Abstract
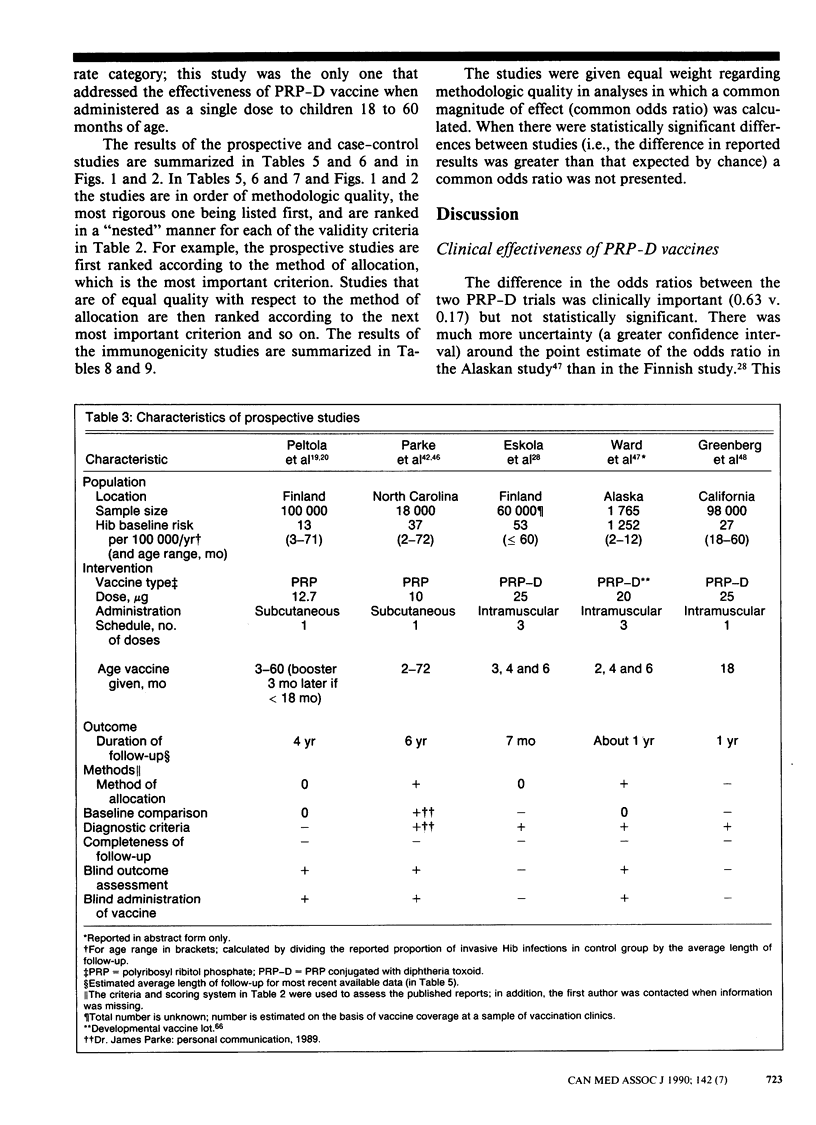
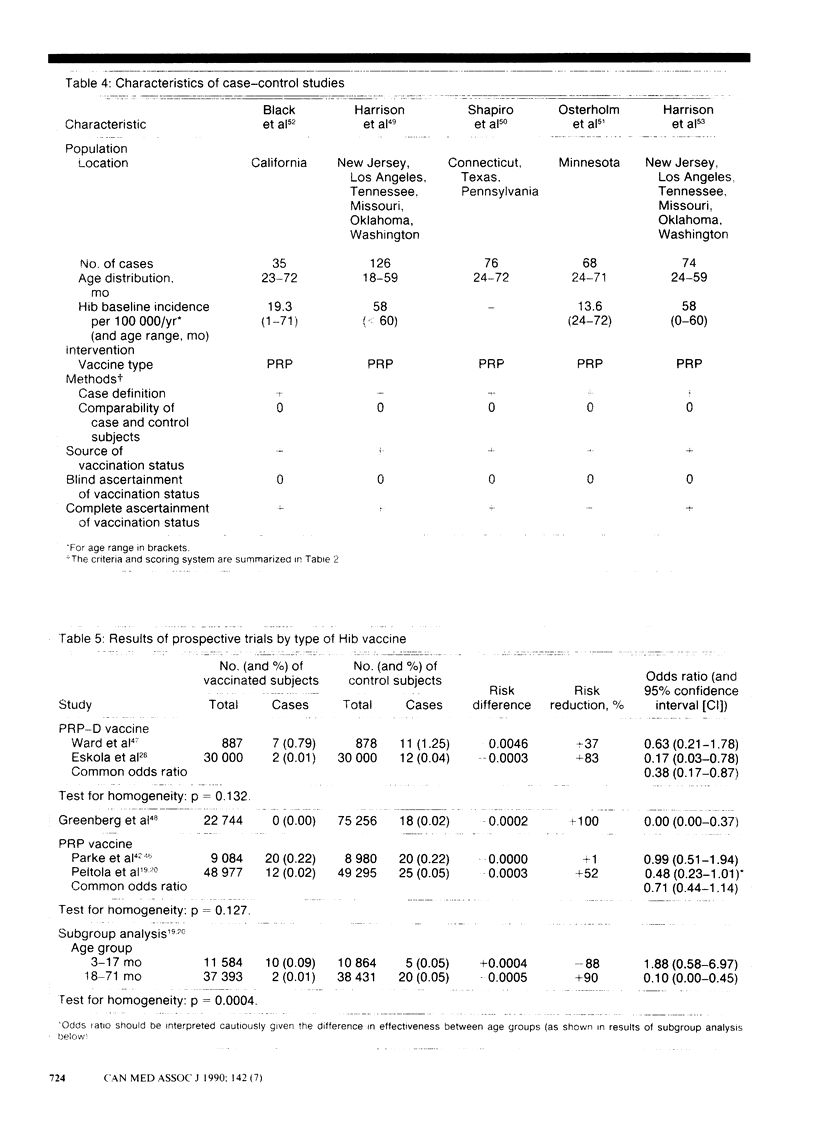
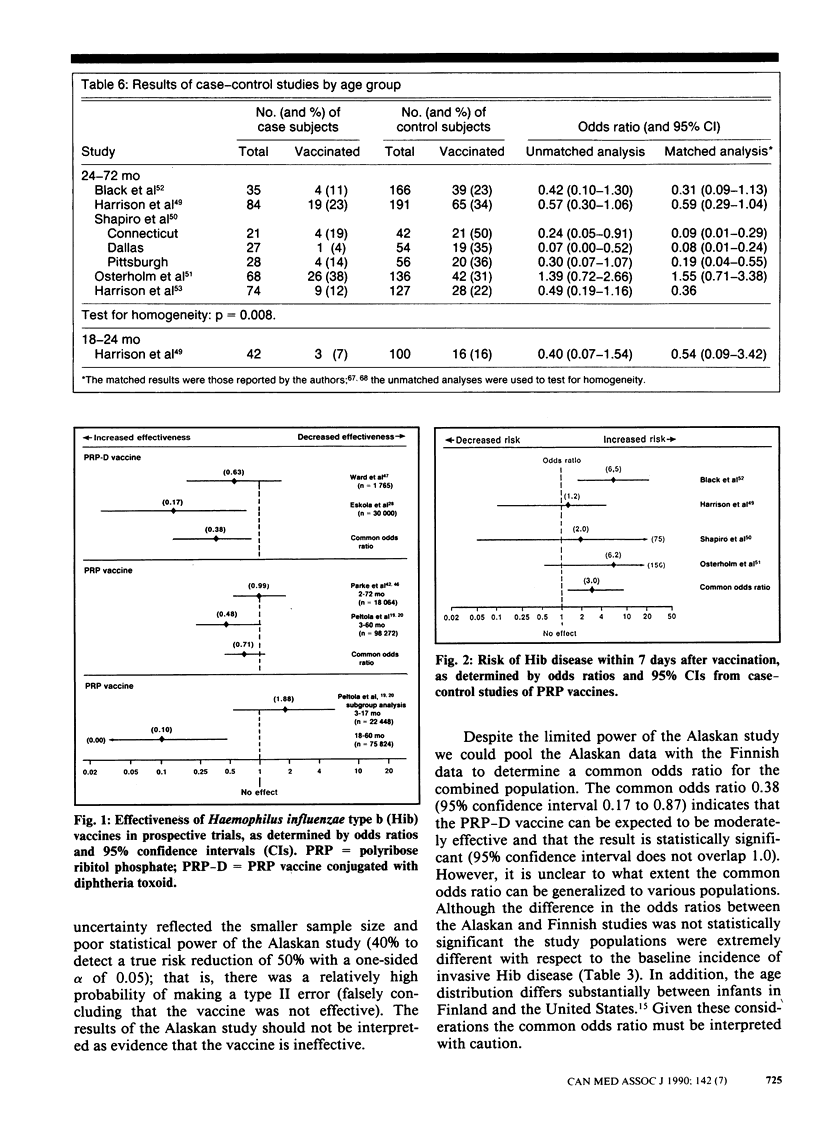
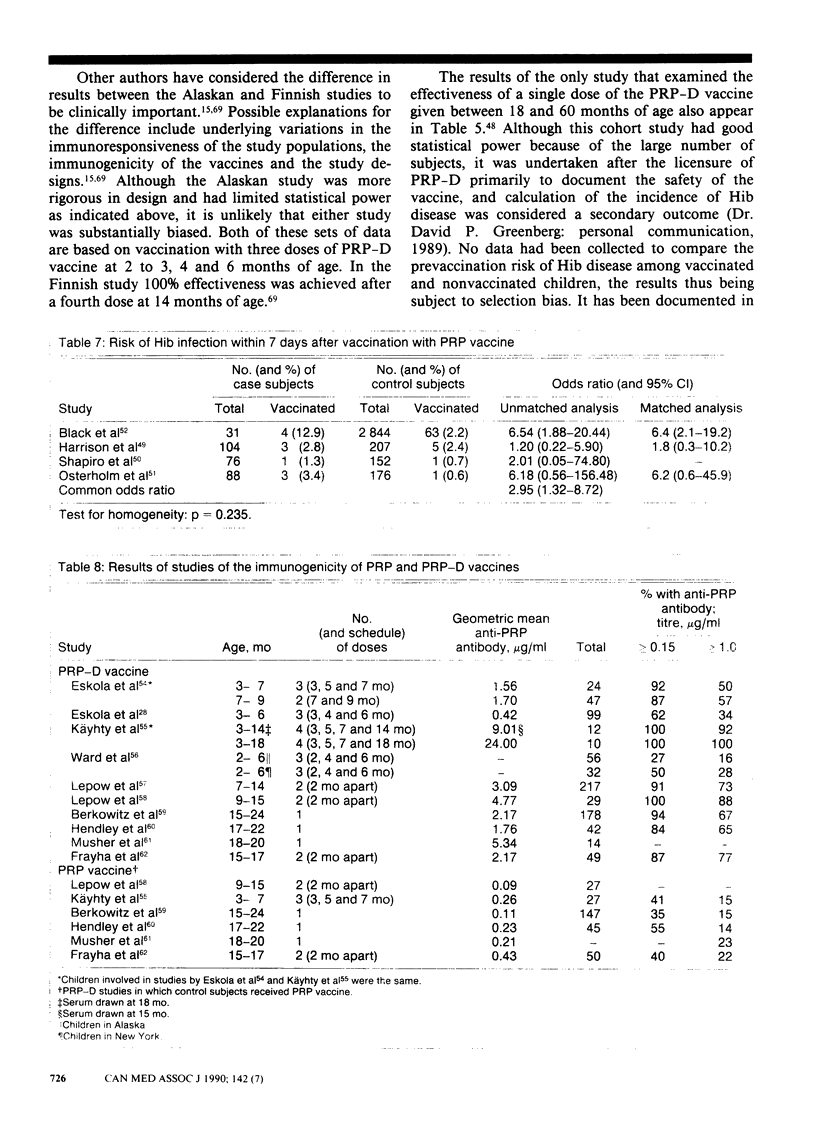
PURPOSE: To determine the clinical effectiveness of Haemophilus influenzae type b (Hib) vaccines. STUDY IDENTIFICATION AND SELECTION: Computerized searches of MEDLINE, EMBASE and SCISEARCH databases were performed, and the reference list of each retrieved article was reviewed. Two prospective clinical trials of Hib polyribosyl ribitol phosphate conjugated with diphtheria toxoid (PRP-D) were identified. In addition, one cohort study of the PRP-D vaccine, two trials of the PRP vaccine, five case-control studies of the PRP vaccine and 10 randomized controlled trials of the immunogenicity of the PRP-D vaccine were identified. DATA EXTRACTION: Study quality was assessed and descriptive information concerning the study populations, the interventions and the outcome measurements was extracted. RESULTS: The difference in the effectiveness of the PRP-D vaccine between the prospective trials, in which a three-dose schedule had been used beginning at 2 to 3 months of age, was clinically important (37% v. 83%) but not statistically significant. The PRP vaccine, which induces lower antibody responses than the PRP-D vaccine does, was clinically effective only in a subgroup of one prospective trial; 90% effectiveness was reported among children 18 to 60 months of age. CONCLUSIONS: Hib vaccine appears to be less effective in high-risk populations. None the less, because of the large variation in baseline risk, the number of children who would have to be vaccinated to prevent one case of invasive Hib disease is substantially less for high-risk than for low-risk populations. The vaccination of children at high risk, such as native children, with the PRP-D vaccine using a four-dose schedule (at 2, 4, 6 and 14 months of age) seems warranted. The currently available evidence does not strongly support a policy of universal vaccination with either a one-dose or a four-dose schedule.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Insel R. A., Porcelli S., Ward J. I. Immunochemical variables affecting radioantigen-binding assays of antibody to Haemophilus influenzae type b capsular polysaccharide in childrens' sera. J Infect Dis. 1987 Oct;156(4):582–590. doi: 10.1093/infdis/156.4.583. [DOI] [PubMed] [Google Scholar]
