Abstract
The endothelium of the adult vasculature is normally quiescent, with the exception of the vasculature of the female reproductive system. However, in response to appropriate stimuli (ie, wound healing, atherosclerosis, tumor growth and metastasis, arthritis) the vasculature becomes activated and grows new capillaries through angiogenesis. We have recently identified a novel endothelial-restricted gene, Egfl7, that encodes a 41-kd secreted protein (Fitch MJ, Campagnolo L, Kuhnert F, Stuhlmann H: Egfl7, a novel epidermal growth factor-domain gene expressed in endothelial cells. Dev Dyn 2004, 230:316–324). Egfl7 is expressed at high levels early during mouse embryonic development and is strictly associated with the vascular bed. In this study, we investigated Egfl7 expression in the quiescent adult vasculature, in the pregnant uterus, and in two different models of arterial injury, namely ballooning and ferric chloride injury. By RNA in situ hybridization, Egfl7 expression in the vasculature was found to be restricted to the endothelium of the capillaries and mature vessels. In the pregnant uterus, increased vascularization was accompanied by up-regulation of Egfl7. On arterial injury, Egfl7 expression was up-regulated in the regenerating endothelium, but not in the neointima. Importantly, the EGFL7 protein acted as a chemoattractant for embryonic endothelial cells and fibroblasts in a cell migration assay. Together, these results suggest that Egfl7 functions in the formation and maintenance of endothelial integrity and that its up-regulation may be a critical component in the reorganization of the vascular bed in response to angiogenic stimuli.
In the adult mammalian organism, the vasculature is normally quiescent. Arterial endothelial cells have an extremely low turnover rate (∼1 in every 105 cells undergoes cell division1). However, adult endothelial cells are not postmitotic and, in response to appropriate stimuli, they can proliferate and form new blood vessels by a process termed angiogenesis.2–4 Angiogenesis describes the formation of new capillaries and larger vessels by sprouting or splitting from pre-existing vessels. Typically, the sprouting of vessels involves activation of quiescent endothelial cells, proteolytic degradation of the extracellular matrix, chemotactic migration, invasion into the surrounding stroma, proliferation and differentiation of endothelial cells, and formation of a new lumen and maturation of the endothelium.2,5–8 This angiogenic sprouting process occurs under physiological conditions during the female reproductive cycle (ovulation, implantation, pregnancy) and wound healing, as well as under pathological conditions in solid tumors and metastases, rheumatoid arthritis, retinopathies, hemangiomas, and psoriasis.2,7,9,10
Several of the key players in both embryonic and adult angiogenesis are vascular-specific growth factor ligands and their tyrosine kinase receptors participating in signaling pathways, including vascular endothelial growth factor and its receptors flk-1 and flt-1, and angiopoietin-1 and -2 and its receptor tie2.4,11 In addition, signaling through ephrins, transforming growth factor-β, and Notch are also crucial for angiogenic remodeling processes.3,12 Although the importance of these signaling pathways for both physiological and pathological angiogenesis has been recognized, many questions remain unanswered. For example, very little is known about how endothelial cell migration, sprouting, and lumen formation is controlled, and how specialized endothelial cells differentiate. Therefore, other factors likely exist that function either independent of, or in the same signaling pathways.
In a genetic screen for early circulatory system genes13,14 we have identified a novel murine gene, epidermal growth factor (EGF)-like domain 7 (Egfl7).15 Egfl7 encodes a secreted protein with an apparent molecular weight of ∼41 kd that contains an amino-terminal signal peptide and two centrally located EGF domains. Expression of Egfl7 during embryonic development is restricted to vascular endothelial cells and their precursors in the blood islands of the visceral yolk sac, largely overlapping with that of PECAM-1/CD31.15 Egfl7 (also named VE-statin, Zneu1, and Notch4-like) has been independently isolated by others.16,17 In a first approach to understanding the biological role of Egfl7 in adults, we studied the expression of Egfl7 in the normal adult vasculature, during angiogenesis in the pregnant uterus, and in models of arterial injury. To elucidate the putative role of EGFL7 in these processes, we investigated whether the protein could function as a chemoattractant for endothelial cells and/or smooth muscle cells. Our results demonstrate that Egfl7 is up-regulated in regenerating endothelium and in angiogenic endothelial cells, and that EGFL7 stimulates migration of endothelial cells. Thus, EGFL7 may play important roles during the formation of the primary plexus and its remodeling during embryogenesis, as well as during adult angiogenesis and vascular injury.
Materials and Methods
Preparation of Adult Mouse Organs and Arteries for RNA in Situ Hybridization and Immunostaining
Adult mouse organs (liver, kidney, lung, heart, brain, skeletal muscle, intestine, uterus, ovary, testes) from CD-1 mice, uteri from 10-week-old nonpregnant females or females at day 7.5 of gestation, and arteries were harvested on ice, washed in phosphate-buffered saline (PBS), and fixed overnight in 4% paraformaldehyde. The following day tissues were dehydrated through an ethanol series and a final 2 × 45 minutes wash in xylene, and paraffin-embedded at 60°C. Material was sectioned at 3 to 4 μm.
RNA in Situ Hybridization
A full-length Egfl7 cDNA probe was generated by reverse transcriptase-polymerase chain reaction using RNA from E11.5 embryos as described.15 The gel-purified polymerase chain reaction product was subcloned in both orientations into pCRII-TOPO vector (Invitrogen, Carlsbad, CA). Sense and anti-sense [α-35S]-UTP riboprobes were synthesized from plasmid DNA that was linearized with BamHI and transcribed from the T7 promoter. A probe specific for flk-1 has been described previously.18 Procedures for RNA in situ hybridization were essentially as described previously.14,19 Briefly, sectioned material was deparaffinized and digested with proteinase K, followed by 16 hours of hybridization with [35S]-radiolabeled riboprobes (final probe concentration was 35 dpm/ml in hybridization buffer). Posthybridization washes of increasing stringency were included to reduce background. Sense control probes were synthesized for all experiments. Slides were dipped in Kodak NBT-2 emulsion (Eastman Kodak, Rochester, NY), air-dried for 1 hour, and exposed for 5 days at 4°C. Material was counterstained with hematoxylin and eosin (H&E), following standard protocols. Dark-field and bright-field photographs were taken using a Zeiss Axioplan2 microscope with an attached digital camera (Axiocam; Zeiss, Thornwood, NY).
