Abstract
Experimental data from human adults or animal models indicate that the Helicobacter pylori-specific immune response is dominated by inflammatory T cells of the Th1 type. To investigate whether a Th1 immune response is established in early H. pylori infection, gastric biopsy samples from 70 children were subjected to immunohistochemical analysis. To this end, T cells, B cells, monocytes, neutrophils, and chemokine receptor 5 (CCR5)-expressing (CCR5+) cells, which are associated with Th1 immune responses, were quantified. Children were classified according to H. pylori status and clinical, laboratory, and macroscopic (during endoscopy) findings, without knowledge of histological findings. Group 1 included 31 H. pylori-infected children, group 2 contained 24 children with other conditions possibly affecting the stomach, and group 3 contained 15 children without verifiable pathological findings in the stomach. Lymphoid follicles were present in 90% of biopsy samples from group 1 and 48% of those from group 2 but absent in group 3 biopsy samples. Intraepithelial T cells and CCR5+ cells were regularly detected in all groups without significant differences. B cells, monocytes, and neutrophils were not found. In contrast, the numbers of lamina propria T cells (P < 0.003) and CCR5+ cells (P < 0.001) were increased significantly in H. pylori-infected children. B cells (in 13 of 66 children) were detected in children with active (n = 11) or previously cleared (n = 2) H. pylori infections but were absent in healthy children. The numbers of monocytes (in 10 of 67 children) did not differ among the groups. Calculations indicated that the majority of gastric T cells express CCR5; this finding is in contrast to the low percentage of CCR5+ T cells in the peripheral circulation. Thus, an increase in the numbers of CCR5+ cells in H. pylori-infected stomach mucosa suggests that this molecule may play an important role in gastric immune responses.
An infection with Helicobacter pylori is one of the most prevalent infectious diseases in the world, affecting over half of the world population. It is acquired during early childhood (50), and without specific therapy, the bacterium usually persists throughout life. The infection of children differs in several aspects from the infection of adults: while spontaneous clearance of the pathogen is a rare event in adults, it seems to be quite common during childhood (17, 47). The degree and activity of gastritis, the extent of H. pylori colonization, and the degree of atrophic gastritis with intestinal metaplasia differ significantly between children and adults (30). In addition, the antral mucosa of symptomatic and asymptomatic H. pylori-infected children shows nodularity, which is the macroscopic correlate of newly formed organized lymphoid tissue (16). This nodularity is noticeably less common in adults. These clinical observations lend support to the hypothesis that the H. pylori-specific immune response may change during progression from the earlier stages of the infection in childhood to the later stages in adulthood. It is generally accepted that infection with H. pylori leads to a predominant Th1 response in the gastric mucosa, which may promote local inflammation (7, 14, 21, 33, 44). Data from H. pylori-infected mice support this contention (14, 33). Previous studies of H. pylori responses in humans investigated adult peripheral blood lymphocytes (25) or used experimental systems that analyzed only gastric T-cell clones (12), single cells isolated from the stomachs of infected adults (44), or cultured stomach explants that were infected in vitro (26).
Chemokines are chemoattractant cytokines that coordinate the migration of leukocytes to sites of inflammation (28). Chemokines are classified into four families (CC, CXC, CX3, and C) based on the positioning of amino acids between the two N-terminal cysteine residues (35). CX3 and C chemokines are each represented by single members. While the group of CXC chemokines acts preferentially on neutrophils, the CC chemokine group is mainly involved in the attraction of lymphocytes (5). The corresponding chemokine receptors are grouped according to the structures of their chemokine ligands. Due to their distinct effector functions, Th1 and Th2 lymphocyte subsets travel to different tissues in a process guided by chemokines and adhesion molecules. Accordingly, they show selective expression of chemokine receptors: human interleukin 4 (IL-4)-producing cells have been shown to express chemokine receptor 3 (CCR3) (36), suggesting that at least a subset of Th2 cells expresses this marker. Other chemokine receptors, including CCR4 and CCR8, are also highly selective for Th2 cells (10, 52). On the other hand, CXCR3 and CCR5 are predominantly expressed by Th1 cells (9). Increased numbers of CCR5-expressing (CCR5+) cells have been observed in a number of Th1-dominated diseases, such as rheumatoid arthritis (29, 49), inflammatory kidney disease (39), multiple sclerosis (6), hepatitis C (41), and inflammatory bowel disease (4). In contrast, CCR5 knockout mice have impaired production of the Th1 cytokine gamma interferon (3). Patients lacking CCR5+ cells due to a homozygous deletion within the CCR5 gene show prolonged renal transplant survival (15), indicating a diminished Th1 response.
