SUMMARY
The composition of extracellular matrix during growth and regression of the neointima was analyzed during healing in a baboon aorto-iliac polytetrafluoroethylene graft. Graft neointimal thickening can be modulated by altering blood flow by construction of downstream arteriovenous fistulas. Normal flow with normal shear stress induces neointimal thickening, whereas high flow with high shear stress upstream of a fistula induces regression of established neointima. The neointima formed under normal shear stress is enriched in hyaluronan and proteoglycans, particularly versican. On the other hand, the neointima near the graft material is enriched in collagen and biglycan. Neointimal regression in response to high shear stress is associated with a loss of proteoglycans as detected by histochemical staining. Immunostaining with an antibody against an ADAMTS cleavage neoepitope of versican increases after switching to high flow, although immunostaining for versican core protein is not appreciably changed by high flow. The present data demonstrate that the graft neointima is enriched with proteoglycans, particularly versican and hyaluronan, as well as collagen, and there is a differential distribution of each. Neointimal atrophy occurs with an apparent loss of proteoglycans and evidence of versican degradation.
Keywords: neointima, proteoglycans, shear stress, smooth muscle cells, versican
Intimal hyperplasia limits the long-term success of arterial grafting, bypass procedures, and stenting (Garratt et al. 1992; Hoffmann et al. 1996). The underlying mechanisms for this include increased smooth muscle cell (SMC) proliferation and migration in combination with extensive synthesis and deposition of extracellular matrix (ECM) by SMCs. The ECM plays an important role in the pathophysiology of neointimal thickening, because it contributes between 60% and 80% to the intimal mass in most forms of vascular lesions (Snow et al. 1990; Garratt et al. 1991; Strauss et al. 1994) and determines the mechanical characteristics of the neointima. ECM also influences the phenotype of neointimal SMC and, therefore, the function of SMCs (Raines 2000). Proteoglycans (versican, biglycan, and decorin) and hyaluronan are ECM molecules that accumulate in topographically distinct patterns within developing atherosclerotic and restenotic lesions [see reviews (Wight 1996; Toole et al. 2002)].
In a baboon model of polytetrafluroethylene (PTFE) graft neointimal growth and regression, the luminal surface of aorto-iliac PTFE grafts becomes fully endothelialized by transmural ingrowth of microvessels from the surrounding granulation tissue (Clowes et al. 1986). The extent of neointimal thickening depends on the rate of blood flow or shear stress. When grafts are allowed to heal under high blood flow or shear stress, grafts form a smaller neointima than those that heal under normal flow or shear stress (Kohler et al. 1991). If high shear stress grafts are returned to normal shear stress, the neointima begins to expand as the result of increased SMC proliferation and ECM production (Geary et al. 1994). In addition, increasing shear stress causes regression of a preexisting intima (Mattsson et al. 1997; Berceli et al. 2002). This is of particular interest because only ~30% of grafts or stented arteries fail because of intimal hyperplasia. Therefore, development of therapeutics designed to cause intimal atrophy would allow the selective treatment of only those patients with stenosis. In contrast, all patients would be required to take therapeutics designed to inhibit intimal hyperplasia. In the present study, we have examined changes in graft intimal ECM with a particular focus on the regressing intima.
Materials and Methods
All chemicals were purchased from Sigma (St Louis, MO) unless otherwise stated. PTFE grafts (internal diameter, 4 mm; internodal distance, 60 μm) were provided by W.L. Gore and Associates, Inc. (Flagstaff, AZ) and polypropylene suture by Davis & Geck (Danbury, CT).
Animal Model
Intimal Induction Model
The induction of neointimal thickening by switching from high to normal flow has been described previously (Figure 1A) (Geary et al. 1994). Briefly, juvenile, male baboons (Papio cynocephalus) received bilateral aorto-iliac PTFE bypass grafts with ligation of the intervening native vessels and bilateral arteriovenous fistula of the superficial femoral vessels. Grafts were allowed to heal for 8 weeks (high-flow condition). Subsequently, the arteriovenous fistula was ligated on one side, returning that side back to normal flow. Intimal tissue from the normal flow and high-flow grafts of animals sacrificed 28 days after fistula ligation was obtained for study (Geary et al. 1994). In these animals, blood flow and shear stress were decreased about four- to fivefold by closing the fistula, and intimal area was increased about fivefold.
Figure 1.
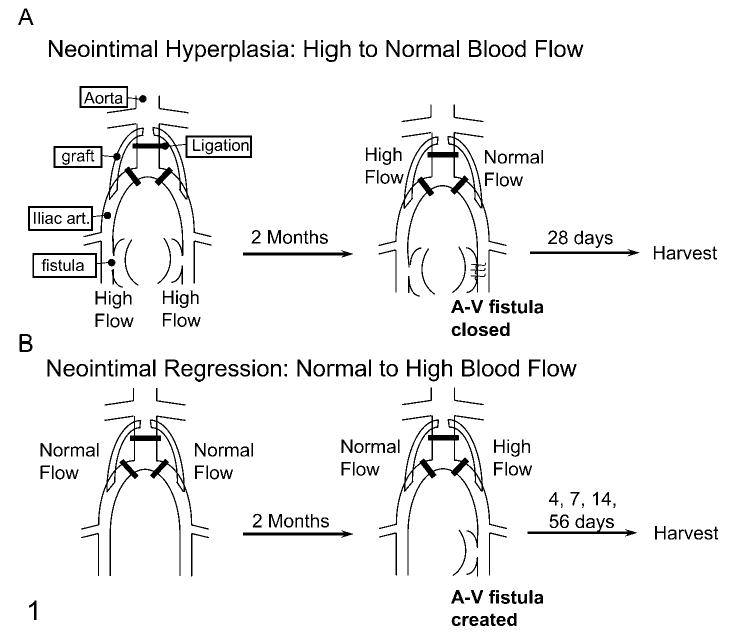
Schematic diagrams of the baboon aorto-iliac PTFE graft models of neointimal hyperplasia (A) and regression (B).