Antibodies and Immunohistochemistry
Rat monoclonal antibody against CD31/PECAM-1 (clone Mec13.3; BD Pharmingen, San Diego, CA) and goat polyclonal antibodies against EphrinB2 (R&D Systems, Minneapolis, MN) were used at a concentration of 2.5 μg/ml. Immunoaffinity-purified rabbit polyclonal antibodies against EGFL715 were used at 1:150 dilution. For immunohistochemistry, slides were deparaffinized in xylene, rehydrated through the ethanol series, and incubated in 0.3% H2O2 in methanol for 30 minutes at room temperature to remove endogenous peroxidase activity. After pretreatment with 30 μg/ml of proteinase K in 0.2 mol/L Tris-HCl, pH 7.2, for 30 minutes at 37°C, slides were incubated with 0.5% Blocking Reagent (New England Nuclear, Boston, MA) for 30 minutes at room temperature, and incubated overnight at 4°C with primary antibodies. Control slides were incubated with rat, rabbit, or goat IgG. After incubation with secondary biotinylated anti-rat or anti-rabbit antibodies, staining was revealed using the Tyramide Amplification System (TSA-Indirect kit; NEN Life Sciences). Slides were counterstained with Mayer’s hematoxylin for 5 minutes, dehydrated, and mounted in Permount mounting medium. For immunofluorescence detection, tissues were embedded in OCT and sectioned in a cryostat at 7 μm. Slides were incubated overnight at 4°C with EphrinB2- and EGFL7-specific antibodies, followed by incubation with secondary antibodies Cy3 goat anti-rabbit (Chemicon, Temecula, CA) and Alexa 488 donkey anti-goat (Molecular Probes, Eugene, OR). Images were taken using a Zeiss Axioplan2 microscope.
Balloon Injury in Rats
Aortic balloon injury was performed in adult Sprague-Dawley male rats (300 to 350 g) under general anesthesia (intraperitoneal ketamine 15 mg/kg) as previously described.20 A no. 2 Fogarty balloon embolectomy catheter was introduced via the femoral artery and advanced to the level of the aortic arch. It was then inflated and withdrawn along the full length of the thoracoabdominal aorta. This procedure was repeated five times. After single injury, the femoral artery was ligated, the incision closed, and the animals returned to their usual diet and routine. After 48 hours, 1 week, 2 weeks, 4 weeks, and 6 weeks from the injury, rats were anesthetized and heparinized, and the ascending aortae were cannulated, perfused with 200 ml of heparinized PBS followed by 200 ml of 4% paraformaldehyde in PBS, pH 7.4, and embedded in paraffin. Noninjured rats were used as controls.
Ferric Chloride Injury of Mouse Carotid Arteries
C57BL/6 mice were anesthetized by inhalation of Metofane (Schering Plough, Kenilworth, NJ), and the left common carotid artery was exposed by making a midline cervical incision. Injury to the vessel was performed by exposing the prepared vessel for 3 minutes to a filter paper (0.5 × 1 mm) soaked in 0.64 mol/L ferric chloride (FeCl3) solution. After injury, flow was monitored for more than 25 minutes using an ultrasonic flow probe (Transonic Systems Inc., Ithaca, NY) to detect thrombotic response to the injury. At the end of the procedure, the incision was closed by suture and animals were allowed to recover at 37°C. The carotid arteries were collected 30 minutes, and 3 days, 7 days, 21 days, and 28 days after injury. Four animals were used for each time point. To harvest the tissue, animals were anesthetized and perfused with zinc formalin. The injured segment of the carotids were excised, fixed 4 hours in zinc formalin, immobilized in 3% agarose, and processed for paraffin embedding.
Human Carotid Artery Biopsies
Human tissues were obtained from excess surgical pathology specimens (atherectomies and endarterectomies from two individuals) at the Mount Sinai Medical Center. Formalin-fixed, paraffin-embedded tissue blocks were serially sectioned at 5 μm onto polylysine-coated slides and stored at room temperature until used. Before staining, the slides were heated at 57°C for 2 hours, rinsed in xylene for 10 minutes, rehydrated in successive rinses of graded ethanol, and placed in PBS.
Cells and Culture Conditions
C167 (murine yolk sac endothelial cells)21 and EOMA (a hemangioendothelioma cell line)22 were obtained from R. Auerbach (Univ. of Wisconsin, Madison, WI), SVEC (a SV40-transformed mouse lymphoid endothelial cell line),23 and bEnd.3 (a mouse brain endothelial cell line immortalized by polyoma middle T)24 were obtained from American Type Culture Collection (Rockville, MD), and HEK293 EBNA125 were obtained from K. Hahn (The Scripps Research Institute, La Jolla, CA). Primary mouse embryonic fibroblasts (MEFs) were prepared from E13.5 CD-1 embryos using standard procedures.26 HEK293, HEK293 EBNA1, C167, SVEC, EOMA, bEnd.3, and MEFs were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Grand Island, NY) supplemented with 10% fetal calf serum. Rat vascular smooth muscle cells (RVSMCs; Cell Applications, Inc., San Diego, CA) were maintained in rat vascular smooth muscle cell medium (Cell Applications). Recombinant human basic fibroblast growth factor and vascular endothelial growth factor were purchased from Preprotech, Inc., Rocky Hill, NJ.