In the study presented here, we investigated whether a Th1 response could be demonstrated in situ in the gastric mucosa of children, in whom an undisturbed cellular microenvironment was preserved. For this purpose, we compared the levels of expression of CCR5 as a surrogate marker of Th1 cells in gastric biopsy samples from H. pylori-infected and noninfected children.
MATERIALS AND METHODS
Patients and tissue sampling.
Children and adolescents younger than 18 years of age and requiring an upper endoscopy for diagnostic purposes and clinical indications were considered for inclusion in the study. At the time of endoscopy, the following information was obtained by using a standardized questionnaire: age; sex; nationality; presence of chronic disease, particularly inflammatory bowel disease, celiac disease, and suspected or proven food allergy; and intake of medication during the preceding 4 weeks. Within 2 days of the upper endoscopy, a 13C-urea breath test (13C-UBT) was performed as described previously (23). Upper endoscopy was performed by the same investigator (S.K.) using a pediatric gastroscope (Olympus PQ20) with a 2.8-mm working channel. Macroscopic findings for the esophagus, stomach, and duodenum were reported in a standardized fashion. Forceps biopsy samples were obtained from different sites for the following purposes: one each from the antrum and corpus for histological analysis and two additional antrum samples for rapid urease testing and culturing of H. pylori. In addition to these routine biopsy samples, one or two biopsy samples of the antrum were obtained for immunohistochemical analysis. After mucus was removed by swabbing the surface of the biopsy samples, the specimens were snap-frozen on dry ice, embedded in Tissue-Tek (Sakura Finetek Europe B.V., Zoeterwoude, The Netherlands), and stored at −80°C until processing.
A child was considered infected with H. pylori when the 13C-UBT and the rapid urease test and/or cultures gave positive results and as noninfected when all three tests gave concordant negative results. Children not fulfilling these criteria were excluded from the study. H. pylori-infected patients with a known immunological disorder (e.g., food allergy, acquired or congenital immunodeficiency, celiac disease, or inflammatory bowel disease) or with intake of antibiotics, acid-suppressing agents, or anti-inflammatory drugs within 4 weeks prior to endoscopy were excluded from the study.
The children were divided into three groups according to H. pylori status, clinical and laboratory findings, history, and macroscopic findings during upper endoscopy. However, classification was made without knowledge of the results of histological and immunohistochemical analyses. Group 1 included all children with proven H. pylori infection. Group 2 included noninfected patients with suspected or proven gastric disease, defined as an abnormal macroscopic appearance of the gastric mucosa (erythema, erosion, ulceration, or nodularity), a known underlying disease, or intake of drugs that may affect the gastric mucosa. This group was considered a disease control group. Group 3 included the remaining H. pylori-negative children, who underwent gastric endoscopy, had no intake of drugs during the preceding 4 weeks, and had no known condition that would affect the stomach. Although patients in group 3 were not all healthy children, they were considered a healthy control group because they had no evidence of gastric disease. Written informed consent was obtained prior to endoscopy. The study protocol was approved by the Ethics Committee of Ludwig-Maximilians University.
Blood sampling.
Prior to endoscopy, peripheral venous blood was drawn from each child for a blood count to exclude anemia. For nine children, residual material from the blood count was used for flow cytometry.
Histological analysis.
Histopathological classification of gastritis was performed by one pathologist (D.A.) using paraffin-embedded sections with hematoxylin-eosin and modified Giemsa staining. The pathologist was blinded for clinical symptoms, diagnoses, and medications. The upgraded Sydney classification (13) was applied and included grading of chronicity, activity, atrophy, intestinal metaplasia, and bacterial density on a scale from 0 to 3 (none, mild, moderate, and severe, respectively).