Intimal Regression Model
Intimal regression was stimulated by switching from normal to high flow as described (Figure 1B) (Berceli et al. 2002). Briefly, an arteriovenous fistula was constructed during a second surgery after 8 weeks of healing from the initial implantation of grafts under normal flow. Tissue from animals euthanized 4, 7, 14 (Berceli et al. 2002), and 56 days (Mattsson et al. 1997) later was obtained for study. In these animals, blood flow and shear stress were increased about fourfold by constructing a fistula. Intimal areas were decreased up to 60% by high shear stress [at 56 days (Mattsson et al. 1997)] with a significant reduction apparent at 7 days [~20% (Berceli et al. 2002)].
Tissue from three to five animals per condition and time were analyzed. Animal care and procedures were conducted at the University of Washington Regional Primate Research Center in accordance with state and federal laws and under protocols approved by the University of Washington Animal Care and Use Committee and the Regional Primate Research Center. Animal care and handling complied with the “Guide for the Care and Use of Laboratory Animals” issued by the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC, National Academies Press, 1996.
Morphological Evaluation
Intimal tissue was fixed in 10% formalin or methyl Carnoy’s, embedded in paraffin, and histological cross-sections were cut. Histochemical characterization of the ECM was performed using Movat’s Pentachrome procedure as described earlier (Movat 1955; Schmidt and Wirtla 1996). The rabbit antiserum to the core protein of human versican was generously provided by Dr. Richard Le Baron (University of Texas at San Antonio, San Antonio, TX) and used at a 1:5000 dilution. The monoclonal antibody to the G3 domain of versican, 2B1, cross-reacts with baboon versican on Western blots (data not presented) and was purchased from Seikagaku America Corporation (East Falmouth, MA). Another rabbit antibody against the βGAG portion of versican V0/V1, which was a kind gift from Dr. Dieter Zimmerman (University of Zurich, Switzerland), was used at 1:250 dilution (Sandy et al. 2001). To detect the ADAMTS-1/4-generated versican cleavage product, an antiserum (JSCDPE, previously described as anti-DPEAAE) against the neoepitope of versican (DPEAAE441) generated by cleavage of the versican core protein by ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motif) -1, -4 was used (Sandy et al. 2001). The antigen-affinity purified JSCDPE was used at 10 ng/ml for immunostaining. Polyclonal rabbit antisera to the core protein of human biglycan (LF-51, 1:500) was a generous gift of Dr. Larry Fisher (Craniofacial and Skeletal Disease Branch of the National Institutes of Health, Bethesda, MD). Sections to be stained for the proteoglycan core proteins of versican and biglycan were digested with chondroitin ABC lyase (ICN Biomedicals; Costa Mesa, CA), 200 mU/ml in 0.05 M Tris/0.06 M sodium acetate/0.05 M sodium chloride/0.1% bovine serum albumin, pH 8.0, 1 hr, 37C. All antibodies were diluted in PBS + 0.1% BSA. Hyaluronan was stained using biotinylated hyaluronan-binding protein kindly provided by Dr. Charles B. Underhill (Georgetown University Medical Center, Washington, DC) (Green et al. 1988).
Results
Normal Shear Stress-mediated Intimal Hyperplasia
Our previous studies demonstrated that switching from high shear stress to normal shear stress (Figure 1A; ~50 dynes/cm2 to ~10 dynes/cm2) increased neointimal area within 4 weeks (Geary et al. 1994). After 4 weeks of normal shear stress, the intima exhibits increased proteoglycan staining (blue color in Movat’s stain; Figure 2B) compared with the high shear stress control (Figure 2A). A gradient of proteoglycan staining is evident with the highest near the lumen and the lowest toward the PTFE graft matrix. In contrast, collagen staining (yellow) is most near the PTFE graft matrix and least near the lumen (Figure 2B). This pattern is also seen with Sirius Red staining in which more tightly packed collagen is evident under polarizing light (Figures 2E and 2F). The neointima formed under high shear stress has tightly packed collagen fibrils as demonstrated under bright field (Field 2C) and under polarized light (Field 2E). Under normal shear stress, the newly formed neointima often shows less tightly packed collagen fibrils (Figure 2D). In addition, the subendothelial layer (arrowheads) shows little collagen staining. Elastic fibers are not observed in either normal or high shear stress intimas (Figures 2A and 2B; elastin stains black).
Figure 2.
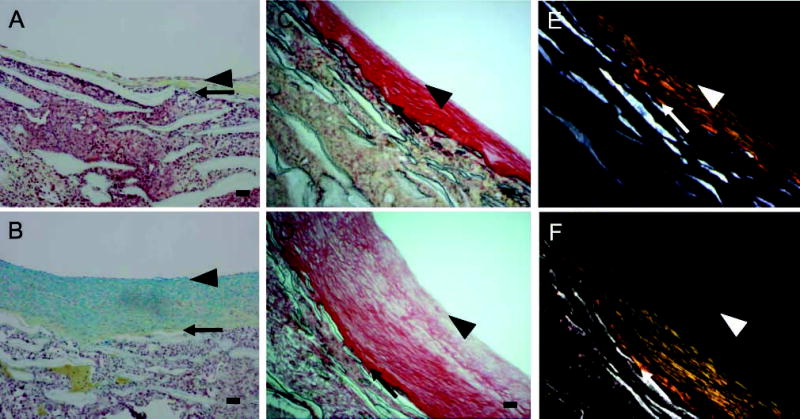
Movat’s pentachrome (A,B) and Sirius Red (C–F) staining of aorto-iliac-PTFE grafts healed for 12 weeks under high flow (A,C,E) or for 8 weeks under high flow and 4 weeks under normal flow (B,D,F). Sirius Red staining was visualized under normal (C,D) or polarizing (E,F) light. Arrows indicate the intima/PTFE boundary, while the arrowheads indicate the subendothelial layer. The Movat’s pentachrome procedure stains proteoglycans blue, collagen yellow, elastin black, and cells red. Bars = 0.1 mm.
