Abstract
Columnar epithelial cells (EC), isolated from the proximal small intestine of the rat, bind ovalbumin (OVA) by a non-specific, cold-inhibitable mechanism and continue to express Ia antigens after 24 hr culture in vitro. Lymph node T cells from rats immunized with OVA proliferate following 18 hr coculture with EC and OVA. This accessory cell function of EC is antigen-specific and is blocked by anti-Ia monoclonal antisera.
Full text
PDF

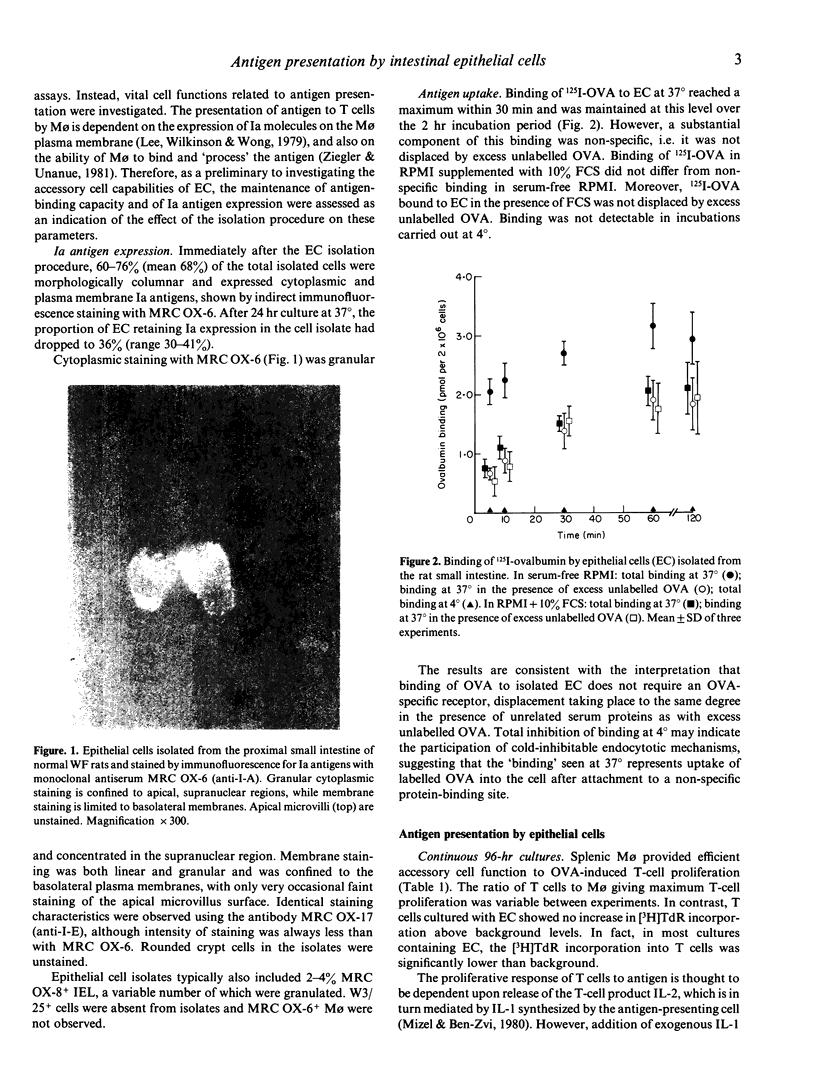
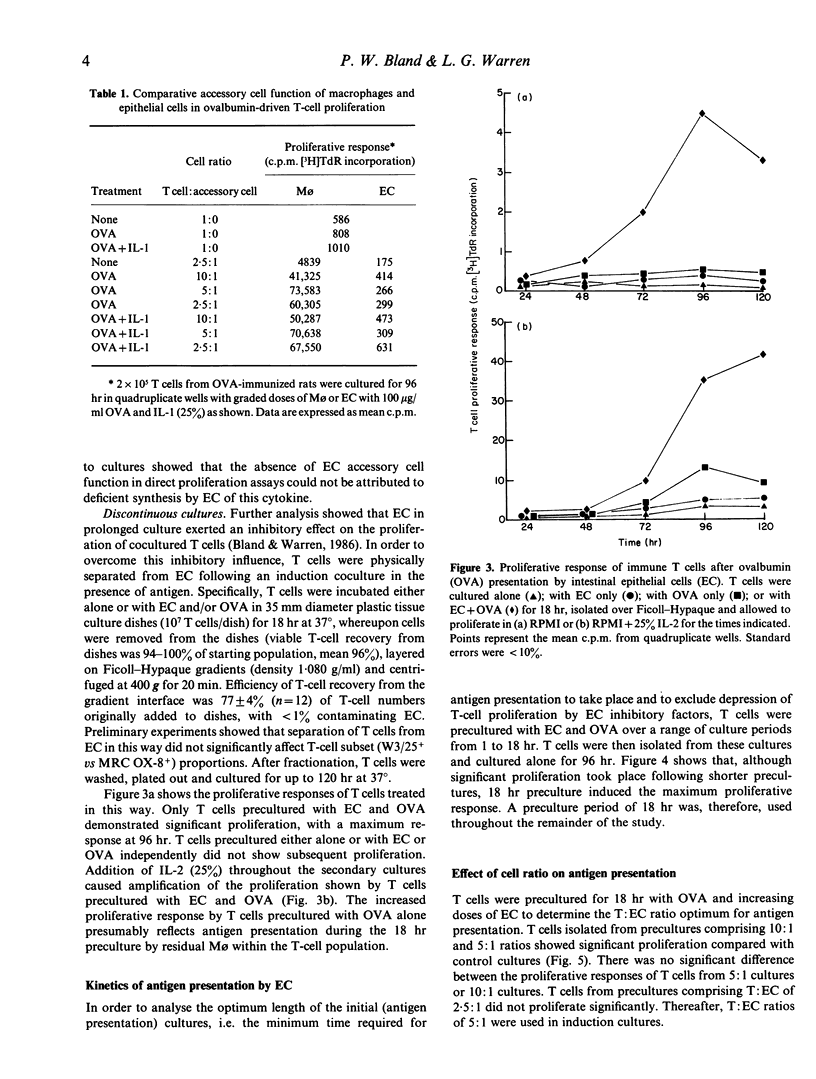
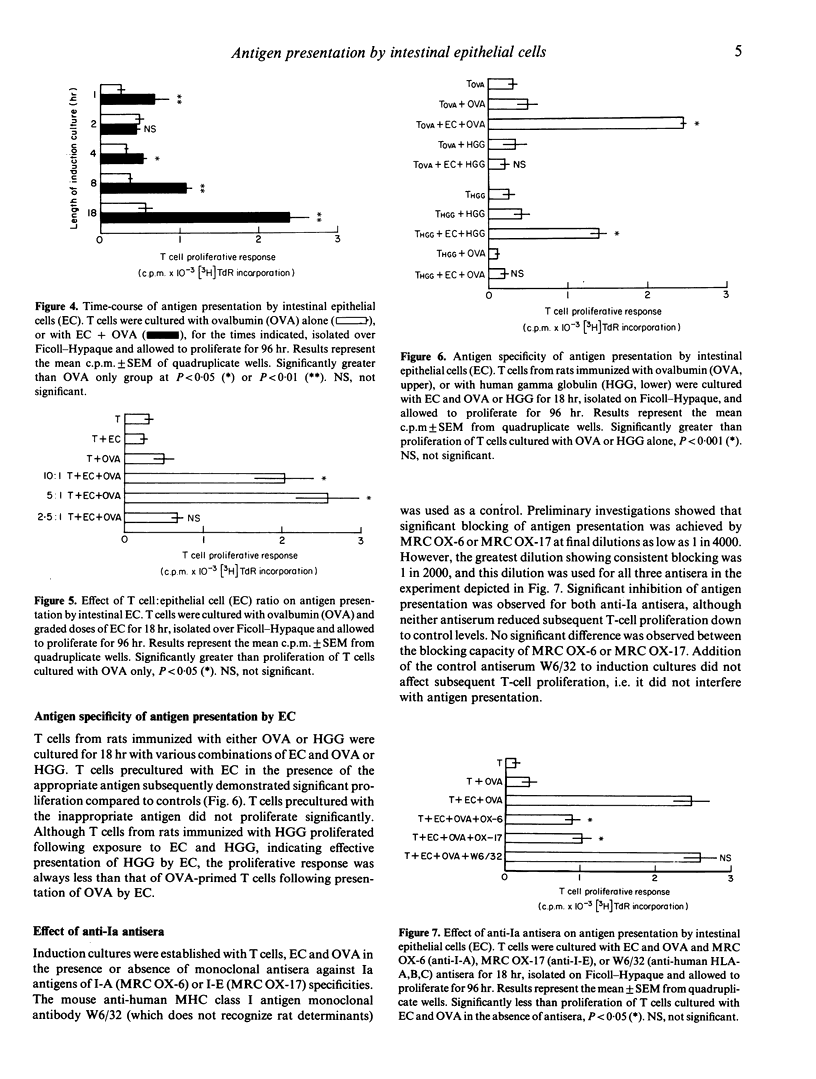


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N., Mason D. W. Induction of Ia antigen in rat epidermal cells and gut epithelium by immunological stimuli. J Exp Med. 1982 Dec 1;156(6):1665–1676. doi: 10.1084/jem.156.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland P. W., Warren L. G. Antigen presentation by epithelial cells of the rat small intestine. II. Selective induction of suppressor T cells. Immunology. 1986 May;58(1):9–14. [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Quaroni A., Kurnick J. T., Bhan A. K. Intraepithelial lymphocytes modulate Ia expression by intestinal epithelial cells. J Immunol. 1984 May;132(5):2244–2252. [PubMed] [Google Scholar]
- Chantot J. F., Saul A. J. A new method for measuring binding of labeled ligands to membrane receptors. Anal Biochem. 1978 Jan;84(1):256–262. doi: 10.1016/0003-2697(78)90508-0. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., Kindle C. S., Shreffler D. C., Cowing C. Differential glycosylation of murine B cell and spleen adherent cell Ia antigens. J Immunol. 1981 Oct;127(4):1478–1484. [PubMed] [Google Scholar]