Immunohistochemical analysis.
Immunohistochemical analysis was performed with air-dried 5-μm cryostat sections of antral mucosa biopsy samples by the indirect immunoperoxidase method. In brief, sections were incubated for 35 min with 40 μl of a primary mouse monoclonal antibody. Optimal dilutions were determined with human tonsil samples (Table 1). Endogenous peroxidase was blocked with blocking reagent (Dako, Hamburg, Germany). After washing, a secondary peroxidase-labeled rabbit anti-mouse immunoglobulin G (IgG) antibody was applied for an additional 30 min. Sections were developed with diaminobenzole under visual control until brownish staining developed. Counterstaining was done with Mayer's Haemalaun (Merck, Darmstadt, Germany). Isotype controls were included. Granulocytes were detected in unblocked sections by the activity of endogenous peroxidase. All biopsy samples were analyzed with the aid of image-processing software (dhs database, version 4.0) by digitizing three nonoverlapping areas (×20 objective; digital 0.5-in.-chip-size TK-C1380 camera [JVC]).
TABLE 1.
Monoclonal antibodies used for immunohistochemical analysis
| Antibody | Specificity | Dilution | Source |
|---|---|---|---|
| M-T910 | CD2 | 1:3 | E. P. Rieber, Dresden, Germany |
| M-T310 | CD4 | 1:3 | E. P. Rieber |
| M-T811 | CD8 | 1:9 | E. P. Rieber |
| M-M42 | CD14 | None (undiluted) | E. P. Rieber |
| M5E2 | CD14 | 1:50 | BD Pharmingen |
| HIB19 | CD19 | 1:80 | BD Pharmingen |
| MC5 | CCR5 | 1:5 | M. Mack, Munich, Germany |
| Isotype control | IgG2a and IgG2b | 1:100 | BD Pharmingen |
| Isotype control | IgG1 | 1:20 | Sigma, Taufkirchen, Germany |
| P 260 | Mouse IgG | 1:50 | Dako |
Intraepithelial cells were quantified as cells per 100 epithelial cells. For the lamina propria, a grid with a side length of 40 μm was used. The lamina propria area was calculated by subtracting the number of intercept points falling into the lamina propria from the number of total intercept points. A total area of 0.625 mm2 was analyzed. Results are expressed as cells per square millimeter.
Flow cytometry.
Three-color flow cytometry was performed with freshly drawn whole-blood samples treated with the anticoagulant EDTA as described elsewhere (18). The following primary antibodies were used: for CD4, allophycocyanin-mouse IgG1 (BD Pharmingen, Heidelberg, Germany); for CD8, phycoerythrin (PE)-cyanin-5-mouse IgG1 (Immunotech, Marseille, France); for CD19, PE-mouse IgG1 (Immunotech); and for CCR5, PE-mouse IgG2a (R&D Systems, Wiesbaden, Germany). As isotype controls, mouse IgG1-fluorescein isothiocyanate (Immunotech) and mouse IgG2a-PE (Immunotech) were used. Cells were immediately analyzed by flow cytometry (Calibur; Becton Dickinson, Heidelberg, Germany), and calculations were performed with CellQuest analysis software. To determine the percentage of chemokine receptor-positive T cells, lymphocytes were first gated according to their forward-scatter and side-scatter characteristics. A second gate was set for CD4+ or CD8+ cells, and the number of chemokine receptor-positive cells was calculated after defining a cutoff value by using an isotype control.
Determination of CCR5 genotype.
CCR5 genotype analysis was performed by PCR with genomic DNA isolated from gastric biopsy samples. Biopsy samples were placed in 1 ml of PCR buffer with nonionic detergents (50 mM KCl, 10 mM Tris-HCl [pH 8.3], 2.5 mM MgCl2, 0.01% [wt/vol] gelatin, 0.45% [vol/vol] Nonidet P-40, 0.45% [vol/vol] Tween 20) and proteinase K (5 μl of a 20-mg/ml stock solution in water). Samples were incubated overnight at 56°C to achieve complete lysis. Proteinase K was inactivated by heating (10 min at 95°C), and 1 μl of the lysate was used directly for PCR analysis. A genomic fragment of the human CCR5 gene spanning a region with a 32-bp deletion was amplified by PCR with primers and conditions described previously (15).