Cell Migration Assays
Preparation of EGFL7-Enriched Medium
HEK293 cells were cultured overnight in a 10-cm dish at a density of 2.5 × 105 cells/ml in DMEM supplemented with 10% fetal calf serum. The following day, the culture medium was replaced by Opti-MEM I reduced serum medium (Invitrogen) containing 1% fetal calf serum, and cells were transfected with 24 μg of the Egfl7 expression vector pCEP4-Pu/AC7-EGFL7-(His)6 vector, or pCEP4-Pu/AC7 empty vector, respectively,15 using lipofectamine 2000 (Invitrogen). Twenty-four hours later, cells were washed twice with PBS and cultured for 20 hours in serum-free DMEM, or in RVSMC basal medium. EGFL7-enriched supernatant medium was harvested, centrifuged at 2000 rpm to remove cells, and snap-frozen in aliquots. Presence of EGFL7 in the supernatant was examined by Western blot using a horseradish peroxidase-conjugated anti-(His)6 antibody (Invitrogen) as described previously.15 EGFL7 protein concentration was estimated as follows: according the manufacturer’s specifications, the anti-(His)6 antibody detects as little as 50 ng of recombinant His-tagged protein. Because 0.9 ml of supernatant was required to detect a band on the Western blot, we estimate that the concentration of EGFL7 in the migration studies is ∼50 ng/ml.
Preparation of EGFL7-Depleted Medium
EGFL7-His-tagged protein was depleted from the culture supernatant by using Talon Dynabeads (Dynal Biotech, Oslo, Norway). Briefly, the beads were washed in 50 mmol/L phosphate buffer (pH 8.0) containing 300 mmol/L NaCl. Dynabeads (65 μl) were mixed with 900 μl of EGFL7-enriched medium at 4°C for 1 hour. The depleted medium was used in the migration assay. Depletion of EGFL7 from medium and its presence on the beads was confirmed by Western blotting.
Migration Assays
For MEF cells, 8-μm trans-well inserts (Costar Corning, Cambridge, MA) were coated with fibronectin (1 μg/ml FN; a gift from M.A. del Pozo, The Scripps Research Institute, La Jolla, CA) and placed into a 24-well plate (Costar Corning). The lower wells were filled with 0.3 ml of culture supernatant that was harvested from HEK293 cells transfected with pCEP4-Pu/AC7-EGFL7-(His)6 vector. Supernatant medium from HEK293 cells transfected with empty vector and EGFL7-depleted medium were used as controls. MEF cells (4 × 104) resuspended in 200 μl of DMEM were added to the top chambers. Migration assays were performed at 37°C for 18 hours; cells were fixed with 10% TCA at 4°C for 1 hour, and stained with Giemsa. Cell migration was determined from the average cell numbers in five fields per filter (counted at ×200 magnification) each from three independent experiments, each performed in duplicate and expressed as mean ± SD. The significance was calculated using the unpaired t-test. Random cell migration, ie, migration in the absence of chemoattractant, was given the arbitrary value of 100%.27 Results were expressed as percent of control migration. For C167, SVEC, EOMA, bEnd.3 endothelial cells, and RVSMCs, the assay was performed as described above, except that 5-μm trans-well inserts coated with 0.2% bovine serum albumin (Sigma) were used for endothelial cells, and 8-μm trans-well inserts coated with 1 μg/ml of FN for RVSMCs, respectively. Fifty ng per ml fibroblast growth factor was used as a positive control for MEFs and RVSMCs, and 50 ng of vascular endothelial growth factor per ml for endothelial cells, respectively.
Results
Differential Levels of Egfl7 Expression in the Quiescent Adult Endothelium
In a previous study, we showed that Egfl7 expression during murine embryogenesis is restricted to endothelial cells of the developing vascular system and their endothelial precursors in the extra-embryonic mesoderm.15 Furthermore, using reverse transcriptase-polymerase chain reaction and Northern blot analysis, Egfl7 transcripts were detected in adult mice in a wide variety of organs as well as in several endothelial cell lines. To investigate the spatial distribution of Egfl7 transcripts in the adult, we have now performed RNA in situ hybridization on sections of adult mouse organs. To assess for endothelial-specific expression in every tissue analyzed, adjacent sections were immunostained for CD31.
Egfl7 expression was detected in the quiescent endothelium of a subset of vessels of every adult mouse tissue examined. Compared to its high level of expression during embryogenesis,15 the overall levels of Egfl7 transcripts detected in the vasculature of adult organs were considerably lower. Egfl7 signal was present in the large vessels infusing the liver [Figure 1, A and E (arrows)] and in small vessels of the brain [Figure 1, D and H (arrows)], and was restricted to the endothelial layer as confirmed by CD31 immunostaining (Figure 1, compare E and I and H and L). In contrast, no Egfl7 signal was detected in hepatocytes or in small vessels infusing the stroma [Figure 1, compare E and I (arrowhead)], and in hematopoietic cells in the vessel lumen (Figure 1, H and L). The highest levels of adult Egfl7 expression were found in the lung, where the silver grains lined the capillaries surrounding the alveolar septa, whereas no signal was detected on the epithelium of the bronchioles (Figure 1; B, F, J). This result correlates well with the high abundance of Egfl7-specific mRNA in the lung detected on Northern blots.15 Interestingly, Egfl7 expression in the heart was localized to the endothelium lining the outflow tract [Figure 1, C and G (arrowhead)] and the atrium wall (not shown). In contrast, no expression above background level was seen in the vessels infusing the myocardium, although staining for CD31 revealed the presence of an intact endothelium (not shown). Expression of Egfl7 in kidney and spleen (not shown) was detected in interstitial spaces between large cells, presumably localized to the flat endothelium of the capillary network. Low levels of Egfl7 signal were detected in the bone marrow within the megakaryocytes (not shown), whereas expression in other hematopoietic cells was at background levels. No Egfl7 expression was detected in peripheral blood. Sections of adult organs that were hybridized to a flk-1-specific riboprobe did not display any signal above background level (not shown).