Strong immunostaining for the proteoglycan versican with the Vc antibody (Figure 3A vs Figure 3B) and with 2B1 (data not presented) is found evenly distributed throughout the neointima formed under high shear stress during the healing phase and also in the intima that develops under normal shear stress. Hyaluronan staining is increased during intimal expansion and follows the staining pattern for versican (Figure 3C vs Figure 3D). Biglycan is detected mostly in the basal part of the high shear stress neointima (Figure 3E) and is not changed appreciably during intimal expansion (Figure 3F). These data all indicate that the ECM of the growing neointima is rich in proteoglycans and hyaluronan.
Figure 3.
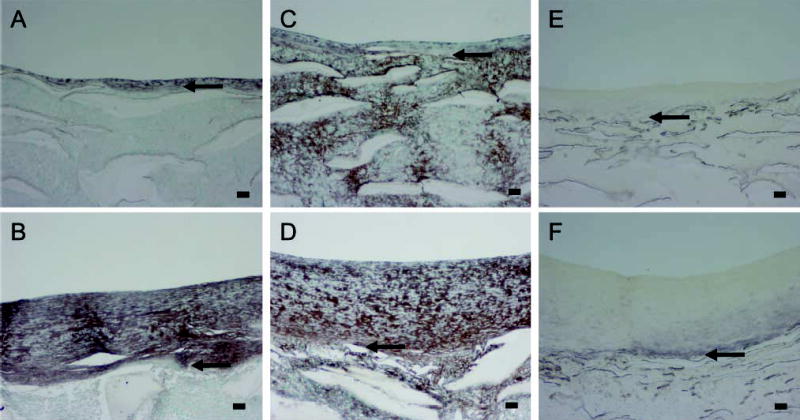
Immunostaining of PTFE grafts healed 12 weeks under high flow (A,C,E) or healed 8 weeks under high flow and 4 weeks under normal flow (B,D,F); versican (A,B); hyaluronan (C,D); biglycan (E,F). Arrows indicate the intima/PTFE boundary. Bars = 0.1 mm.
High Shear Stress-mediated Regression of the Neointima
To examine ECM changes during neointimal regression, blood flow through the grafts, which had healed for 2 months under normal blood flow, was switched to high flow for 4, 7, 14, and 56 days (Figure 1B). Shear stress increased about fourfold [~10 dynes/cm2 to 40–60 dynes/cm2 (Berceli et al. 2002; Mattsson et al. 1997)]. Regression of the neointima is significant by 7 days, and there is a clear gradient of cell density with fewer cells present in the deep intima (Berceli et al. 2002). There is a distinct loss of proteoglycan staining apparent by day 14 (blue stain in Figure 4A vs Figure 4B, normal vs high), which is maintained through day 56 (Figure 5A vs Figure 5B). This proteoglycan stain is lost upon treatment of sections with chondroitin ABC lyase, but not with streptococcal hyaluronidase (results not shown). On the other hand, there is no evidence of a loss in collagen by Sirius Red staining at either 14 (data not shown) or 56 days (Figure 5C vs Figure 5D and polarizing light Figure 5E vs Figure 5F). Immunostaining of the core protein of versican with Vc (Figure 6A vs Figure 6B, normal vs high) or 2B1 (data not presented) and staining of hyaluronan (Figure 6C vs Figure 6D, normal vs high) is not altered by high shear up to day 56. Immunoreactive decorin core protein is not detectable in the intima, although there is a strong signal in the tissue surrounding the graft matrix (data not presented). Finally, immunostaining of biglycan core protein is observed in the deep intima. Although biglycan staining is more intense under high shear stress (Figure 6E vs Figure 6F, normal vs high), it appears that, as the intima regresses, biglycan is simply concentrated in a smaller area. At no time did we observe elastic fibers in the intima.
Figure 4.
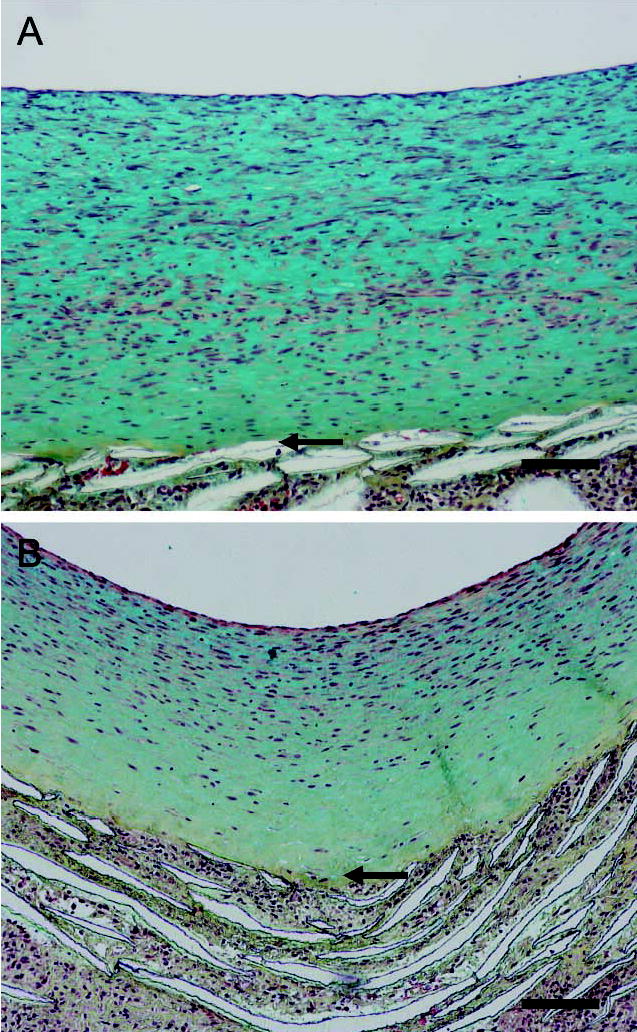
Movat’s pentachrome staining of aorto-iliac-PTFE grafts healed under normal flow conditions for 8 weeks and then an additional 2 weeks under normal (A) or high flow (B). Bars = 0.1 mm.
Figure 5.