- Green D. R., Gold J., St Martin S., Gershon R., Gershon R. K. Microenvironmental immunoregulation: possible role of contrasuppressor cells in maintaining immune responses in gut-associated lymphoid tissues. Proc Natl Acad Sci U S A. 1982 Feb;79(3):889–892. doi: 10.1073/pnas.79.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hanson D. G. Ontogeny of orally induced tolerance to soluble proteins in mice. I. Priming and tolerance in newborns. J Immunol. 1981 Oct;127(4):1518–1524. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Lee K. C., Wilkinson A., Wong M. Antigen-specific murine T-cell proliferation: role of macrophage surface Ia and factors. Cell Immunol. 1979 Nov;48(1):79–90. doi: 10.1016/0008-8749(79)90101-1. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Dallman M., Barclay A. N. Graft-versus-host disease induces expression of Ia antigen in rat epidermal cells and gut epithelium. Nature. 1981 Sep 10;293(5828):150–151. doi: 10.1038/293150a0. [DOI] [PubMed] [Google Scholar]
- Mayrhofer G., Pugh C. W., Barclay A. N. The distribution, ontogeny and origin in the rat of Ia-positive cells with dendritic morphology and of Ia antigen in epithelia, with special reference to the intestine. Eur J Immunol. 1983 Feb;13(2):112–122. doi: 10.1002/eji.1830130206. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Ben-Zvi A. Studies on the role of lymphocyte-activating factor (Interleukin 1) in antigen-induced lymph node lymphocyte proliferation. Cell Immunol. 1980 Sep 1;54(2):382–389. doi: 10.1016/0008-8749(80)90218-x. [DOI] [PubMed] [Google Scholar]
- Ngan J., Kind L. S. Suppressor T cells for IgE and IgG in Peyer's patches of mice made tolerant by the oral administration of ovalbumin. J Immunol. 1978 Mar;120(3):861–865. [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. 3. Suppression of plaque-forming cell responses in vitro by supernatant fluids from concanavalin A-activated spleen cell cultures. J Immunol. 1974 Apr;112(4):1360–1368. [PubMed] [Google Scholar]
