consumption O2, which was not paired with sucrose. However, any such learned preferences are unlikely to have had much impact in these studies. First, in Holland (1983, Exp. 4) a preference for O1 should have been reflected in more consumption of O1 than of O2 in the control group E as well as in the potentiation Group C; no such preference was observed. Second, there was no evidence for a contribution of such a preference when a real flavor was present in Group Real of Experiment 1 of the present series. Third, in Experiment 2, potentiation by an odor-activated flavor representation was just as large when that flavor was a nonpreferred saline solution as when it was a highly-preferred sucrose solution. Fourth, in Experiment 3, similar effects were observed with potentiation of a taste aversion by an odor representation. In that case, it seems unlikely that a preference would develop for a flavor presented in compound with an odor, given that previous unpublished data (described in Experiment 1) showed that each flavor alone is preferred to the flavor + odor compounds.
In the absence of other accounts based on preexposure, it then remains to be determined why associatively-activated event representations only modulate learning about CSs that do not themselves activate event representations, or alternately, why learning about CSs that activate representations of other events is apparently immune to modulation by other associatively-activated event representations, despite being normally susceptible to modulation by real associated events. This determination would seem to depend on the mechanisms by which potentiation may occur, but it seems fair to say that the major accounts for potentiation provide little insight.
One account, offered by Durlach and Rescorla (1980) is that potentiation is mediated by the formation of odor-taste associations. Within this view, although the rate of odor-illness association is low, both odor-taste and taste-illness learning is rapid. Thus, odor-taste associations permit the odor to access the illness reinforcer indirectly, through a process akin to second-order conditioning. In support of this view, Durlach and Rescorla (1980) found that post-illness extinction of the taste aversion eliminated the evidence for potentiation of the associated odor. It is difficult to see how the present results could be consistent with such a mechanism of potentiated odor aversion learning. For example, it seems unlikely that, in Group One of Experiment 2, the potentiation of an odor aversion mediated by odor-flavor association would be stronger for O2, which was paired only with a CS-activated representation of a flavor, and only on the conditioning trial, than for O1, which had also been previously paired with that flavor itself.
A second account for the potentiation of odor aversion learning is that the presence of a flavor serves some “catalytic” role in enhancing direct odor-illness associations. For example, Palermino et al. (1980) and Rusiniak et al. (1979) suggested that pairing an odor and a taste changes the nature of the odor, endowing it with the memorial properties of a flavor at the time of illness. To deal with the present results, one would have to posit that an associatively-activated representation of a flavor can also endow an odor with taste properties, but only if that odor was not already associated with a flavor, which amounts to little more than a restatement of the present results in that hypothetical context. Furthermore, doubt is cast on the plausibility of such an account by the comparability of the results of Experiments 2 and 3, which used the same odor-taste compound cues in the preexposure phase, but paired either odor or taste compounds with toxin. Clearly, the observation of potentiated aversion learning did not depend on special properties of taste or odor, including any differences in salience (e.g., Bouton et al., 1986; Slotnick et al., 1997). Similarly, Capaldi et al. (2004) showed that even relatively dilute flavors, such as the saline solution used in the present experiments, have substantial olfactory properties, which can contribute to flavor aversion learning. Thus, the nature of cue-facilitated aversion learning is still poorly understood.
A third account for potentiated aversion learning (e.g., Kucharski and Spear, 1985; Rescorla, 1981) blends aspects of the preceding two accounts. Within this account, presentations of an odor + taste compound produce a configural stimulus representation, which is moderately associable with illness. The display of aversion to elements of that compound depends on the generalization between an element and the compound. If the advantage in conditioning acquired to the compound because of its greater salience or associability than an individual element is large enough to overcome the generalization decrement between compound and element, then potentiation would be observed. In principle, depending on the amounts of associability gain and generalization decrement that occur, this approach could account for observations of mutual potentiation, mutual overshadowing, and potentiation of learning about one cue and overshadowing of the other. Although this account might then deal with the symmetrical results of Experiments 2 and 3 of the present series, it is not clear how it would handle the remaining results a priori. To account for the selective potentiation in Group One and no potentiation in Group Two in these experiments, one would have to assume that the generalization between the compound in training and a single element in testing is reduced by prior presentation of the element in compound with another cue. Although this may well be true, it is not clear on what basis such an assumption could be made.