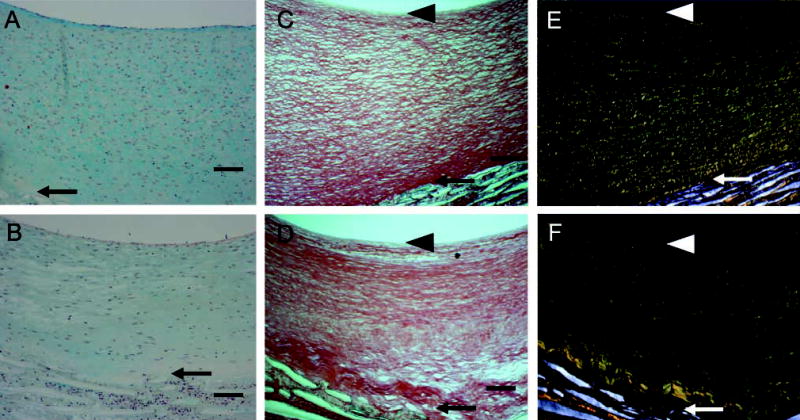
Movat’s pentachrome (A,B) and Sirius Red (C–F) staining of aorto-iliac-PTFE grafts healed for 16 weeks under normal flow (A,C,E) or for 8 weeks under normal flow and 8 weeks under high flow (B,D,F). Sirius Red staining was visualized under normal (C,D) or polarizing (E,F) light. Arrows indicate the intima/PTFE boundary, while the arrowheads indicate the subendothelial layer. The Movat’s pentachrome procedure stains proteoglycans blue, collagen yellow, elastin black, and cells red. Bars = 0.1 mm.
Figure 6.
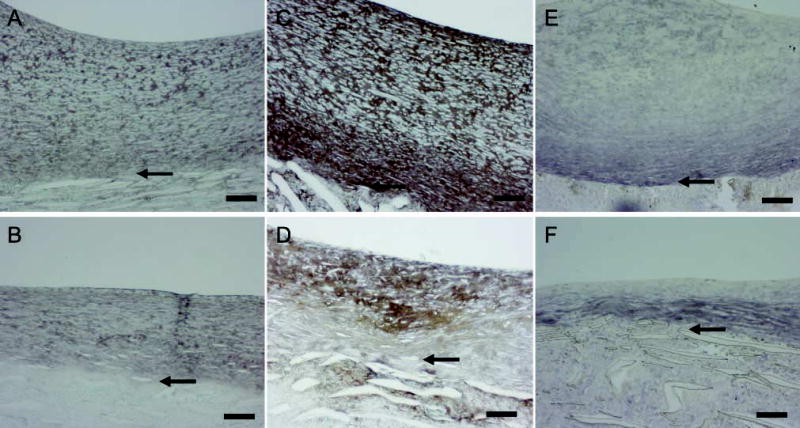
Immunostaining of PTFE grafts healed 16 weeks under normal flow (A,C,E) or healed 8 weeks under normal flow and 8 weeks under high flow (B,D,F); versican (A,B); hyaluronan (C,D); biglycan (E,F). Bars = 0.1 mm.
The loss of proteoglycan staining without a loss of versican core protein staining raises the possibility that the antibodies are staining both intact and partially degraded core proteins. To examine this possibility, we performed immunostaining using JSCDPE, an antibody against the sequence DPEAAE in an ADAMTS-specific cleavage product of versican (Sandy et al. 2001). This antibody intensely stains the region of the intima nearer the graft in both normal and high shear stress intimas (Figures 7A and 7B), demonstrating that versican cleavage products are normally present. Staining for DPE is increased by high shear at 4 days (Figure 7B vs Figure 7A, high vs normal shear stress), but not at later times (data not presented). A 70-kD protein is detected with the antibody to DPEAAE on western blots of graft intimal extracts (insert of Figure 7C). This is the N-terminal ADAMTS cleavage product of versican V1 (Sandy et al. 2001). Western blot analysis shows that the 70-kD DPE fragment of versican is increased by high shear stress at 4 days (Figure 7C). We also stained sections with an affinity-purified antibody to the βGAG domain to which GAG chains are attached. Immunoreactive βGAG domain is not altered by high shear stress at 14 days (data not presented), but is decreased at day 56 (Figure 8A vs Figure 8B). Because neither staining with Vc (active against epitopes in the N terminus as well as C terminus) nor 2B1 (active against an epitope in the C terminus) is altered at 56 days, the βGAG domain data suggest a preferential loss of the glycosaminoglycan-bearing region of versican after the ADAMTS-like cleavage at day 4.
Figure 7.
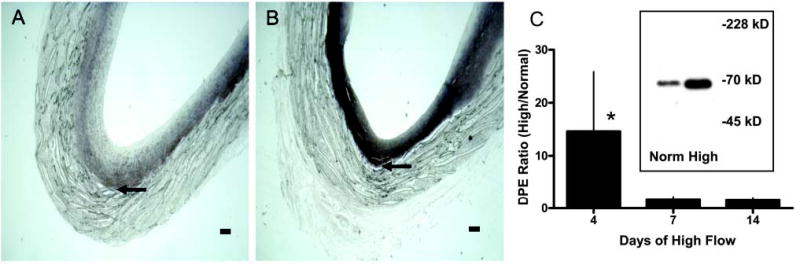
Immunostaining for the versican neoepitope DPEAAE of aorto-iliac-PTFE grafts healed under normal flow conditions for 8 weeks and then an additional 4 days under normal (A) or high (B) flow. Bars = 0.1 mm. Western blots (inset panel C) of graft intimal extracts were probed with JSCDPE, and the 70-kD band was scanned for quantitation (C). *p<0.05 high vs normal flow; n=4–6.
Figure 8.