Figure 1.
Expression of Egfl7 in organ sections of adult CD-1 mice. A mouse-specific anti-sense Egfl7 [α-35S]-UTP-labeled riboprobe, corresponding to the full-length Egfl7 cDNA, indicates levels of gene expression in the endothelium of liver (A, E), lung (B, F), outflow tract of the heart (C, G), and brain (D, H). Squares indicate regions magnified in E to H. Sections adjacent to A to D were immunostained for CD31/PECAM1 (I–L). All sections were counterstained with H&E, and photographed under dark field at ×20 (A–D) or bright field at ×63 magnification (E–L), respectively.
Egfl7 Expression Is Up-Regulated in the Reproductive Tract during Pregnancy
In the reproductive apparatus of a 10-week-old female mouse, Egfl7 transcripts were restricted to the capillary spaces (Figure 2). Interestingly, higher in situ signals were detected in the vessels surrounding the lumen compared to the vasculature infusing the outermost layers of the uterus (Figure 2, compare A and D). In the pregnant uterus undergoing extensive angiogenesis, Egfl7 was strongly up-regulated, and its expression remained strictly associated with the endothelium surrounding the enlarged vessels of the deciduae (Figure 2, B and E). In addition, high levels of EGFL7 protein were detected in the endothelium of the pregnant uterus using a polyclonal antibody specific for EGFL7 (Figure 2C). Furthermore, abundant Egfl7 expression was found in the placenta of a conceptus at 16.5 days of gestation in the capillary network of both maternal and fetal origin (not shown).
Figure 2.
Expression of Egfl7 in pregnant and normal uterus. A–F: Normal and pregnant mouse uteri were paraffin-embedded, sectioned, and analyzed for EGFL7 expression both by RNA in situ hybridization and immunohistochemistry. A: Normal, nonpregnant uterus; B: 7.5-dpc pregnant uterus hybridized with the Egfl7 anti-sense riboprobe. D, E: CD31/PECAM1 staining of sections adjacent to A and B, respectively. C: Section adjacent to that shown in B, immunostained using an antibody against EGFL7. F: Section was incubated with generic rabbit IgG as a control for immunohistochemistry. Sections C to F were counterstained with H&E. G, H: Cryostat sections from normal, nonpregnant uterus were double immunostained for EphrinB2 (G) and EGFL7 (H). Images shown in G and H depict a peripheral area of the uterus. *, Uterus lumen. All sections were photographed using an Axiocam camera (Zeiss) under bright field (A–F) or dark field (G, H). Original magnifications: ×20 (A, B, D, E); ×40 (C, F, G, H).
We further investigated if the observed expression of Egfl7 in a subset of vessels in adult tissues is due to restriction to arterial vessels or veins. For this, a section from the nonpregnant uterus was double stained using antibodies specific for EGFL7 and EphrinB2, an arterial-specific marker. Expression of EphrinB2 was confined to the large arterial vessel shown in the field of view from the peripheral area of the uterus (Figure 2G, arrow), whereas EGFL7 was detected in the same artery (Figure 2H, arrow), as well as in smaller veins (Figure 2H, arrowheads).
Up-Regulation of Egfl7 in Response to Balloon Injury
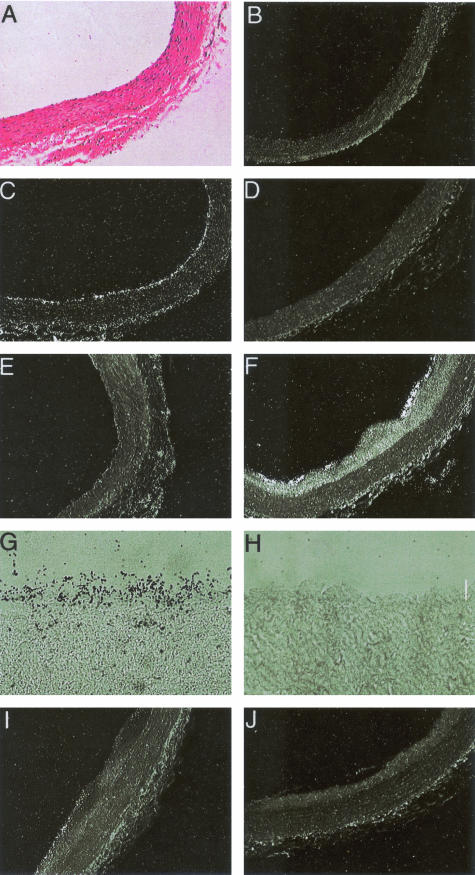
Next we examined the expression of Egfl7 mRNA in regenerating endothelium during response to balloon injury of rat aortas. In normal arteries, Egfl7 signal was distributed uniformly in the endothelium, and was also detected in small patches surrounding adventitial capillaries (Figure 3, compare A to C). No expression was found in the medial layer of smooth muscle cells (SMCs). In contrast to Egfl7, no signal was seen in the endothelium of control arteries using a flk-1-specific riboprobe (Figure 3D). Forty-eight hours after denudation of the endothelium, Egfl7 transcripts were absent on the luminal surface or in the medial SMC (Figure 3E). Two weeks after injury, there was substantial intimal hyperplasia and almost complete endothelial regeneration. This was accompanied by intense Egfl7 expression localized in the endothelium, but not in the SMCs of the developing intima or the media (Figure 3, F and G). A decrease in Egfl7 expression in the regenerating endothelium was seen at 4 and 6 weeks after injury (Figure 3I). No flk-1-specific transcripts were detected at 2 or 4 weeks after injury in the regenerating endothelium or SMCs (Figure 3, H and J).