Holland (1983) cast his general observations of the functions of associatively-activated event representations into the rubric of Wagner’s (1981) SOP theory. Within this theory, excitatory associations are formed between two events to the extent that their memorial representations are jointly processed in a working memory system while in states of focal activity (A1). An event representation enters the A1 state by presentation of the event itself, but its processing in that state can be curtailed if, when the event is presented, that representation is already active in working memory in a more marginal state of activity (A2). Event representations enter the A2 state either by passive decay from the A1 state, or by associative priming by an another event represented in working memory. These features of the model allow it to account for a variety of associatively-based cue competition phenomena. For example, in the case of blocking (Kamin, 1969), prior pairings of one CS with the US allows that CS to prime a representation of the US into the A2 state. This priming reduces processing of the US in the A1 state, which is needed for the establishment of new associations involving that US. Thus, when a new cue is added to the pretrained element in a blocking experiment, its representation is paired with a US that is only minimally processed in the A1 state, and so that cue acquires little association with the US.
Within SOP, the associatively-activated event representations discussed here are A2-state representations. To deal with the potentiation of odor aversion by an associatively-activated taste representation, one could posit that an A2-state representation of a flavor, like an A1-state flavor representation, can enhance the formation of associations between the A1-state odor and illness representations. For example, in Experiment 2, O1F1 compound preexposure in Groups One and Two endows O1 and F1 each with the ability to prime a representation of the other element into the A2 state. On the aversion conditioning trial, O1 presentation would prime F1 into the A2 state, ostensibly permitting F to potentiate conditioning of both O1 and O2. However, within Wagner’s theory, both A1- and A2-state events can prime representations of their associates into the A2 state. In Experiment 2, once primed into the A2 state by O1, F1 might also prime O1 into the A2 state, which might curtail processing of O1 in the A1 state, and thus reduce its association with illness. The same fate would befall O2 in Group Two, because of O2’s prior associations with F2. But in Group One, in which O2 was not previously paired with a flavor, O2 would remain in the A1 state and hence have more opportunity for association with illness.
This approach can be viewed as a formalization of the account offered in the discussion of the results of Experiment 3. Although it handles many aspects of the present data, it fails to account for the results of Group Real in Experiment 1. If, because of its past associations with F1, O1 is processed in the A1 state less than O2, then O1 should be less well-conditioned than O2 whether a real (A1-state) flavor is present or not. But in Experiment 1 the presence of a real flavor associate potentiated both O1 and O2. One might posit different likelihoods of potentiation and associative competition among these event representations, depending on whether the potentiator was in the A1 state (as in Group Real) or the A2 state, but at this point this strategy seems unduly complex and post hoc. Also problematic is the possibility that in the interval between presentation of the solutions and the onset of illness, all of the event representations would have decayed into the A2 state. Thus, more quantitative modeling of the potentiated aversion paradigm would be necessary before unambiguous predictions about the limitations to representation-mediated learning could be generated from SOP.
Previously, I have emphasized the common functions that associatively-activated event representations and their referents may serve. In several series of experiments, those representations mimicked the actions of their referents in many conditioning phenomena including acquisition (Holland, 1981), extinction (Holland & Forbes, 1982b), occasion-setting (Holland & Forbes, 1982a), selective association (Holland, 1981), overshadowing (Holland, 1983), and potentiation (Holland, 1983). Data like those presented in this article demonstrate limits to this functional substitutability of CS for US. In some respects, these limitations seem adaptive. For example, immunity of a CS to the effects of representations it activates avoids a potential paradox in experimental extinction. If a CS activates a representation of the US, then functional CS-US pairings would occur even if delivery of the US was discontinued, and extinction might never occur. Better understanding of the ways in which information derived from currently-available stimuli and that retrieved from memory may be basic to our understanding of the nature of learning and memory.