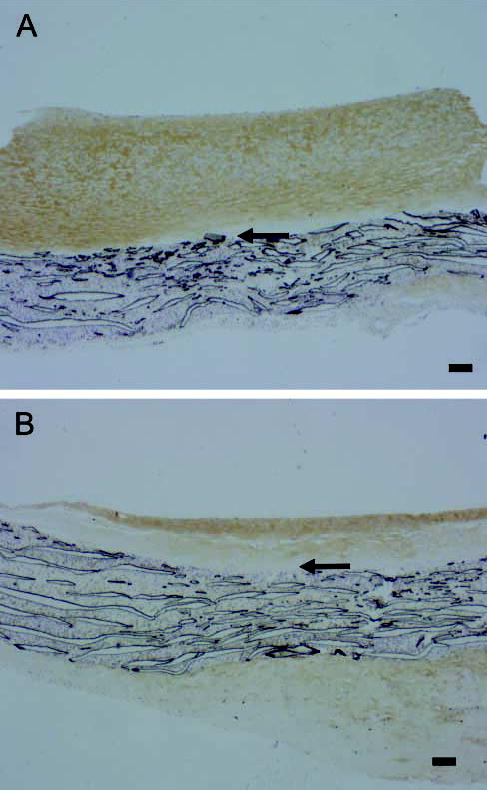
Immunostaining for the βGAG domain of versican in aorto-iliac-PTFE grafts healed under normal flow conditions for 8 weeks and then an additional 56 days (A,B) under normal (A) or high (B) flow. Arrows indicate the intima/PTFE boundary. Bars = 0.1 mm.
Discussion
We have shown that changes in the ECM are a significant aspect of the process of intimal hyperplasia and regression in this non-human primate graft model. An important observation is that under all conditions studied the graft neointima is rich in hyaluronan and versican and lacks elastin. As such, it is similar to the hyaluronan-, versican-rich, elastin fiber-poor intimal ECM produced after angioplasty (Nikkari et al. 1994; Riessen et al. 1996; Wight et al. 1997; Geary et al. 1998; Imanaka-Yoshida et al. 2001) and after stent placement (Finn et al. 2002; Inoue et al. 2002). These intimas also demonstrate a gradient of ECM proteins. The luminal side of the intima tends to be richer in glycosaminoglycans, while the deeper intima contains more collagen (Snow et al. 1990; Stary 1990; Inoue et al. 2002). High versican and low elastin fiber content (none was observed in the baboon graft intima) as seen in restenotic lesions (Wight et al. 1997) may be the result of the chondroitin sulfate chains of versican disrupting elastin fiber assembly [discussed by (Merrilees et al. 2002)]. In addition to the similarity in ECM, PTFE grafts and stented arteries also share a fixed geometry and a foreign body response with high macrophage content in the graft material or near stent wires with increased cell death and decreased cell density near the foreign materials (Golden et al. 1991; Kollum et al. 1997; Akimaro et al. 2002; Berceli et al. 2002; Inoue et al. 2002). Also, at late times most cell proliferation occurs in grafts and stented arteries in SMCs near the endothelial cells of the lumen (Berceli et al. 2002; Inoue et al. 2002). Thus, data from this PTFE graft model may be of particular relevance to the problem of restenosis in stented arteries as well as in synthetic grafts.
Intimal Hyperplasia
In the growing neointima, after the switch to normal blood flow there is a relative enrichment in hyaluronan and proteoglycans compared with the high-flow controls. The lack of a clear change in staining of the major arterial proteoglycan versican in the face of increased proteoglycan histochemical staining suggests the possibility that the glycosaminoglycan portion of the proteoglycans may be selectively altered. For example, it may be that the length of the glycosaminoglycan chains attached to the core protein is increased. Such an effect has been observed after arterial injury in the rabbit (Srinivasan et al. 1993). In addition, PDGF, which plays a critical role in graft intimal hyperplasia (Davies et al. 2000), increases the chain length of glycosaminoglycans in versican in vitro (Schonherr et al. 1991,1997). A role for hyaluronan and versican in intimal hyperplasia beyond a simple structural role is suggested by the observation that each has been shown to promote SMC proliferation and migration (Evanko et al. 1999). Of interest, the G1 and G3 domains of versican have mitogenic activity in vitro (Zhang et al. 1998; Yang et al. 1999), and the G3 domain decreases apoptosis via binding to the β1 integrin (Wu et al. 2002). Both effects would contribute to an increased number of cells (Yang et al. 1999). In our graft model, SMCs proliferate in the subendothelial zone (Berceli et al. 2002) of the PTFE graft matrix where there is less collagen and more proteoglycans. In contrast, the collagen content appears to be the highest and proteoglycan content the lowest in the deep intima, a region where SMC apoptosis occurs (Berceli et al. 2002). Biglycan is also found mostly in the deep intima, similar to the codistribution of biglycan and collagen seen in atherosclerotic and restenotic lesions (Riessen et al. 1994).
Intimal Atrophy
Our data suggest that ECM degradation contributes significantly to high shear stress-mediated intimal atrophy in baboon PTFE grafts. We have previously shown that overall nuclear density does not change during atrophy, suggesting that as cells are lost a portion of ECM is also lost (Geary et al. 1994; Mattsson et al. 1997). This is consistent with the concept of an ECM “domain” for SMC (Snow et al. 1990) that is relatively constant in volume but may have different components depending on location and experimental treatment. Proteolytic cleavage of versican is increased at 4 days as demonstrated by increased staining with JSCDPE. In addition, there are discrete low-molecular-weight versican fragments in both arterial samples (Sandy et al. 2001; Formato et al. 2004) (Kinsella, Evanko, and Wight, unpublished observations) and the baboon graft intima (Kenagy et al., unpublished data) indicating that versican undergoes proteolytic processing in vivo. Discrete proteolytic clips like that at E441A442 by the ADAMTS-like activity may be necessary for further activity by proteases, as observed for aggrecan (Arner 2002) and for the sequential action of plasmin and matrix metalloproteinases (MMPs) on matrix collagen (Montgomery et al. 1993) and of plasmin and heparinases on matrix heparan sulfates (Bar-Ner et al. 1986). We have previously demonstrated increased serine protease (including plasmin) activity against versican in extracts of day 4 high-flow intimas (Kenagy et al. 2002). Also, although MMPs do degrade versican (Halpert et al. 1996; Kenagy et al. 2002), there is no evidence of MMP activity against versican in these extracts suggesting that endogenous MMP inhibitors such as TIMP 1 block the MMPs in these extracts (Kenagy et al. 2002). By 14 days, a loss of proteoglycan staining in the graft intima is evident. The loss of proteoglycans may be responsible for a “collapse” of the collagen matrix onto itself (Figure 3 and Figure 4). These results are consistent with our previous observation that nuclear density in the graft intima is increased only after 14 days of high shear stress and only near the lumen where the loss of glycosaminoglycans is most evident (Berceli et al. 2002).