Figure 3.
Expression of Egfl7 and flk-1 in untreated control and balloon-injured rat arterial sections. A: Untreated control rat artery stained with H&E. B: Control sense Egfl7 riboprobe labeled with [35S]-UTP on untreated control rat artery. C, E, F, G, and I: Mouse-specific anti-sense Egfl7 riboprobe labeled with [35S]-UTP indicate levels of gene expression in an untreated control rat artery (C) and the balloon-injured rat arteries at 48 hours after injury (E), 2 weeks after injury (F, G), and 4 weeks after injury (I). D, H, J: Anti-sense flk-1 riboprobes labeled with [35S]-UTP indicate gene expression in control rat arteries (D), 2 weeks after injury (H), and 4 weeks after injury (J). Photomicrographs under bright field (A) and under dark field (B–F, I, and J). G and H were stained with toluidine blue. Original magnifications: ×10 (A–F, I, J); ×40 (G, H).
Up-Regulation of Egfl7 in Response to Ferric Chloride Injury
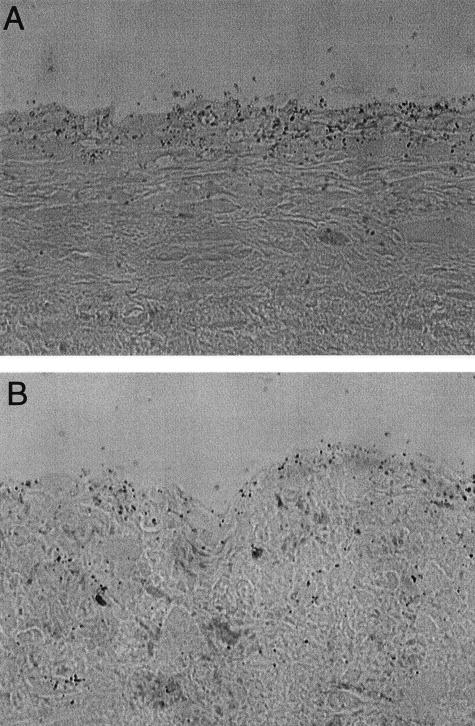
The regulation of Egfl7 expression was studied in a second model of arterial injury, namely endothelial denudation by ferric chloride in mice. In this model system, mice are treated locally with FeCl3 to induce vascular injury and thrombosis.28 The endothelium of a noninjured control carotid artery was immunoreactive to antibodies against CD31 and expressed low levels of Egfl7 mRNA (Figure 4, A and B). Thirty minutes after injury, the carotid wall area where FeCl3 was administered showed complete lack of residual endothelium (Figure 4, C and Q), and no Egfl7 signal above background level could be detected (Figure 4D). At 3 and 7 days after injury, Egfl7 expression levels were significantly up-regulated throughout the whole endothelium (Figure 4; E to H). In addition, at 3 days after injury, a high in situ signal was also detected on endothelial cells lining the luminal side of the thrombus [Figure 4, F (arrow) and R], and at 7 days after injury Egfl7 expression was detected within the thrombus and co-localized with CD31 expression (Figure 4, compare G and H, arrows). At this stage, wound-healing processes also occur in the adventitia where we detected high levels of Egfl7 signal localized to sites of newly formed vessels (Figure 4H, arrowheads). Egfl7 signal appeared to be restricted to endothelial cells. No Mac3-positive cells (macrophages) were detected in the thrombus at 7 days after injury, whereas few macrophages were observed in the area underneath the endothelial cell layer (data not shown). Egfl7 expression was absent in erythrocytes. At 3 weeks after injury, high levels of Egfl7 expression were detected only in the endothelium lining the site of the neointimal formation (Figure 4, I and L, arrows), whereas Egfl7 expression in the luminal endothelium was comparable to that found in the control noninjured carotid (Figure 4, compare L with B). At 4 weeks after injury the carotid showed levels of Egfl7 expression similar to a normal, noninjured artery (Figure 4, compare M and N with A and B).
Figure 4.
Expression of Egfl7 in FeCl3-denuded mouse carotid arteries. B, D, F–H, L, and N: Egfl7 RNA in situ hybridization on mouse carotids using the same probe as in Figures 1 and 2. P: A sense riboprobe was used as control. Sections adjacent to those used for in situ hybridization were immunostained for CD31/PECAM1 (A, C, E, G, I, M, Q, P). O: Generic rat IgG was used as control for immunohistochemistry. Q and P: Magnification of the areas delimited by the squares in C and E, respectively. Abbreviations on the bottom right-hand corner represent the number of days after the injury.
Egfl7 Expression in Human Carotid Artery Plaques
Egfl7 expression was also examined in human atherosclerotic plaques. Substantial levels of Egfl7 expression were detected in the luminal endothelium, but not in the underlying SMCs (Figure 5, A and B). Immunohistochemical staining of parallel sections using anti-human smooth muscle-specific α-actin antibodies, or endothelial-specific anti-vWF antibodies, respectively, confirmed that Egfl7 mRNA was restricted to the endothelium (not shown).
Figure 5.
Expression of EGFL7 in human arterial plaques. Human-specific anti-sense EGFL7 riboprobe labeled with [35S]-UTP indicates levels of gene expression in carotid artery samples from patients 7079 (A) and 2799 (B).