Acknowledgments
Experiment 1 was performed at Duke University, and was described casually in Holland (1990). Thanks to Vanessa McKenna, Rose Baker, Souvik Chatterjee, and Mary Keough for their assistance in data collection.
Footnotes
Support for Experiments 2–4 was provided by NIH grant MH65879; support for Experiment 1 was provided by NSF grant BNS-8513603.
References
- Batsell WR, Blankenship AA. Beyond potentiation: Synergistic conditioning in flavor-aversion learning. Brain and Mind. 2003;3:383–408. [Google Scholar]
- Batsell WR, Paschall GY, Gleason DI, Batson JD. Taste preconditioning augments odor aversion learning. Journal of Experimental Psychology: Animal Behavior Processes. 2001;27:30–47. [PubMed] [Google Scholar]
- Bouton ME, Dunlap CM, Swartzentruber D. Potentiation of taste by another taste during compound aversion learning. Animal Learning & Behavior. 1987;15:433–438. [Google Scholar]
- Bouton ME, Jones DL, McPhillips SA, Swartzentruber D. Potentiation and overshadowing in odor-aversion learning: Role of method of odor presentation, the distal-proximal cue distinction, and the conditionability of odor. Learning and Motivation. 1986;17:115–138. [Google Scholar]
- Bouton ME, Whiting MR. Simultaneous odor-taste and taste-taste compounds in poison-avoidance learning. Learning and Motivation. 1982;13:472–494. [Google Scholar]
- Capaldi ED, Hunter MJ, Privitera GJ. Odor of taste stimuli in conditioned “taste” aversion learning. Behavioral Neuroscience. 2004;118:1400–1408. doi: 10.1037/0735-7044.118.6.1400. [DOI] [PubMed] [Google Scholar]
- Davey GCL, McKenna I. The effects of postconditioning revaluation of CS1 and UCS following Pavlovian second-order electrodermal conditioning in humans. Quarterly Journal of Experimental Psychology. 1983;35B:125–133. doi: 10.1080/14640748308400899. [DOI] [PubMed] [Google Scholar]
- Durlach PJ, Rescorla RA. Potentiation rather than overshadowing in flavor aversion learning: An analysis in terms of within compound associations. Journal of Experimental Psychology: Animal Behavior Processes. 1980;6:175–187. [PubMed] [Google Scholar]
- Fantino, E.(1977). Conditioned reinforcement: Choice and information. In W.K. Honig & J.E.R. Staddon (Eds.), Handbook of operant behavior (313–339). Englewood Cliffs, N.J.: Prentice-Hall.
- Hall, G. (1991). Perceptual and associative learning Oxford, England: Clarendon Press.