- Anderson P., Peter G., Johnston R. B., Jr, Wetterlow L. H., Smith D. H. Immunization of humans with polyribophosphate, the capsular antigen of Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):39–44. doi: 10.1172/JCI106794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Pichichero M. E., Insel R. A. Immunization of 2-month-old infants with protein-coupled oligosaccharides derived from the capsule of Haemophilus influenzae type b. J Pediatr. 1985 Sep;107(3):346–351. doi: 10.1016/s0022-3476(85)80504-7. [DOI] [PubMed] [Google Scholar]
- Berkowitz C. D., Ward J. I., Meier K., Hendley J. O., Brunell P. A., Barkin R. A., Zahradnik J. M., Samuelson J., Gordon L. Safety and immunogenicity of Haemophilus influenzae type b polysaccharide and polysaccharide diphtheria toxoid conjugate vaccines in children 15 to 24 months of age. J Pediatr. 1987 Apr;110(4):509–514. doi: 10.1016/s0022-3476(87)80540-1. [DOI] [PubMed] [Google Scholar]
- Black S. B., Shinefield H. R., Hiatt R. A., Fireman B. H. Efficacy of Haemophilus influenzae type b capsular polysaccharide vaccine. Pediatr Infect Dis J. 1988 Mar;7(3):149–156. doi: 10.1097/00006454-198803000-00003. [DOI] [PubMed] [Google Scholar]
- Bricker S. L., Langlais R. P., van Dis M. L. Dominant craniometaphyseal dysplasia. Literature review and case report. Dentomaxillofac Radiol. 1983;12(2):95–100. doi: 10.1259/dmfr.1983.0016. [DOI] [PubMed] [Google Scholar]
- Bulpitt C. J. Subgroup analysis. Lancet. 1988 Jul 2;2(8601):31–34. doi: 10.1016/s0140-6736(88)92956-x. [DOI] [PubMed] [Google Scholar]
- Cates K. L. Haemophilus influenzae type b polysaccharide vaccine. Pediatr Ann. 1986 Jun;15(6):461-3, 467-8. doi: 10.3928/0090-4481-19860601-10. [DOI] [PubMed] [Google Scholar]