Determination of CagA tyrosine phosphorylation and IL-8 production.
For measurement of CagA tyrosine phosphorylation, AGS cells (ATCC CRL-1739) were grown in six-well plates (9.5 cm2) and infected with H. pylori at a multiplicity of infection of 100. Adherent bacteria were removed by washing five times in phosphate-buffered saline (PBS). Cells were suspended in 1 ml of ice-cold PBS* (PBS, 1 mM EDTA, 1 mM o-vanadate, 1 mM phenylmethylsulfonyl fluoride, 1 μM leupeptin, 1 μM pepstatin), collected by centrifugation, and resuspended in 3 μl of PBS*. Lysates of infected cells were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis by using a minigel apparatus (Bio-Rad) and were blotted onto a polyvinylidene difluoride membrane at 1 mA/cm2 by using a semidry blot system (Biotec Fischer). For detection of CagA, the filters were blocked with 3% bovine serum albumin in 50 mM Tris-HCl (pH 7.5)-150 mM NaCl and incubated with antiserum AK257 (anti-CagA) (34). Alkaline phosphatase-coupled protein A was used to visualize the antibody bound by decomposition of nitroblue tetrazolium. For detection of tyrosine phosphorylation of proteins, a blot was blocked overnight in 50 mM Tris (pH 7.4)-200 mM NaCl- 0.1% Tween 20- 5% milk powder. For detection of tyrosine phosphorylation of CagA, phosphotyrosine-specific monoclonal antibody PY99 was applied (Santa Cruz Biotechnology, Inc.).
A sandwich enzyme-linked immunosorbent assay (38) was used for the quantitative determination of IL-8. The anti-IL-8 capture antibody was bound to a 96-well microtiter plate. After four washing steps and blocking of nonspecific binding sites, 100 μl of cell culture supernatant was tested per well. Each sample was tested in duplicate, and the assay was standardized with 0 to 800 pg of recombinant human IL-8/ml. After overnight incubation (4°C) and four washing steps, the wells were incubated with biotinylated secondary anti-IL-8 antibody G265-8 (0.5 μg/ml in blocking buffer-10% PBS) for 1 to 2 h at room temperature. A streptavidin-alkaline phosphatase conjugate was added, and the mixture was incubated for 1 h at 37°C. After four washing steps, the substrate was added, and the mixture was incubated for 20 min at 37°C in the dark before the absorbance at 405 nm was measured with an enzyme-linked immunosorbent assay reader.
Statistical analysis.
The nonparametric Kruskal-Wallis test was applied to calculate differences among the three groups. The Mann-Whitney U test was used to test differences between two groups. A probability of ≤0.05 was regarded as statistically significant. The Spearman rho test was performed to test the correlation among scores of Sydney classification parameters, CagA expression, and cell subsets. A correlation was considered positive when correlation coefficient r was greater than 0.3. Statistical analysis was performed with the SPSS statistical program (version 10; SPSS Inc., Chicago, Ill.) and with the expert advice of a statistician (P. Dirschedl, Institute of Biometry and Epidemiology, Ludwig-Maximilians University).
RESULTS
Patient characteristics.
