Abstract
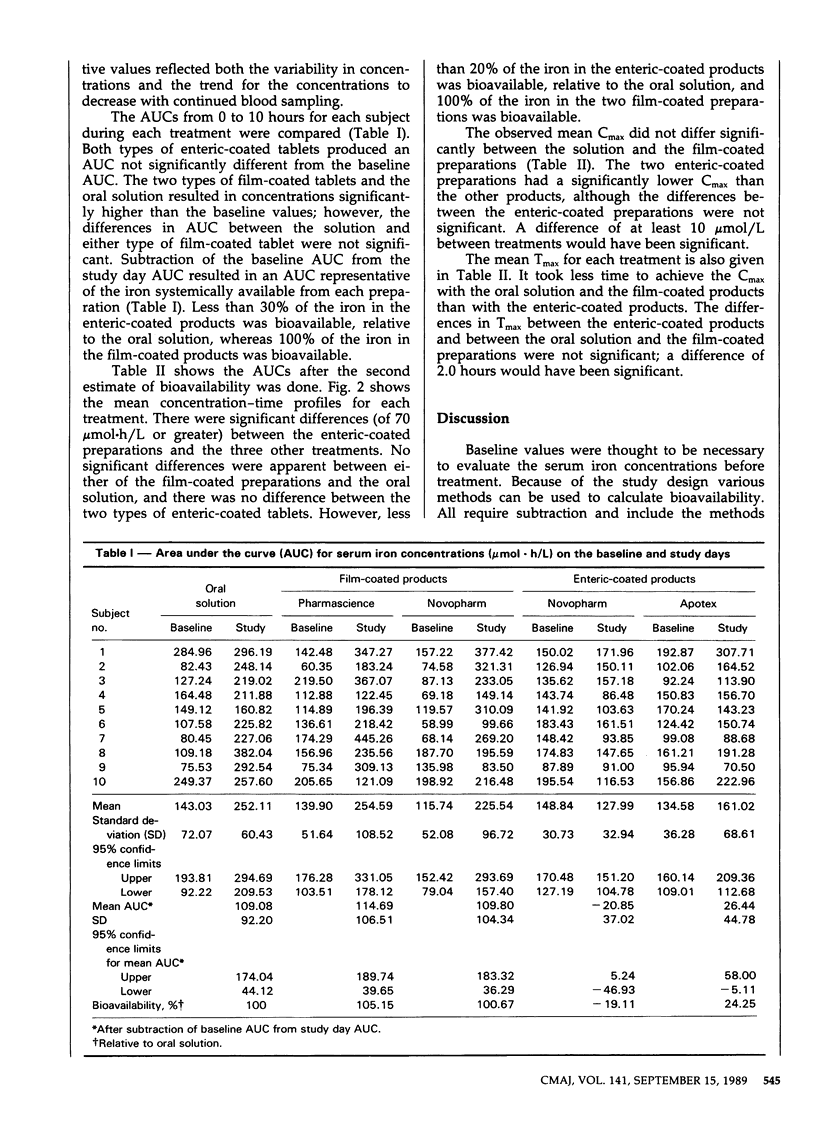
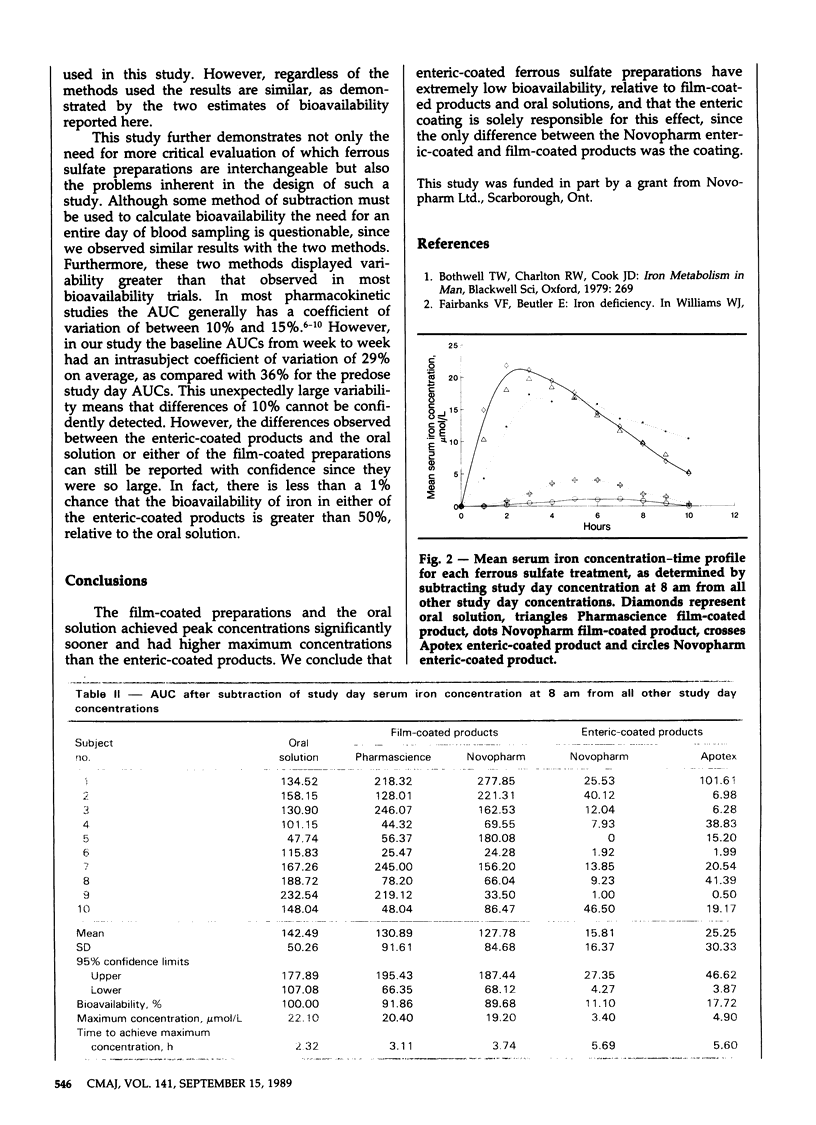
The bioavailability of iron in five ferrous sulfate preparations was studied in 10 healthy male volunteers. The preparations were an oral solution, two types of film-coated tablets and two types of enteric-coated tablets. Blood samples were drawn hourly from 8 am to 6 pm on the day before each study day to assess baseline serum iron concentrations and on the study day. Spectrophotometry was used to measure the serum iron concentrations. The area under the curve (AUC), the maximum concentration and the time to achieve the maximum concentration were compared by analysis of variance. The enteric-coated preparations resulted in AUCs less than 30% of the AUC for the oral solution. The two film-coated products produced AUCs essentially equivalent to that of the oral solution. We conclude that the bioavailability of iron in the enteric-coated preparations was low, relative to that of the film-coated products and the oral solution, and that these products should not be considered interchangeable.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boggs D. R. Fate of a ferrous sulfate prescription. Am J Med. 1987 Jan;82(1):124–128. doi: 10.1016/0002-9343(87)90387-1. [DOI] [PubMed] [Google Scholar]