- Claesson B. A., Trollfors B., Lagergard T., Taranger J., Bryla D., Otterman G., Cramton T., Yang Y., Reimer C. B., Robbins J. B. Clinical and immunologic responses to the capsular polysaccharide of Haemophilus influenzae type b alone or conjugated to tetanus toxoid in 18- to 23-month-old children. J Pediatr. 1988 May;112(5):695–702. doi: 10.1016/s0022-3476(88)80684-x. [DOI] [PubMed] [Google Scholar]
- Cochi S. L., Broome C. V., Hightower A. W. Immunization of US children with Hemophilus influenzae type b polysaccharide vaccine. A cost-effectiveness model of strategy assessment. JAMA. 1985 Jan 25;253(4):521–529. [PubMed] [Google Scholar]
- Cochi S. L., Broome C. V. Vaccine prevention of Haemophilus influenzae type b disease: past, present and future. Pediatr Infect Dis. 1986 Jan-Feb;5(1):12–19. doi: 10.1097/00006454-198601000-00003. [DOI] [PubMed] [Google Scholar]
- Cochi S. L., Fleming D. W., Hightower A. W., Limpakarnjanarat K., Facklam R. R., Smith J. D., Sikes R. K., Broome C. V. Primary invasive Haemophilus influenzae type b disease: a population-based assessment of risk factors. J Pediatr. 1986 Jun;108(6):887–896. doi: 10.1016/s0022-3476(86)80922-2. [DOI] [PubMed] [Google Scholar]
- Dajani A. S., Asmar B. I., Thirumoorthi M. C. Systemic Haemophilus influenzae disease: an overview. J Pediatr. 1979 Mar;94(3):355–364. doi: 10.1016/s0022-3476(79)80571-5. [DOI] [PubMed] [Google Scholar]
- Daum R. S., Sood S. K., Osterholm M. T., Pramberg J. C., Granoff P. D., White K. E., Granoff D. M. Decline in serum antibody to the capsule of Haemophilus influenzae type b in the immediate postimmunization period. J Pediatr. 1989 May;114(5):742–747. doi: 10.1016/s0022-3476(89)80130-1. [DOI] [PubMed] [Google Scholar]
- Eskola J., Käyhty H., Peltola H., Karanko V., Mäkelä P. H., Samuelson J., Gordon L. K. Antibody levels achieved in infants by course of Haemophilus influenzae type B polysaccharide/diphtheria toxoid conjugate vaccine. Lancet. 1985 May 25;1(8439):1184–1186. doi: 10.1016/s0140-6736(85)92863-6. [DOI] [PubMed] [Google Scholar]
- Eskola J., Peltola H., Takala A. K., Käyhty H., Hakulinen M., Karanko V., Kela E., Rekola P., Rönnberg P. R., Samuelson J. S. Efficacy of Haemophilus influenzae type b polysaccharide-diphtheria toxoid conjugate vaccine in infancy. N Engl J Med. 1987 Sep 17;317(12):717–722. doi: 10.1056/NEJM198709173171201. [DOI] [PubMed] [Google Scholar]