The major glycosaminoglycan in vascular tissue is chondroitin sulfate, and the only reported mechanism for degradation of chondroitin sulfate in mammalians is via the hyaluronidases. Hyal-1 degrades chondroitin sulfate at a lower rate than hyaluronan, but Hyal-4 has been reported to be a specific chondroitinase, although no data were given (Csoka et al. 2001). Nothing is known about the regulation of these enzymes after arterial injury or during graft healing, although it is interesting that levels of both heparan sulfate and chondroitin sulfate decrease within 6 hr after arterial injury with peak loss at 4 days (Bingley et al. 2001). The increased ADAMTS activity at day 4 cleaves versican into fragments with and without chondroitin sulfate chains. Immunostaining of the βGAG domain of versican, but not of the C-terminal (2B1) or of the N-terminal (JSCDPE) domains, is decreased by high shear stress at late times only after proteoglycan staining decreases at 14 days. This suggests the possibility that the βGAG fragment is degraded only after its chondroitin sulfate chains are removed.
In conclusion, the neointimal ECM of prosthetic vascular grafts in baboons contains large amounts of versican and hyaluronan. These molecules accumulate under normal shear stress and are removed during atrophy induced by high shear stress. Versican is actively removed by proteolytic cleavage of the core protein by one or more of the proteases of the “aggrecanase” type, namely, ADAMTS-1, -4, -5, -8, -9, and -15. The present findings support the hypothesis that deposition or removal of proteoglycans, in particular versican, mediates control of ECM-volume. Among the proteoglycans expressed in the vasculature, versican is the most abundant (Theocharis et al. 2001) and carries the most glycosaminoglycan chains (>15). Thus, it contributes considerably to the water content and volume of the intima, making its deposition and removal an effective mechanism for expansion or regression of the graft-intima. Serial quantitative angiography in humans performed up to 3 years following coronary artery stenting has indicated spontaneous intimal regression (Asakura et al. 1998; Kuroda et al. 2002). Studies in pigs and rats also show regression of in-stent restenosis over time (Kim et al. 2000; Finn et al. 2002). The mechanism of this effect is not known, but a role for shear stress is supported by the inverse correlation between shear stress and lumenal area in stented coronary arteries (Wentzel et al. 2001). Spontaneous regression may also relate to changes in the ECM, in which relative levels of versican decrease and levels of collagen increase (Imanaka-Yoshida et al. 2001; Finn et al. 2002). Thus, strategies to block versican deposition or induce versican cleavage may be of clinical value.
Acknowledgments
Supported by U.S. Public Health Service (HL30946, RR00166 and HL07828) and ZymoGenetics Inc. PTFE grafts were provided by W.L. Gore & Associates and polypropylene suture by Davis & Geck. Dr Fischer is recipient of a Schering Research Foundation postdoctoral fellowship.
We thank Drs Randolph Geary, Scott Berceli, Mark Davies, and Erney Mattsson for their work in originally producing the archived graft material we have studied. We thank Robert A. Underwood for his help with the polarizing light microscopy.
References
- Akimaro KF, Nishibe T, Miyazaki K, Watanabe S, Flores J, Yasuda K. Induction of apoptosis after stent implantation in canine portal vein. Ann Vasc Surg. 2002;16:456–461. doi: 10.1007/s10016-001-0085-9. [DOI] [PubMed] [Google Scholar]
- Arner EC. Aggrecanase-mediated cartilage degradation. Curr Opin Pharmacol. 2002;2:322–329. doi: 10.1016/s1471-4892(02)00148-0. [DOI] [PubMed] [Google Scholar]
- Asakura M, Ueda Y, Nanto S, Hirayama A, Adachi T, Kitakaze M, Hori M, et al. Remodeling of in-stent neointima, which became thinner and transparent over 3 years—serial angiographic and angioscopic follow-up. Circulation. 1998;97:2003–2006. doi: 10.1161/01.cir.97.20.2003. [DOI] [PubMed] [Google Scholar]
- Bar-Ner M, Mayer M, Schirrmacher V, Vlodavsky I. Involvement of both heparanase and plasminogen activator in lymphoma cell-mediated degradation of heparan sulfate in the subendothelial extracellular matrix. J Cell Physiol. 1986;128:299–306. doi: 10.1002/jcp.1041280223. [DOI] [PubMed] [Google Scholar]
- Berceli SA, Davies MG, Kenagy RD, Clowes AW. Flow-induced neointimal regression in baboon polytetrafluoroethylene grafts is associated with decreased cell proliferation and increased apoptosis. J Vasc Surg. 2002;36:1248–1255. doi: 10.1067/mva.2002.128295. [DOI] [PubMed] [Google Scholar]
- Bingley JA, Hayward IP, Campbell GR, Campbell JH. Relationship of glycosaminoglycan and matrix changes to vascular smooth muscle cell phenotype modulation in rabbit arteries after acute injury. J Vasc Surg. 2001;33:155–164. doi: 10.1067/mva.2001.109774. [DOI] [PubMed] [Google Scholar]
- Clowes AW, Kirkman TR, Reidy MA. Mechanisms of arterial graft healing. Rapid transmural capillary ingrowth provides a source of intimal endothelium and smooth muscle in porous PTFE prostheses. Am J Pathol. 1986;123:220–230. [PMC free article] [PubMed] [Google Scholar]
- Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20:499–508. doi: 10.1016/s0945-053x(01)00172-x. [DOI] [PubMed] [Google Scholar]