EGFL7 Stimulates Migration of Endothelial Cells and MEFs
EGFL7 is targeted for secretion through the endoplasmic reticulum and the Golgi apparatus. However, in cells transiently transfected with an Egfl7 expression plasmid, secreted protein levels were low when compared with that of intracellular protein.15 To test if EGFL7 acts as a chemoattractant in a cell migration assay, we sought to purify EGFL7 protein from several cell lines with high transfection efficiencies (HEK293, HEK293 EBNA1, C167, and CHO) that were transiently transfected with a His-tagged Egfl7 expression vector. However, purification of EGFL7 from cell extracts or culture supernatant has been unsuccessful to date because the protein is labile and becomes readily inactivated during purification steps. Thus in our migration assays, we have used culture supernatant harvested from transiently transfected HEK293 cells. EGFL7 protein was detected in the supernatant using a horseradish peroxidase-conjugated anti-(His)6 antibody, albeit in low amounts when compared to cell extracts (Figure 6A). EGFL7 protein concentration for the cell migration studies was estimated as between 30 to 60 ng per ml (see Materials and Methods).
Figure 6.
Cell migration studies. A: Detection of EGFL7 in culture supernatant and cell extracts from HEK293 cells transfected with a His-tagged Egfl7 vector, using immunoblotting with anti-His antibody. Two days after transfection, culture supernatant (lane 1) was harvested, and cell extract was prepared (lane 2). Media (900 μl) was TCA-precipitated, and the protein detected using an horseradish peroxidase-conjugated anti-(His)6 antibody. B: MEFs (40,000 cells) were placed on the 8-μm (1 μg/ml FN-coated) trans-well inserts and inserted into wells that contained control medium, EGFL7-conditioned, or EGFL7-depleted media, respectively. Eighteen hours later, cells that had migrated to the lower side of the membrane were fixed, stained, and counted. Cell migration was determined from the average cell numbers in five fields per filter (counted at ×200 magnification) each from three independent experiments, each performed in duplicate and expressed as mean ± SD. Random cell migration, ie, migration in the absence of chemoattractant, was given the arbitrary value of 100%, and results are expressed as percent of control migration. C: Migration of C167 cells in response to EGFL7 is shown. Trans-well inserts (5 μm in diameter) were coated with 0.2% bovine serum albumin. D: Migration of RVSMC in response to EGFL7 is shown. Trans-well inserts (8 μm in diameter) were coated with 0.1 mg/dl FN. Methods and statistical evaluation in C and D were as described in B.
Because Egfl7 mRNA is expressed at high levels in the vascular endothelium during mouse development, angiogenesis, and vascular injury, we examined the effect of EGFL7 on the migration of murine yolk sac endothelial cells (C167), MEFs, and RVSMCs, using a trans-well assay. Random cell migration, ie, migration in response to mock-transfected supernatant, was given the arbitrary value of 100%.27 Results were expressed as percent of control migration. EGFL7-containing medium significantly increased migration of MEFs to ∼1.5-fold (P = 0.01) greater than control, whereas a threefold increase was observed in the presence of 50 ng/ml of basic fibroblast growth factor that was used as a positive control (P = 0.0003) (Figure 6B). The chemotactic effect was specific for EGFL7, because media depleted of EGFL7 abrogated stimulation of MEF migration (P = 0.03). EGFL7 depletion was confirmed by Western blotting (data not shown). Importantly, significantly higher levels of cell migration were observed for C167 endothelial cells using EGFL7-containing supernatant (1.9-fold, P = 0.0002) (Figure 6C), and depletion of EGFL7 abrogated this stimulation (P = 0.002). C167 cell migration was stimulated to a lesser extent in the presence of 50 ng/ml of vascular endothelial growth factor (1.4-fold, P = 0.0137). These data suggest that the effect of EGFL7 is more pronounced on yolk sac endothelial cells as compared to MEF cells. Similarly, EGFL7-specific stimulation of cell migration was detected with other mouse endothelial cell lines, including SVEC (1.5-fold, P = 0.002), EOMA, and bEnd.3 (not shown). We also tested RVSMCs because they are juxtaposed to the endothelium in the mature vessels. However, no difference was observed in cell migration between control and EGFL7-containing supernatant (Figure 6D), indicating that RVSMCs do not migrate in response to EGFL7. In this experiment, basic fibroblast growth factor (50 ng/ml) was used as a positive control29 and resulted in a 1.4-fold increase of RVSMC migration (P = 0.007). Similar results for cell migration were obtained in experiments using Boyden chambers (data not shown). Together, our results show that EGFL7 functions as a chemoattractant for embryonic and adult endothelial cells and fibroblasts, consistent with its possible role as an autocrine and paracrine factor involved in the organization of the vascular bed.