A total of 70 children were included in this study: 38 males and 32 females with a median age of 9.5 years and a range of 0.9 to 17.5 years. Thirty-one patients (17 females) were infected with H. pylori and formed group 1. All patients in group 1 had positive results for the 13C-UBT and the rapid urease test; all but three had positive cultures. Their median age was 10.7 years, with a range of 3.5 to 17 years. The remaining 39 children were not infected with H. pylori, based on concordant negative results in all diagnostic tests. Twenty-four of these noninfected patients were considered disease controls and formed group 2 (12 females; median age, 7.2 years, and range, 0.9 to 17.5 years). Sixteen of the 24 children had a chronic inflammatory disease of the digestive tract: 4 children had Crohn's disease or ulcerative colitis, 6 patients had proven or highly suspected food allergy, 5 patients had celiac disease with flat duodenal mucosa, and 1 child had had chronic varioliform gastritis with associated autoimmune thyroiditis for 2 years. Five children had been successfully treated for H. pylori infection within the preceding 12 months. The remaining three children had gastric erythema in the antrum: one patient had H. heilmannii infection, and the two remaining patients had severe reflux esophagitis, delayed gastric emptying, and gastric erythema due to biliary reflux. Fifteen noninfected children (9 females; median age, 6.2 years, and range, 1.6 to 15.7 years) had no indication of gastric involvement and were assigned to group 3. Seven of them had clinical symptoms suggestive of reflux esophagitis; however, their esophagus was without pathological macroscopic findings. Three children with previous reflux esophagitis underwent control endoscopy after weaning from medication. These children had neither clinical nor endoscopic signs of esophagitis. One child was investigated because of refusal to eat, but esophagitis or other pathological findings could not be demonstrated. One child had achalasia, and one had suspected celiac disease, which was not confirmed with duodenal biopsy samples. One severely mentally retarded child underwent endoscopy to rule out reflux disease and received a percutanous endoscopic gastrostoma because of malnutrition.
Histopathological analysis.
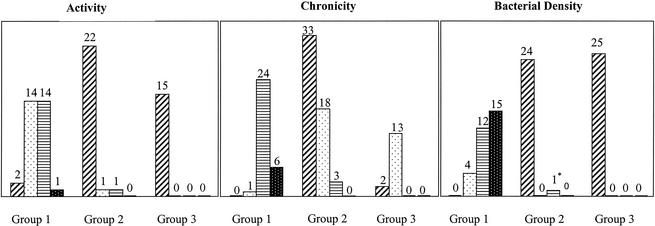
The results of the histopathological grading for activity and chronicity of gastritis and bacterial density are summarized in Fig. 1. Higher grades of activity and chronicity were found only in H. pylori-infected children. Mild atrophy (grade 1) was observed in four, two, and three patients in groups 1, 2, and 3, respectively. Intestinal metaplasia was not observed in any of the patients. Lymphoid follicles were present in 28 H. pylori-infected children and in 10 patients in group 2, whereas no follicles were seen in children without gastric disease.
FIG. 1.
Sydney classification for the three patient groups. Bars for each group indicate, from left to right, grades 0, 1, 2, and 3, respectively. Numbers above bars indicate numbers of patients in groups. The asterisk indicates one child who was infected with H. heilmannii but not with H. pylori.
Intraepithelial lymphocytes.
The numbers of intraepithelial T cells were not significantly different among the groups. CD8+ cells (median values, 6.2, 6.3, and 7.0% in groups 1, 2, and 3, respectively) clearly predominated over CD4+ T cells (median values, 2.2, 0.9, and 1.7% in groups 1, 2, and 3, respectively). The CD4/CD8 ratio was slightly, but not significantly, higher in the H. pylori-infected group than in the other two groups (0.44 versus 0.29). B cells, defined as CD19-staining cells, monocytes, and neutrophils, were not observed within the epithelium. CCR5+ cells were demonstrated in the epithelium of 64 of 70 children. No differences were observed among the groups (median values, 3.2, 2.5, and 3.1% of cells in groups 1, 2, and 3, respectively, with a range of 0 to 10.3% of cells). The children lacking CCR5+ cells belonged to groups 2 and 3. In four of these children, CCR5+ cells were also not detected in the lamina propria.
Lamina propria lymphocytes.