- Davies MG, Owens EL, Mason DP, Lea H, Tran PK, Vergel S, Hawkins SA, et al. Effect of platelet-derived growth factor receptor-α and -β blockade on flow-induced neointimal formation in endothelialized baboon vascular grafts. Circ Res. 2000;86:779–786. doi: 10.1161/01.res.86.7.779. [DOI] [PubMed] [Google Scholar]
- Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- Finn AV, Gold HK, Tang A, Weber DK, Wight TN, Clermont A, Virmani R, et al. A novel rat model of carotid artery stenting for the understanding of restenosis in metabolic diseases. J Vasc Res. 2002;39:414–425. doi: 10.1159/000064518. [DOI] [PubMed] [Google Scholar]
- Formato M, Farina M, Spirito R, Maggioni M, Guarino A, Cherchi GM, Biglioli P, et al. Evidence for a proinflammatory and proteolytic environment in plaques from endarterectomy segments of human carotid arteries. Arterioscler Thromb Vasc Biol. 2004;24:129–135. doi: 10.1161/01.ATV.0000104013.71118.53. [DOI] [PubMed] [Google Scholar]
- Garratt KN, Edwards WD, Kaufmann UP, Vlietstra RE, Holmes DR., Jr Differential histopathology of primary atherosclerotic and restenotic lesions in coronary arteries and saphenous vein bypass grafts: analysis of tissue obtained from 73 patients by directional atherectomy. J Am Coll Cardiol. 1991;17:442–448. doi: 10.1016/s0735-1097(10)80113-5. [DOI] [PubMed] [Google Scholar]
- Garratt KN, Holmes DR, Jr, Bell MR, Berger PB, Kaufmann UP, Bresnahan JF, Vlietstra RE. Results of directional atherectomy of primary atheromatous and restenosis lesions in coronary arteries and saphenous vein grafts. Am J Cardiol. 1992;70:449–454. doi: 10.1016/0002-9149(92)91188-a. [DOI] [PubMed] [Google Scholar]
- Geary RL, Kohler TR, Vergel S, Kirkman TR, Clowes AW. Time course of flow-induced smooth muscle cell proliferation and intimal thickening in endothelialized baboon vascular grafts. Circ Res. 1994;74:14–23. doi: 10.1161/01.res.74.1.14. [DOI] [PubMed] [Google Scholar]
- Geary RL, Nikkari ST, Wagner WD, Williams JK, Adams MR, Dean RH. Wound healing: a paradigm for lumen narrowing after arterial reconstruction. J Vasc Surg. 1998;27:96–106. doi: 10.1016/s0741-5214(98)70296-4. [DOI] [PubMed] [Google Scholar]
- Golden MA, Au YPT, Kirkman TR, Wilcox JN, Raines EW, Ross R, Clowes AW. Platelet-derived growth factor activity and mRNA expression in healing vascular grafts in baboons. Association in vivo of platelet-derived growth factor mRNA and protein with cellular proliferation. J Clin Invest. 1991;87:406–414. doi: 10.1172/JCI115011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SJ, Tarone G, Underhill CB. Distribution of hyaluronate and hyaluronate receptors in the adult lung. J Cell Sci. 1988;90:145–156. doi: 10.1242/jcs.90.1.145. [DOI] [PubMed] [Google Scholar]
- Halpert I, Sires UI, Roby JD, Potter-Perigo S, Wight TN, Shapiro SD, Welgus HG, et al. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci USA. 1996;93:9748–9753. doi: 10.1073/pnas.93.18.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann R, Mintz GS, Dussaillant GR, Popma JJ, Pichard AD, Satler LF, Kent KM, et al. Patterns and mechanisms of in-stent restenosis—a serial intravascular ultrasound study. Circulation. 1996;94:1247–1254. doi: 10.1161/01.cir.94.6.1247. [DOI] [PubMed] [Google Scholar]
- Imanaka-Yoshida K, Matsuura R, Isaka N, Nakano T, Sakakura T, Yoshida T. Serial extracellular matrix changes in neointimal lesions of human coronary artery after percutaneous transluminal coronary angioplasty: clinical significance of early tenascin-C expression. Virch Arch Int J Pathol. 2001;439:185–190. doi: 10.1007/s004280000390. [DOI] [PubMed] [Google Scholar]
- Inoue S, Koyama H, Miyata T, Shigematsu H. Pathogenetic heterogeneity of in-stent lesion formation in human peripheral arterial disease. J Vasc Surg. 2002;35:672–678. doi: 10.1067/mva.2002.122021. [DOI] [PubMed] [Google Scholar]
- Kenagy RD, Fischer JW, Davies MG, Berceli SA, Hawkins SM, Wight TN, Clowes AW. Increased plasmin and serine proteinase activity during flow-induced intimal atrophy in baboon PTFE grafts. Arterioscler Thromb Vasc Biol. 2002;22:400–404. doi: 10.1161/hq0302.105376. [DOI] [PubMed] [Google Scholar]
- Kim WH, Hong MK, Virmani R, Kornowski R, Jones R, Leon MB. Histopathologic analysis of in-stent neointimal regression in a porcine coronary model. Coronary Artery Dis. 2000;11:273–277. doi: 10.1097/00019501-200005000-00011. [DOI] [PubMed] [Google Scholar]
- Kohler TR, Kirkman TR, Kraiss LW, Zierler BK, Clowes AW. Increased blood flow inhibits neointimal hyperplasia in endothelialized vascular grafts. Circ Res. 1991;69:1557–1565. doi: 10.1161/01.res.69.6.1557. [DOI] [PubMed] [Google Scholar]
- Kollum M, Kaiser S, Kinscherf R, Metz J, Kubler W, Hehrlein C. Apoptosis after stent implantation compared with balloon angioplasty in rabbits—role of macrophages. Arterioscler Thromb Vasc Biol. 1997;17:2383–2388. doi: 10.1161/01.atv.17.11.2383. [DOI] [PubMed] [Google Scholar]
- Kuroda N, Kobayashi Y, Nameki M, Kuriyama N, Kinoshita T, Okuno T, Yamamoto Y, et al. Intimal hyperplasia regression from 6 to 12 months after stenting. Am J Cardiol. 2002;89:869–872. doi: 10.1016/s0002-9149(02)02205-1. [DOI] [PubMed] [Google Scholar]
- Mattsson EJR, Kohler TR, Vergel SM, Clowes AW. Increased blood flow induces regression of intimal hyperplasia. Arterioscler Thromb Vasc Biol. 1997;17:2245–2249. doi: 10.1161/01.atv.17.10.2245. [DOI] [PubMed] [Google Scholar]