Discussion
The EGF domain gene Egfl7 was identified in a genetic screen for early circulatory system genes in mouse embryonic stem cells and embryos.13,15 Its restricted expression during embryogenesis in the vascular endothelium and its precursors in the yolk sac blood islands suggested that Egfl7 plays a role during endothelial lineage determination, and possibly during embryonic vasculogenesis and angiogenesis. Egfl7-specific transcripts were found in most adult organs tested, and in primary and transformed endothelial cell lines.15
In this report, we show that Egfl7 expression is down-regulated throughout the quiescent endothelium of the adult vasculature except of the lung, and that its expression is up-regulated during physiological angiogenesis and during vascular injury. Using RNA in situ hybridization and immunofluorescence, Egfl7 mRNA and protein were detected in the vasculature in all adult tissues examined. As during embryogenesis, Egfl7 expression is restricted to the endothelium of the adult quiescent vasculature, albeit at reduced levels. No expression was detected in the intimal smooth muscle cells. Interestingly, Egfl7 mRNA levels varied between different organs, and within a given organ between different vascular beds. For example, in the heart significant expression is detected in the outflow tract and in the endothelium lining the atrium. In contrast, expression in the capillaries infusing the myocardium is at undetectable levels, both at the RNA and protein levels. In the adult lung, capillary endothelial expression of Egfl7 remains throughout the organ at similar high levels as in the embryonic lung buds.15 Of interest, Egfl7 expression is not restricted to the venous or arterial endothelium. We do not know the underlying cause for its differential expression in adult endothelium, but it likely reflects the molecular identities of different vascular beds, as evidenced in recent gene expression and proteomic profiling studies.30–33 Of note, expression of other early endothelial markers such as flk-1 in the quiescent adult endothelium varies and becomes down-regulated to low or undetectable levels in the endothelium of many organs, including brain, lung, thymus, bone marrow, uterus, and testes.34–38
Transient up-regulation of Egfl7 expression was detected during arterial injury. The wave of Egfl7 expression correlated with re-endothelialization of the vessel lumen, and highest expression was detected in areas corresponding to extensive endothelial proliferation and migration. In both injury models, expression remained restricted to the endothelium and was not detectable in intimal smooth muscle cells. In support of our animal studies, we also detected high Egfl7 levels in the luminal endothelium of human atherosclerotic plaques. Our results therefore suggest that Egfl7 is a specific marker for adult endothelial cells within the arterial wall that are in an activated state of proliferation, migration, and remodeling. In addition, Egfl7 up-regulation occurs in physiological angiogenesis associated with pregnancy as well. Thus, endothelial cells in the extensively vascularized decidua of the pregnant uterus were found to express high levels of Egfl7 mRNA, in agreement with previous reports.16,17 We extended these studies here by comparing the spatial expression pattern in the nonpregnant and pregnant uterus. High amounts of Egfl7 transcripts were detected in vessels and capillaries of the endometrium, which is directly influenced by hormone levels and is responsive to angiogenesis during implantation of the embryo. In contrast, the larger subserosal vessels showed overall lower Egfl7 expression. It would be of interest to investigate if endometrial Egfl7 expression is modulated during the estrus cycle. Future studies in which Egfl7 up-regulation in these model systems is blocked by siRNA or neutralizing antibodies will shed light on a possible causative relationship between Egfl7 and adult angiogenesis.
At this point, little is known about the biological role of Egfl7 in the adult endothelium. In this study we show for the first time that EGFL7 acts as a chemoattractant for embryonic endothelial cells and, to a lesser extent, for embryonic fibroblasts, whereas no effect on VSMC migration was observed. Studies by two other groups failed to detect an effect of EGFL7 on endothelial cell migration.16,17 However, Soncin and colleagues16 showed that EGFL7 represses platelet-derived growth factor-induced smooth muscle cell migration, and they termed the gene accordingly VE-statin. It is possible that these differences reflect a differential chemotactic response of mouse versus human endothelial cells to EGFL7. In fact, we did not detect stimulation of human umbilical vein endothelial cell migration (S. Chitnis, L. Campagnolo, and H. Stuhlmann, unpublished results). This difference could provide a tool for isolating a receptor and/or proteins in the extracellular matrix that functionally interact with EGFL7. Alternatively, the amounts of secreted EGFL7 protein might be suboptimal for cell migration studies. Clearly, using purified EGFL7 protein will be crucial to further clarify this issue.
Based on its expression profile and chemotactic properties presented in this study, we propose that EGFL7 is a secreted chemoattractant that recruits angioblasts, endothelial cells, and supporting cells to active sites of vessel formation under conditions of normal and pathological angiogenesis. This process does not appear to affect intimal smooth muscle cells. It will be important to determine whether the roles of Egfl7 during adult and embryonic angiogenesis are similar or different. Clues about a crucial role during vascular development came from recent knock-down studies in the Zebrafish embryo demonstrating that Egfl7 is required for tube formation during angiogenesis.17 Loss- and gain-of-function experiments in mice are under way in our laboratory to determine whether Egfl7 has a similar function in mammals. In this context it is important to note that a paralogous gene, Egfl8, exists in mammals but not Zebrafish, with mostly neuronal expression (E. Kleinmann and H. Stuhlmann, unpublished).17
It is tempting to speculate that EGFL7 protein might be a potential target to modulate endothelial cell proliferation in conditions of normal and pathological angiogenesis, independent of the intimal smooth muscle cells. In addition, regulatory elements within the Egfl7 promoter may prove useful tools for treating vascular disorders by targeting gene expression specifically to proliferating endothelial cells.
Acknowledgments
We thank Wendy LeVine for excellent technical support with mouse husbandry and histology.
Footnotes
Address reprint requests to Heidi Stuhlmann, Ph.D., Department of Cell Biology, Division of Vascular Biology, The Scripps Research Institute, Mail CVN-26, 10550 North Torrey Pines Rd., La Jolla, CA 92037. E-mail: hstuhlm@scripps.edu.
Supported by grants from the National Institutes of Health (R29 HD31534 and R21 HL072270 to H.S. and training grant T32 HL07695 to M.J.F. and S.C.) and the American Heart Association (grant-in-aid 9950585N to H.S.).
Present address of L.C.: Dip.to Sanita Pubblica e Biologia Cellulare, Universita Degli Studi di Roma “Tor Vergata,” Rome, Italy; S.K.: Dept. of Nephrology and Rheumatology, Georg August University of Goettingen, Goettingen, Germany; and M.B.T.: Dept. of Medicine, University of Rochester, Rochester, NY.