- Gilsdorf J. R. Haemophilus influenzae type b vaccine efficacy in the United States. Pediatr Infect Dis J. 1988 Mar;7(3):147–148. doi: 10.1097/00006454-198803000-00001. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Osterholm M. T. Safety and efficacy of Haemophilus influenzae type b polysaccharide vaccine. Pediatrics. 1987 Oct;80(4):590–592. [PubMed] [Google Scholar]
- Granoff D. M., Shackelford P. G., Pandey J. P., Boies E. G. Antibody responses to Haemophilus influenzae type b polysaccharide vaccine in relation to Km(1) and G2m(23) immunoglobulin allotypes. J Infect Dis. 1986 Aug;154(2):257–264. doi: 10.1093/infdis/154.2.257. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Sheetz K., Pandey J. P., Nahm M. H., Rambeck J. H., Jacobs J. L., Musser J., Selander R. K., Kabeer M., Murphy T. V. Host and bacterial factors associated with Haemophilus influenzae type b disease in Minnesota children vaccinated with type b polysaccharide vaccine. J Infect Dis. 1989 May;159(5):908–916. doi: 10.1093/infdis/159.5.908. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Suarez B. K., Pandey J. P., Shackelford P. G. Genes associated with the G2m(23) immunoglobulin allotype regulate the IgG subclass responses to Haemophilus influenzae type b polysaccharide vaccine. J Infect Dis. 1988 Jun;157(6):1142–1149. doi: 10.1093/infdis/157.6.1142. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., Hansen E. J. Coprecipitation of lipopolysaccharide and the 39,000-molecular-weight major outer membrane protein of Haemophilus influenzae type b by lipopolysaccharide-directed monoclonal antibody. Infect Immun. 1985 Sep;49(3):819–827. doi: 10.1128/iai.49.3.819-827.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Rutherford B. E., Malazdrewicz R., MacFarlane N., Pillay N., Tate R. B., Nicolle L. E., Postl B. D., Stiver H. G. Haemophilus influenzae meningitis in Manitoba and the Keewatin District, NWT: potential for mass vaccination. CMAJ. 1988 Oct 15;139(8):743–747. [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Robertson S. M., Gulig P. A., Frisch C. F., Haanes E. J. Immunoprotection of rats against Haemophilus influenzae type B disease mediated by monoclonal antibody against a haemophilus outer-membrane protein. Lancet. 1982 Feb 13;1(8268):366–368. doi: 10.1016/s0140-6736(82)91394-0. [DOI] [PubMed] [Google Scholar]