- Bunting P. S., Aggarwal M. Interference from renal dialysis patients' specimens in a direct method for serum iron. Clin Chem. 1983 Jun;29(6):1106–1108. [PubMed] [Google Scholar]
- Chiou W. L. Ferrous sulfate. J Am Pharm Assoc. 1977 Jun;17(6):377–380. doi: 10.1016/s0003-0465(16)34221-5. [DOI] [PubMed] [Google Scholar]
- Stolley P. D., Strom B. L. Sample size calculations for clinical pharmacology studies. Clin Pharmacol Ther. 1986 May;39(5):489–490. doi: 10.1038/clpt.1986.85. [DOI] [PubMed] [Google Scholar]
- Upton R. A., Thiercelin J. F., Guentert T. W., Wallace S. M., Powell J. R., Sansom L., Riegelman S. Intraindividual variability in theophylline pharmacokinetics: statistical verification in 39 of 60 healthy young adults. J Pharmacokinet Biopharm. 1982 Apr;10(2):123–134. doi: 10.1007/BF01062330. [DOI] [PubMed] [Google Scholar]
- Walker S. E., Paton T. W., Iazzetta J. Single dose cross-over theophylline bioavailability study. Br J Clin Pract. 1983 Jan;37(1):23–27. [PubMed] [Google Scholar]