- Merrilees MJ, Lemire JM, Fischer JW, Kinsella MG, Braun KR, Clowes AW, Wight TN. Retrovirally mediated overexpression of versican V3 by arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injury. Circ Res. 2002;90:481–487. doi: 10.1161/hh0402.105791. [DOI] [PubMed] [Google Scholar]
- Montgomery AMP, De Clerck YA, Langley KE, Reisfeld RA, Mueller BM. Melanoma-mediated dissolution of extracellular matrix: contribution of urokinase-dependent and metalloproteinase-dependent proteolytic pathways. Cancer Res. 1993;53:693–700. [PubMed] [Google Scholar]
- Movat HZ. Demonstration of all connective tissue elements in a single section. Arch Pathol. 1955;60:289–295. [PubMed] [Google Scholar]
- Nikkari ST, Järveläinen HT, Wight TN, Ferguson M, Clowes AW. Smooth muscle cell expression of extracellular matrix genes after arterial injury. Am J Pathol. 1994;144:1348–1356. [PMC free article] [PubMed] [Google Scholar]
- Raines EW. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int J Exp Pathol. 2000;81:173–182. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riessen R, Isner JM, Blessing E, Loushin C, Nikol S, Wight TN. Regional differences in the distribution of the proteoglycans biglycan and decorin in the extracellular matrix of atherosclerotic and restenotic human coronary arteries. Am J Pathol. 1994;144:962–974. [PMC free article] [PubMed] [Google Scholar]
- Riessen R, Wight TN, Pastore C, Henley C, Isner JM. Distribution of hyaluronan during extracellular matrix remodeling in human restenotic arteries and balloon-injured rat carotid arteries. Circulation. 1996;93:1141–1147. doi: 10.1161/01.cir.93.6.1141. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, et al. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276:13372–13378. doi: 10.1074/jbc.M009737200. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Wirtla J. Modification of movat pentachrome stain with improved reliability of elastin staining. J Histotechnol. 1996;19:325–327. [Google Scholar]
- Schonherr E, Jarvelainen HT, Sandell LJ, Wight TN. Effects of platelet-derived growth factor and transforming growth factor-beta1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J Biol Chem. 1991;266:17640–17647. [PubMed] [Google Scholar]
- Schonherr E, Kinsella MG, Wight TN. Genistein selectively inhibits platelet-derived growth factor-stimulated versican biosynthesis in monkey arterial smooth muscle cells. Arch Biochem Biophys. 1997;339:353–361. doi: 10.1006/abbi.1996.9854. [DOI] [PubMed] [Google Scholar]
- Snow AD, Bolender RP, Wight TN, Clowes AW. Heparin modulates the composition of the extracellular matrix domain surrounding arterial smooth muscle cells. Am J Pathol. 1990;137:313–330. [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SR, Xu JH, Vijayagopal P, Radhakrishnamurthy B, Berenson GS. Injury to the arterial wall of rabbits produces proteoglycan variants with enhanced low-density lipoprotein-binding property. Biochim Biophys Acta. 1993;1168:158–166. doi: 10.1016/0005-2760(93)90120-x. [DOI] [PubMed] [Google Scholar]
- Stary HC. The sequence of cell and matrix changes in atherosclerotic lesions of coronary arteries in the first forty years of life. Eur Heart J. 1990;11(Suppl E):3–19. doi: 10.1093/eurheartj/11.suppl_e.3. [DOI] [PubMed] [Google Scholar]
- Strauss BH, Chisholm RJ, Keeley FW, Gotlieb AI, Logan RA, Armstrong PW. Extracellular matrix remodeling after balloon angioplasty injury in a rabbit model of restenosis. Circ Res. 1994;75:650–658. doi: 10.1161/01.res.75.4.650. [DOI] [PubMed] [Google Scholar]
- Theocharis AD, Tsolakis I, Hjerpe A, Karamanos NK. Human abdominal aortic aneurysm is characterized by decreased versican concentration and specific downregulation of versican iso-form V-0. Atherosclerosis. 2001;154:367–376. doi: 10.1016/s0021-9150(00)00504-9. [DOI] [PubMed] [Google Scholar]
- Toole BP, Wight TN, Tammi MI. Hyaluronan-cell interactions in cancer and vascular disease. J Biol Chem. 2002;277:4593–4596. doi: 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]
- Wentzel JJ, Krams R, Schuurbiers JCH, Oomen JA, Kloet J, Van der Giessen WJ, Serruys PW, et al. Relationship between neointimal thickness and shear stress after wallstent implantation in human coronary arteries. Circulation. 2001;103:1740–1745. doi: 10.1161/01.cir.103.13.1740. [DOI] [PubMed] [Google Scholar]
- Wight TN (1996) The vascular extracellular matrix. In Fuster V, ed. Atherosclerosis and Coronary Artery Disease. New York, Raven Press, 421–440
- Wight TN, Lara S, Riessen R, Le Baron R, Isner J. Selective deposits of versican in the extracellular matrix of restenotic lesions from human peripheral arteries. Am J Pathol. 1997;151:963–973. [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Chen L, Zheng PS, Yang BB. beta 1-Integrin-mediated glioma cell adhesion and free radical-induced apoptosis are regulated by binding to a C-terminal domain of PG-M/versican. J Biol Chem. 2002;277:12294–12301. doi: 10.1074/jbc.M110748200. [DOI] [PubMed] [Google Scholar]
- Yang BL, Zhang Y, Cao L, Yang BB. Cell adhesion and proliferation mediated through the G1 domain of versican. J Cell Biochem. 1999;72:210–220. doi: 10.1002/(sici)1097-4644(19990201)72:2<210::aid-jcb5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao L, Yang BL, Yang BB. The G3 domain of versican enhances cell proliferation via epidermal growth factor-like motifs. J Biol Chem. 1998;273:21342–21351. doi: 10.1074/jbc.273.33.21342. [DOI] [PubMed] [Google Scholar]