- Hall G. Learning about associatively-activated stimulus representations: Implications for acquired equivalence and perceptual learning. Animal Learning & Behavior. 1996;24:233–255. [Google Scholar]
- Hall G. Learned changes in the sensitivity of stimulus representations: associative and nonassociative mechanisms. Quarterly Journal of Experimental Psychology. 2003;56B:43–55. doi: 10.1080/02724990244000151. [DOI] [PubMed] [Google Scholar]
- Harris JA, Shand FL, Carroll LQ, Westbrook RF. Persistence of preference for a flavor presented in simultaneous compound with sucrose. Journal of Experimental Psychology: Animal Behavior Processes. 2004;30:177–189. doi: 10.1037/0097-7403.30.3.177. [DOI] [PubMed] [Google Scholar]
- Heth CD. Simultaneous and backward fear conditioning as a function of number of CS-UCS pairings. Journal of Experimental Psychology: Animal Behavior Processes. 1976;2:117–129. doi: 10.1037//0097-7403.2.2.117. [DOI] [PubMed] [Google Scholar]
- Holland PC. Acquisition of representation mediated conditioned food aversions. Learning and Motivation. 1981;12:1–18. doi: 10.1016/j.lmot.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Representation mediated overshadowing and potentiation of conditioned aversions. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:1–13. [Google Scholar]
- Holland PC. Event representation in Pavlovian conditioning: Image and action. Cognition. 1990;37:105–131. doi: 10.1016/0010-0277(90)90020-k. [DOI] [PubMed] [Google Scholar]
- Holland PC, Forbes DT. Effects of compound or element preexposure on compound flavor aversion conditioning. Animal Learning & Behavior. 1980;8:199–203. [Google Scholar]
- Holland PC, Forbes DT. Control of conditional discrimination performance by CS evoked event representations. Animal Learning & Behavior. 1982a;10:249–256. [Google Scholar]
- Holland PC, Forbes DT. Representation mediated extinction of flavor aversions. Learning and Motivation. 1982b;13:454–471. [Google Scholar]
- Kamin, L. J. (1969). Predictability, surprise, attention, and conditioning. In B. Campbell & R. Church (Eds.), Punishment and Aversive Behavior (279–298).New York: Appleton Century Crofts.
- Konorski, J. (1948). Conditioned reflexes and neuron organization Cambridge: Cambridge University Press.
- Kucharski D, Spear NE. Potentiation and overshadowing in preweanling and adult rats. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:15–34. doi: 10.1037//0097-7403.11.1.15. [DOI] [PubMed] [Google Scholar]
- Le Pelley ME. The role of associative history in models of associative learning: A selective review and a hybrid model. Quarterly Review of Experimental Psychology. 2004;57B:193–243. doi: 10.1080/02724990344000141. [DOI] [PubMed] [Google Scholar]
- LoLordo, V.M., & Droungas, A. (1989). Selective associations and adaptive specialization: Taste aversions and phobias. In S.B. Klein & R.R (eds.), Contemporary learning theories: instrumental conditioning theory and the impact of biological constraints on learning (pp. 145–179). Hillsdale, NJ: Erlbaum.
- Lubow RE, Wagner M, Weiner I. The effects of compound stimulus preexposure of two elements differing in salience on the acquisition of conditioned suppression. Animal Learning & Behavior. 1982;10:483–489. [Google Scholar]
- Mackintosh, N. J. (1974). The psychology of animal learning New York: Academic Press.
- McLaren IPL, Mackintosh NJ. An elemental model of associaitve learning: I. Latent inhibition and perceptual learning. Animal Learning & Behavior. 2000;28:211–246. [Google Scholar]
- Palermino CC, Rusiniak KW, Garcia J. Flavor illness aversions: the peculiar role of odor and taste in memory for poison. Science. 1980;208:753–755. doi: 10.1126/science.7367891. [DOI] [PubMed] [Google Scholar]
- Rescorla, R. A. (1981). Simultaneous associations. In P. Harzem, & M. Zeiler (Eds.), Advances in the Study of Behavior, (Vol. 2). New York: Wiley, 1981.
- Rescorla RA. Pavlovian conditioning: It’s not what you think it is. American Psychologist. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- Rusiniak K, Hankins W, Garcia J, Brett L. Flavor illness associations: Potentiation of odor by taste in rats. Behavioral and Neural Biology. 1979;25:1–17. doi: 10.1016/s0163-1047(79)90688-5. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Westbrook RF, Darling FMC. What the rat’s nose tells the rat’s mouth: long delay aversion conditioning with aqueous odors and potentiation of tastes by odors. Animal Learning & Behavior. 1997;25:357–369. [Google Scholar]
- Wagner, A. R. (1981). SOP: A model of automatic memory processing in animal behavior. In N. E. Spear & R. R. Miller (Eds.), Information processing in animals: Memory mechanisms (5–47). Hillsdale, N. J.: Erlbaum.


