Abstract
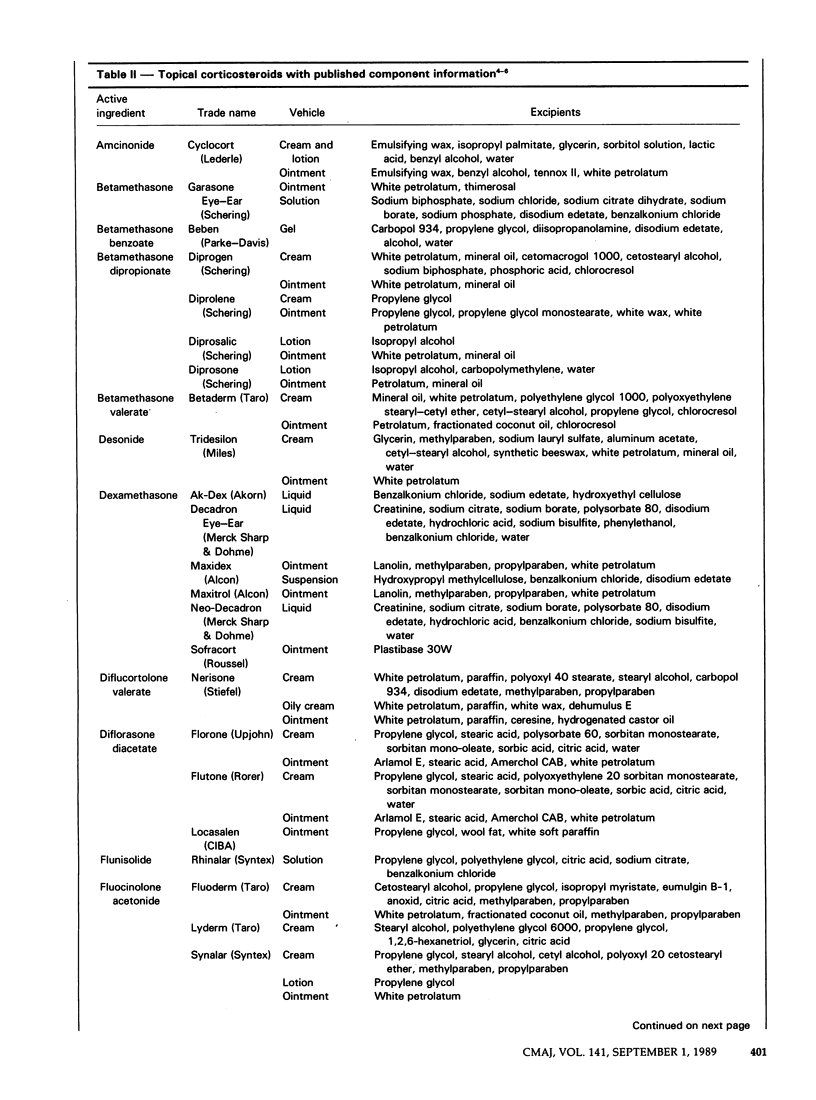
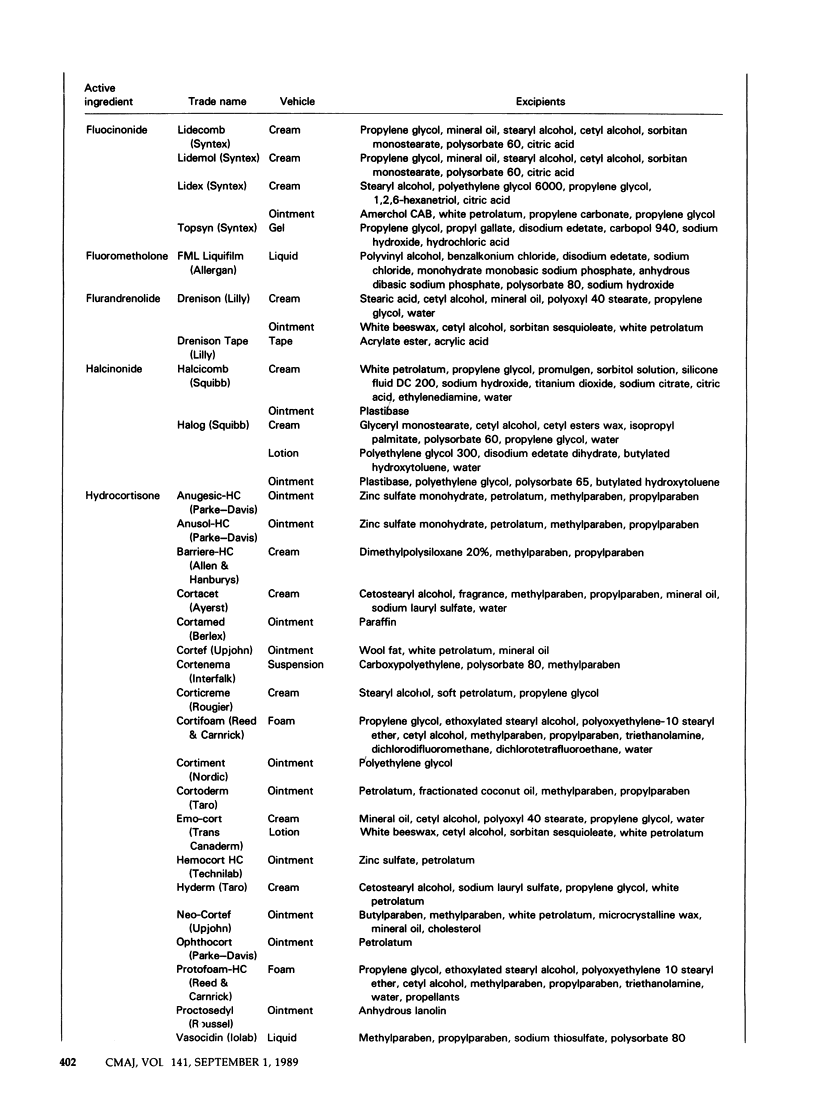
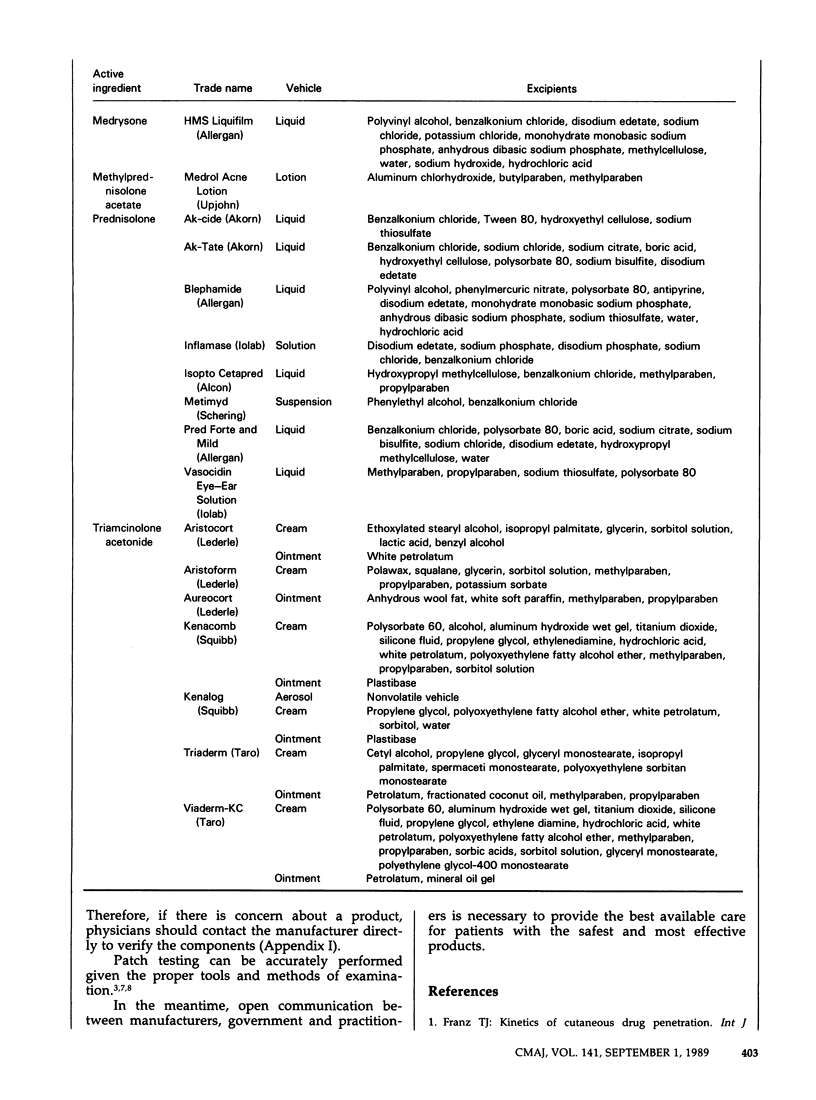
Topical corticosteroids are widely used for the treatment of dermatoses in Canada. The effects of the various nontherapeutic components of these formulations are less well known than those of the active ingredients and may cause adverse reactions. Information on the components is fragmentary and is scattered throughout the literature. We have attempted to consolidate this information into one source. Recent provincial legislation requiring the generic substitution of interchangeable products and the nondisclosure of all ingredients in product labelling hinder the search for an excipient that has caused an adverse reaction. Practitioner participation in the Cutaneous Adverse Reaction Registry of the Canadian Dermatology Association will identify sensitizing excipients and will support efforts by the profession to obtain more effective and safer products.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Stoughton R. B. Are generic formulations equivalent to trade name topical glucocorticoids? Arch Dermatol. 1987 Oct;123(10):1312–1314. [PubMed] [Google Scholar]
- Sulzberger M. B. The patch test--who should and should not use it and why. Contact Dermatitis. 1975;1(2):117–119. doi: 10.1111/j.1600-0536.1975.tb05337.x. [DOI] [PubMed] [Google Scholar]