- Harrison L. H., Broome C. V., Hightower A. W. Haemophilus influenzae type b polysaccharide vaccine: an efficacy study. Haemophilus Vaccine Efficacy Study Group. Pediatrics. 1989 Aug;84(2):255–261. [PubMed] [Google Scholar]
- Harrison L. H., Broome C. V., Hightower A. W., Hoppe C. C., Makintubee S., Sitze S. L., Taylor J. A., Gaventa S., Wenger J. D., Facklam R. R. A day care-based study of the efficacy of Haemophilus b polysaccharide vaccine. JAMA. 1988 Sep 9;260(10):1413–1418. [PubMed] [Google Scholar]
- Hay J. W., Daum R. S. Cost-benefit analysis of two strategies for prevention of Haemophilus influenzae type b infection. Pediatrics. 1987 Sep;80(3):319–329. [PubMed] [Google Scholar]
- Hendley J. O., Wenzel J. G., Ashe K. M., Samuelson J. S. Immunogenicity of Haemophilus influenzae type b capsular polysaccharide vaccines in 18-month-old infants. Pediatrics. 1987 Sep;80(3):351–354. [PubMed] [Google Scholar]
- Istre G. R., Conner J. S., Broome C. V., Hightower A., Hopkins R. S. Risk factors for primary invasive Haemophilus influenzae disease: increased risk from day care attendance and school-aged household members. J Pediatr. 1985 Feb;106(2):190–195. doi: 10.1016/s0022-3476(85)80285-7. [DOI] [PubMed] [Google Scholar]
- Kafidi K. T., Rotschafer J. C. Bacterial vaccines for splenectomized patients. Drug Intell Clin Pharm. 1988 Mar;22(3):192–197. doi: 10.1177/106002808802200302. [DOI] [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käyhty H., Eskola J., Peltola H., Stout M. G., Samuelson J. S., Gordon L. K. Immunogenicity in infants of a vaccine composed of Haemophilus influenzae type b capsular polysaccharide mixed with DPT or conjugated to diphtheria toxoid. J Infect Dis. 1987 Jan;155(1):100–106. doi: 10.1093/infdis/155.1.100. [DOI] [PubMed] [Google Scholar]