- Richman L. K., Graeff A. S., Strober W. Antigen presentation by macrophage-enriched cells from the mouse Peyer's patch. Cell Immunol. 1981 Jul 15;62(1):110–118. doi: 10.1016/0008-8749(81)90304-x. [DOI] [PubMed] [Google Scholar]
- Richman L. K., Graeff A. S., Yarchoan R., Strober W. Simultaneous induction of antigen-specific IgA helper T cells and IgG suppressor T cells in the murine Peyer's patch after protein feeding. J Immunol. 1981 Jun;126(6):2079–2083. [PubMed] [Google Scholar]
- Scott H., Solheim B. G., Brandtzaeg P., Thorsby E. HLA-DR-like antigens in the epithelium of the human small intestine. Scand J Immunol. 1980;12(1):77–82. doi: 10.1111/j.1365-3083.1980.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Mason D. Y., Jewell D. P. Expression of HLA-DR antigens by colonic epithelium in inflammatory bowel disease. Clin Exp Immunol. 1983 Sep;53(3):614–618. [PMC free article] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding D. M., Williamson S. I., Koopman W. J., McGhee J. R. Preferential induction of polyclonal IgA secretion by murine Peyer's patch dendritic cell-T cell mixtures. J Exp Med. 1984 Sep 1;160(3):941–946. doi: 10.1084/jem.160.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M., Walker W. A. Food proteins and gut mucosal barrier I. Binding and uptake of cow's milk proteins by adult rat jejunum in vitro. Am J Physiol. 1984 May;246(5 Pt 1):G556–G562. doi: 10.1152/ajpgi.1984.246.5.G556. [DOI] [PubMed] [Google Scholar]
- Stokes C. R., Swarbrick E. T., Soothill J. F. Genetic differences in immune exclusion and partial tolerance to ingested antigens. Clin Exp Immunol. 1983 Jun;52(3):678–684. [PMC free article] [PubMed] [Google Scholar]
- Vives J., Parks D. E., Weigle W. O. Immunologic unresponsiveness after gastric administration of human gamma-globulin: antigen requirements and cellular parameters. J Immunol. 1980 Oct;125(4):1811–1816. [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]