References
- Hobson B, Denekamp J. Endothelial proliferation in tumors and normal tissues: continuous labeling studies. Br J Cancer. 1984;49:405–413. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Rossant J, Howard L. Signaling pathways in vascular development. Annu Rev Cell Dev Biol. 2002;18:541–573. doi: 10.1146/annurev.cellbio.18.012502.105825. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Folkman J, D’Amore PA. Bloods vessel formation: what is its molecular basis. Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–651. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;16(Suppl 6):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Rossant J, Hirashima M. Vascular development and patterning: making the right choices. Curr Opin Genet Dev. 2003;13:408–412. doi: 10.1016/s0959-437x(03)00080-7. [DOI] [PubMed] [Google Scholar]
- Xiong J-W, Battaglino R, Leahy A, Stuhlmann H. Large-scale screening for developmental genes in embryonic stem cells and embryoid bodies using retroviral entrapment vectors. Dev Dyn. 1998;212:181–197. doi: 10.1002/(SICI)1097-0177(199806)212:2<181::AID-AJA4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Leahy A, Xiong J-W, Kuhnert F, Stuhlmann H. Use of developmental marker genes to define temporal and spatial patterns of differentiation during embryoid body formation. J Exp Zool. 1999;284:67–81. doi: 10.1002/(sici)1097-010x(19990615)284:1<67::aid-jez10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Fitch MJ, Campagnolo L, Kuhnert F, Stuhlmann H. Egfl7, a novel epidermal growth factor-domain gene expressed in endothelial cells. Dev Dyn. 2004;230:316–324. doi: 10.1002/dvdy.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soncin F, Mattot V, Lionneton F, Spruyt N, Lepretre F, Begue A, Stehelin D. VE-statin, an endothelial repressor of smooth muscle cell migration. EMBO J. 2003;22:5700–5711. doi: 10.1093/emboj/cdg549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY, De Sauvage FJ, Ye W. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754–758. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- Sassoon D, Rosenthal N. Detection of messenger RNA by in situ hybridization. Methods Enzymol. 1993;225:384–404. doi: 10.1016/0076-6879(93)25027-y. [DOI] [PubMed] [Google Scholar]
- Marmur JD, Rossikhina M, Guha A, Fyfe B, Friedrich V, Mendlowitz M, Nemerson Y, Taubman MB. Tissue factor is rapidly induced in arterial smooth muscle after balloon injury. J Clin Invest. 1993;91:2253–2259. doi: 10.1172/JCI116452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SJ, Greer P, Auerbach R. Isolation and propagation of yolk-sac-derived endothelial cells from a hypervascular transgenic mouse expressing a gain-of-function fps/fes proto-oncogene. In Vitro Cell Dev Biol Anim. 1996;32:292–299. doi: 10.1007/BF02723062. [DOI] [PubMed] [Google Scholar]
- Obeso J, Weber J, Auerbach R. A hemangioendothelioma-derived cell line: its use as a model for the study of endothelial cell biology. Lab Invest. 1990;63:259–269. [PubMed] [Google Scholar]
- O’Connell KA, Edidin M. A mouse lymphoid endothelial cell line immortalized by simian virus 40 binds lymphocytes and retains functional characteristics of normal endothelial cells. J Immunol. 1990;144:521–525. [PubMed] [Google Scholar]
- Montesano R, Pepper MS, Mohle-Steinlein U, Risau W, Wagner EF, Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990;62:435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- Kohfeldt E, Maurer P, Vannahme C, Timpl R. Properties of the extracellular calcium binding module of the proteoglycan testican. FEBS Lett. 1997;414:557–561. doi: 10.1016/s0014-5793(97)01070-3. [DOI] [PubMed] [Google Scholar]
- Matise MP, Auerbach W, Joyner AL. Production of targeted embryonic stem cell clones. Joyner AL, editor. New York: Oxford University Press,; Gene TargetingA Practical Approach. 2000:pp 101–132. [Google Scholar]
- Degryse B, Resnati M, Rabbani SA, Villa A, Fazioli F, Blasi F. Src-dependence and pertussis-toxin sensitivity of urokinase receptor-dependent chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. Blood. 1999;94:649–662. [PubMed] [Google Scholar]
- Konstantinides S, Schafer K, Thinnes T, Loskutoff DJ. Plasminogen activator inhibitor-1 and its cofactor vitronectin stabilize arterial thrombi after vascular injury in mice. Circulation. 2001;103:576–583. doi: 10.1161/01.cir.103.4.576. [DOI] [PubMed] [Google Scholar]
- Sato Y, Hamanaka R, Ono J, Kuwano M, Rifkin DB, Takaki R. The stimulatory effect of PDGF on vascular smooth muscle cell migration is mediated by the induction of endogenous basic FGF. Biochem Biophys Res Commun. 1991;174:1260–1266. doi: 10.1016/0006-291x(91)91557-s. [DOI] [PubMed] [Google Scholar]
- Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA. 2003;100:10623–10628. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre CJ, Mancuso M, Maas K, McLean JW, Baluk P, McDonald DM. Expression of genes involved in vascular development and angiogenesis in endothelial cells of adult lung. Am J Physiol. 2003;285:H1917–H1938. doi: 10.1152/ajpheart.00983.2002. [DOI] [PubMed] [Google Scholar]
- Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, Detmar M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, Testa JE, Schnitzer JE. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429:629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NPH, Risau W, Ullrich A. High affinity vascular endothelial growth factor binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Matthews W, Jordan CT, Gavin M, Jenkins NA, Copeland NG, Lemischka IR. A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc Natl Acad Sci USA. 1991;88:9026–9030. doi: 10.1073/pnas.88.20.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer C, Breier G, Risau W, Plate KH. Up-regulation of flk-1/vascular endothelial growth factor receptor 2 by its ligand in a cerebral slice culture system. Cancer Res. 1997;57:3852–3859. [PubMed] [Google Scholar]
- Viglietto G, Romano A, Maglione D, Rambaldi M, Paoletti I, Lago CT, Califano D, Monaco C, Mineo A, Santelli G, Manzo G, Botti G, Chiapetta G, Persico MG. Neovascularization in human germ cell tumors correlates with a marked increase in the expression of the vascular endothelial growth factor but not the placenta-derived growth factor. Oncogene. 1996;13:577–587. [PubMed] [Google Scholar]
- Chakraborty I, Das SK, Dey SK. Differential expression of vascular endothelial growth factor and its receptor mRNAs in the mouse uterus around the time of implantation. J Endocrinol. 1995;147:339–352. doi: 10.1677/joe.0.1470339. [DOI] [PubMed] [Google Scholar]