- Laupacis A., Sackett D. L., Roberts R. S. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. 1988 Jun 30;318(26):1728–1733. doi: 10.1056/NEJM198806303182605. [DOI] [PubMed] [Google Scholar]
- Lenoir A. A., Pandey J. P., Granoff D. M. Antibody responses of black children to Haemophilus influenzae type b polysaccharide-Neisseria meningitidis outer-membrane protein conjugate vaccine in relation to the Km(1) allotype. J Infect Dis. 1988 Jun;157(6):1242–1245. doi: 10.1093/infdis/157.6.1242. [DOI] [PubMed] [Google Scholar]
- Lepow M. L., Barkin R. M., Berkowitz C. D., Brunell P. A., James D., Meier K., Ward J., Zahradnik J. M., Samuelson J., McVerry P. H. Safety and immunogenicity of Haemophilus influenzae type b polysaccharide-diphtheria toxoid conjugate vaccine (PRP-D) in infants. J Infect Dis. 1987 Oct;156(4):591–596. doi: 10.1093/infdis/156.4.591. [DOI] [PubMed] [Google Scholar]
- Lepow M. L., Samuelson J. S., Gordon L. K. Safety and immunogenicity of Haemophilus influenzae type b-polysaccharide diphtheria toxoid conjugate vaccine in infants 9 to 15 months of age. J Pediatr. 1985 Feb;106(2):185–189. doi: 10.1016/s0022-3476(85)80284-5. [DOI] [PubMed] [Google Scholar]
- Lepow M. Clinical trials of the Haemophilus influenzae type b capsular polysaccharide-diphtheria toxoid conjugate vaccine. Pediatr Infect Dis J. 1987 Aug;6(8):804–807. doi: 10.1097/00006454-198708000-00042. [DOI] [PubMed] [Google Scholar]
- MANTEL N., HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959 Apr;22(4):719–748. [PubMed] [Google Scholar]
- Marchant C. D., Band E., Froeschle J. E., McVerry P. H. Depression of anticapsular antibody after immunization with Haemophilus influenzae type b polysaccharide-diphtheria conjugate vaccine. Pediatr Infect Dis J. 1989 Aug;8(8):508–511. doi: 10.1097/00006454-198908000-00007. [DOI] [PubMed] [Google Scholar]
- Marwick C. Hib vaccine efficacy trials continue; data needed about use in younger children. JAMA. 1989 Apr 14;261(14):2015–2019. doi: 10.1001/jama.261.14.2015. [DOI] [PubMed] [Google Scholar]
- Mortimer E. A., Jr Efficacy of Haemophilus b polysaccharide vaccine: an enigma. JAMA. 1988 Sep 9;260(10):1454–1455. [PubMed] [Google Scholar]