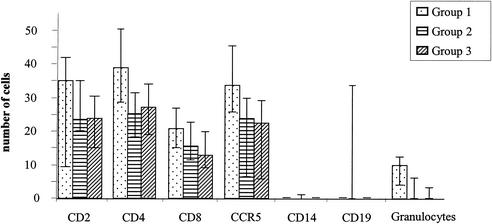
All patient biopsy samples had T cells in the lamina propria, with a predominance of CD4+ cells. The numbers of CD4+ and CD8+ cells were significantly higher in H. pylori-infected children (Fig. 2). However, the CD4/CD8 ratio was not altered among the groups. No significant differences were found between disease controls and healthy children. B cells were present in 13 of 66 children. Eleven of these children were infected with H. pylori. The two H. pylori-negative children whose biopsy samples stained for B cells in the lamina propria had been successfully treated for an H. pylori infection within the preceding 12 months. Most biopsy samples had very low numbers of B cells (median, 0%; range, 0 to 117%). Three children with unusually high numbers of B cells in their biopsy samples were siblings from an Afghani family. CD14+ cells were present in only 10 of 67 children and were distributed in all groups without apparent differences. In contrast, CCR5+ cells could be demonstrated in 66 of 70 children, and there was a highly significant difference between H. pylori-positive and -negative individuals (Fig. 2). The four children lacking CCR5+ cells in the lamina propria also lacked intraepithelial CCR5+ cells. Two children each belonged to group 2 and group 3. Individuals homozygous for the 32-bp deletion in the CCR5 gene express a truncated protein that cannot be expressed at the cellular surface (11, 37). Therefore, CCR5 genotyping was performed for these four children to determine whether this deletion could have accounted for the lack of CCR5+ cells. This analysis revealed that one individual in each group was heterozygous for the deletion. Therefore, the lack of CCR5+ cells was not due to the presence of homozygous mutant alleles.
FIG. 2.
Cells per square millimeter in the lamina propria. Bars indicate interquartile ranges.
To determine whether the increase in the numbers of CCR5+ cells was specific for H. pylori infection, we calculated the ratios of CCR5+ cells to T cells for the different groups. No significant changes in the CCR5+ cell/T-cell ratios were observed. As would be expected, neutrophil numbers differed highly significantly between infected and noninfected children (Fig. 2).
Association of CCR5+ cells with lymphocyte subsets.
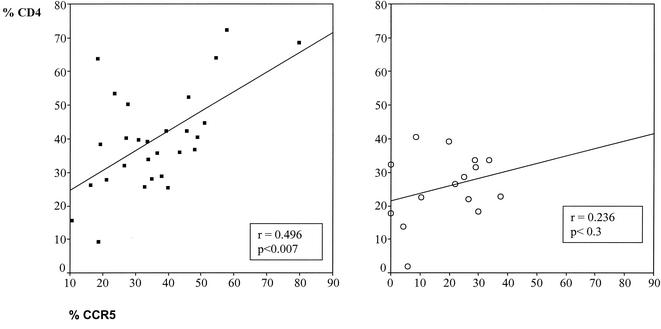
We further analyzed whether CCR5+ cells correlated with the presence of other cells. No significant correlations were found within the epithelium. In contrast, CCR5+ cells showed highly significant correlations with CD4+ cells (r = 0.63; P < 0.001) and CD8+ cells (r = 0.48; P < 0.001) in the lamina propria. A lower correlation was also found for B cells (r = 0.31; P = 0.01). To exclude bias due simply to higher cell numbers in H. pylori-infected individuals, we analyzed the association of CCR5+ populations with other lymphocyte populations in individual groups (Fig. 3). A strong correlation was still found for T cells in groups 1 and 2. No correlation was found in the healthy children.
FIG. 3.
Correlation of CCR5+ and CD4+ cells in the lamina propria. (Left) Group 1. (Right) Group 3. The Spearman rho test was used.
Correlation of CCR5 expression between lamina propria and peripheral blood.