- Murphy T. V. Haemophilus b polysaccharide vaccine: need for continuing assessment. Pediatr Infect Dis J. 1987 Aug;6(8):701–703. doi: 10.1097/00006454-198708000-00001. [DOI] [PubMed] [Google Scholar]
- Musher D. M., Watson D. A., Lepow M. L., McVerry P., Hamill R., Baughn R. E. Vaccination of 18-month-old children with conjugated polyribosyl ribitol phosphate stimulates production of functional antibody to Haemophilus influenzae type b. Pediatr Infect Dis J. 1988 Mar;7(3):156–159. doi: 10.1097/00006454-198803000-00004. [DOI] [PubMed] [Google Scholar]
- Orenstein W. A., Bernier R. H., Dondero T. J., Hinman A. R., Marks J. S., Bart K. J., Sirotkin B. Field evaluation of vaccine efficacy. Bull World Health Organ. 1985;63(6):1055–1068. [PMC free article] [PubMed] [Google Scholar]
- Oxman A. D., Guyatt G. H. Guidelines for reading literature reviews. CMAJ. 1988 Apr 15;138(8):697–703. [PMC free article] [PubMed] [Google Scholar]
- Parke J. C., Jr Capsular polysaccharide of Haemophilus influenzae type b as a vaccine. Pediatr Infect Dis J. 1987 Aug;6(8):795–798. doi: 10.1097/00006454-198708000-00040. [DOI] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Sivonen A., Mäkelä H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977 Nov;60(5):730–737. [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Virtanen M., Mäkelä P. H. Prevention of Hemophilus influenzae type b bacteremic infections with the capsular polysaccharide vaccine. N Engl J Med. 1984 Jun 14;310(24):1561–1566. doi: 10.1056/NEJM198406143102404. [DOI] [PubMed] [Google Scholar]
- Redmond S. R., Pichichero M. E. Hemophilus influenzae type b disease. An epidemiologic study with special reference to day-care centers. JAMA. 1984 Nov 9;252(18):2581–2584. doi: 10.1001/jama.252.18.2581. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber J. R., Barrus V., Cates K. L., Siber G. R. Functional characterization of human IgG, IgM, and IgA antibody directed to the capsule of Haemophilus influenzae type b. J Infect Dis. 1986 Jan;153(1):8–16. doi: 10.1093/infdis/153.1.8. [DOI] [PubMed] [Google Scholar]
- Sherry B., Emanuel I., Kronmal R. A., Smith A. L., Char L. F., Gale J. L., Walkley E. Interannual variation of the incidence of Haemophilus influenzae type b meningitis. JAMA. 1989 Apr 7;261(13):1924–1929. [PubMed] [Google Scholar]
- Smith P. G., Rodrigues L. C., Fine P. E. Assessment of the protective efficacy of vaccines against common diseases using case-control and cohort studies. Int J Epidemiol. 1984 Mar;13(1):87–93. doi: 10.1093/ije/13.1.87. [DOI] [PubMed] [Google Scholar]
- Tai J. Y., Vella P. P., McLean A. A., Woodhour A. F., McAleer W. J., Sha A., Dennis-Sykes C., Hilleman M. R. Haemophilus influenzae type b polysaccharide-protein conjugate vaccine. Proc Soc Exp Biol Med. 1987 Feb;184(2):154–161. doi: 10.3181/00379727-184-42460. [DOI] [PubMed] [Google Scholar]
- Takala A. K. Epidemiologic characteristics and risk factors for invasive Haemophilus influenzae type b disease in a population with high vaccine efficacy. Pediatr Infect Dis J. 1989 Jun;8(6):343–346. doi: 10.1097/00006454-198906000-00002. [DOI] [PubMed] [Google Scholar]
- Ward J. I., Brenneman G., Lepow M., Lum M., Burkhart K., Chiu C. Y. Haemophilus influenzae type b anticapsular antibody responses to PRP-pertussis and PRP-D vaccines in Alaska native infants. J Infect Dis. 1988 Oct;158(4):719–723. doi: 10.1093/infdis/158.4.719. [DOI] [PubMed] [Google Scholar]
- Ward J. I., Lum M. K., Hall D. B., Silimperi D. R., Bender T. R. Invasive Haemophilus influenzae type b disease in Alaska: background epidemiology for a vaccine efficacy trial. J Infect Dis. 1986 Jan;153(1):17–26. doi: 10.1093/infdis/153.1.17. [DOI] [PubMed] [Google Scholar]
- Weinberg G. A., Einhorn M. S., Lenoir A. A., Granoff P. D., Granoff D. M. Immunologic priming to capsular polysaccharide in infants immunized with Haemophilus influenzae type b polysaccharide-Neisseria meningitidis outer membrane protein conjugate vaccine. J Pediatr. 1987 Jul;111(1):22–27. doi: 10.1016/s0022-3476(87)80336-0. [DOI] [PubMed] [Google Scholar]
- Weinberg G. A., Granoff D. M. Polysaccharide-protein conjugate vaccines for the prevention of Haemophilus influenzae type b disease. J Pediatr. 1988 Oct;113(4):621–631. doi: 10.1016/s0022-3476(88)80369-x. [DOI] [PubMed] [Google Scholar]