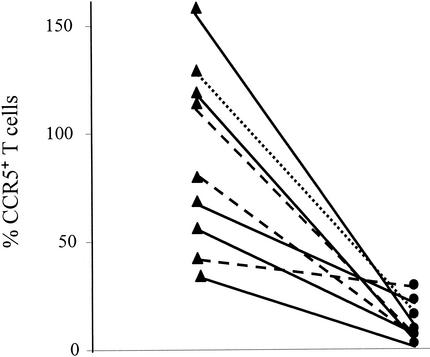
The expression of CCR5 on peripheral blood lymphocytes was compared with the expression in the lamina propria for nine patients. Six, two, and one patients belonged to groups 1, 2, and 3, respectively. In peripheral blood, the percentage of CCR5+ T cells ranged from 1.2 to 28.2% (median, 6.55%). Since monocytes were very rare or absent in the lamina propria, it was presumed that CCR5 expression in the gastric mucosa was essentially due to T cells. The biopsy samples with numbers of CCR5+ cells higher than the sum of CD4+ and CD8+ cells had higher numbers of B cells, a fact which most likely accounted for the additional CCR5+ cells. A comparison of the values for peripheral T cells with those for lamina propria T cells revealed much higher numbers of CCR5+ T cells in the lamina propria (Fig. 4), suggesting specific accumulation or selective expansion of these cells in the lamina propria.
FIG. 4.
CCR5+ T cells in lamina propria (triangles) and peripheral blood (circles) for group 1 (solid line), group 2 (dashed line), and group 3 (dotted line).
Correlation with CagA status.
From 24 H. pylori-infected patients, the respective H. pylori strain was isolated and analyzed for its capacity to induce IL-8 secretion and to translocate and phosphorylate the CagA protein in AGS epithelial cells. Both phenotypes are dependent on an intact cag pathogenicity island (PAI), which encodes a functional type IV secretion system. Fifteen isolated strains were able to induce IL-8 secretion and to translocate and tyrosine phosphorylate CagA. Both markers showed a positive and very strong correlation with the numbers of neutrophils in the lamina propria (for IL-8, r was 0.562 and P was <0.005; for Tyr-P, r was 0.589 and P was <0.002). In contrast, the numbers of CCR5+ cells and all other lymphocyte subsets did not correlate significantly with the presence or absence of a functional cag PAI.
DISCUSSION
The present work demonstrates that CCR5+ cells and T cells are regularly present in gastric mucosa and that their levels increase noticeably in inflamed gastric mucosa. Previous studies investigating the immune response in H. pylori-infected gastric mucosa in humans were performed mainly with adults. However, the gastric immune response of adults can be altered by factors not related to the infection, including exposure to alcohol, tobacco, or pharmaceutical agents (43). Therefore, we investigated the gastric mucosa of children, in the majority of whom these factors could be largely excluded. Some studies have classified patients according to histological criteria only (40, 48). This procedure might introduce selection bias, since higher numbers of immunohistochemically stained cells are expected in tissues that are rich in inflammatory cells. For this reason, we based our patient classification on the results of three diagnostic tests. Because we wanted to exclusively assess the immune response to H. pylori, we excluded all infected children with an additional underlying condition that may have influenced gastric inflammation. The division of noninfected children into disease controls and healthy controls was based on the clinical history and endoscopic findings, but we did not consider the results of histological analyses. The finding of lymphoid follicles, which clearly represent a pathological finding in the stomach, in 90% of the H. pylori-infected children and in 42% of the disease controls but in none of the healthy controls confirmed, retrospectively, the assignment of the patients to the three groups. Group 2 included five children with recently eradicated H. pylori infection. It seemed most reasonable to include these children in group 2, since cellular infiltrates may persist for several months after successful treatment of the infection. Exclusion of these children from the calculation, however, did not alter the results (data not shown). H. pylori infection is mainly acquired in early childhood, but symptoms develop only after a longer period of infection. This explains why the children in group 1 were significantly older (P ≤ 0.04) than the noninfected children. However, a relationship between age and numbers of CCR5+ cells seems unlikely, since CCR5 expression within each group did not correlate with age. There were also differences in nationality, since group 1 included more children of non-German origin than the other two groups. However, analysis within the groups revealed no correlation of CCR5 expression with nationality.
Intraepithelial T cells were regularly detected, with a predominance of CD8+ cells. A slight but nonsignificant increase in the CD4/CD8 ratio was observed in infected gastric mucosa. This finding is in line with the findings of Hatz et al. (19) but contradicts those of other studies in which significant increases in the numbers of T cells (24) or CD8+ cells (20) were found in H. pylori-infected mucosa. In contrast, the numbers of lamina propria CD4+ and CD8+ T cells increased significantly during H. pylori infection, with a consistent predominance of CD4+ cells, corresponding to the findings of Hatz et al. (19). The CD4/CD8 ratio was not significantly different among the groups, in contrast to studies that reported a selective enrichment of CD4+ cells (40) or CD8+ cells (20) in infected individuals. These cited studies were performed exclusively with adults and had substantial methodological differences that may have contributed to the divergent results. B cells were rarely detected in the biopsy samples from the children. However, when B cells were present, they were almost exclusively found in children with active or recent H. pylori infection.
Detailed studies of the local and systemic immune responses against H. pylori have led to the concept that infection with H. pylori elicits a predominant Th1 immune response (21, 33, 44). Both in vitro and in vivo studies have shown that CCR5 expression is associated with a Th1 response (4, 9, 27, 32, 45). Recent studies revealed CCR5 expression on isolated lymphocytes of the human jejunum (1, 31). Nevertheless, only indirect evidence suggests that CCR5+ cells may be also involved in gastric inflammatory disease, on the basis of the finding that the levels of the CCR5 ligands MIP-1α (2, 42) and RANTES (22, 43, 51) increase during H. pylori infection. However, the binding of MIP-1α or RANTES is not restricted to CCR5+ cells. Furthermore, monocytes and macrophages express CCR5 in addition to T cells. In our study, high numbers of CCR5+ cells were present in inflamed gastric mucosa, with a strong statistical correlation between T cells and CCR5 expression in the lamina propria of H. pylori-infected children and those with suspected gastric disease. In healthy children, this correlation did not exist, a result which is possibly due to the overall lower cell numbers in healthy gastric mucosa. Taking into account that macrophages were absent or very rare, we assume that CCR5 expression was primarily restricted to T cells; differentiation between CD4+ and CD8+ cells could not be made. In four H. pylori-negative children, CCR5+ cells were absent in both the epithelium and the lamina propria. The two children carrying one allele with a CCR5 mutation had average numbers of CD2+ T cells in both tissue compartments (intraepithelial lymphocytes, 4 to 8%; lamina propria, 19 to 23 cells/mm2). Thus, the absence of CCR5+ cells in these children could not be explained by an overall absence of infiltrating lymphocytes in the tissues. Other polymorphisms in the CCR5 gene that lead to reduced expression of the protein were not examined in these children, since they are very rare mutations (8, 46).
The expression of CCR5 on high numbers of cells in the gastric mucosa contrasted with the considerably lower numbers of peripheral CCR5+ T cells. A high percentage of organ-infiltrating CCR5+ T cells has also been described for Th1-associated diseases, such as hepatitis C (41) and different forms of arthritis (29). Whether this finding represents selective recruitment of CCR5+ memory T cells, local upregulation of CCR5 expression on T cells, or local proliferation of such T cells in this microenvironment is unknown at present. The cagA PAI, which consists of multiple genes, seems not to be involved in the regulation of CCR5 expression. An intact PAI induces gastric expression of the CXC chemokines IL-8, ENA-78, and GRO-α, while that of the CCR5 ligand RANTES appears to be independent of the PAI (43).
In conclusion, the present study shows that H. pylori leads to major changes in cellular compositions in the lamina propria but not in the gastric epithelium. Furthermore, it provides evidence that CCR5+ cells are constitutively present in healthy gastric mucosa. The increased numbers of CCR5+ cells in H. pylori-infected stomach mucosa indicate that this molecule may play an important role in gastric mucosal immune responses. Taking into consideration that CCR5 is a Th1-associated molecule, we speculate that the gastric mucosa is primarily capable of exerting Th1 immune responses irrespective of the nature of the intruding pathogen.
Acknowledgments
We thank M. Schleicher for providing access to hardware with installed dhs database software.
This work was supported by grants from the Else-Kröner-Fresenius Stiftung, the Friedrich-Baur-Stiftung, and Hochschulsonderprogramm III of Ludwig-Maximilians University.
REFERENCES
- 1.Agace, W. W., A. I. Roberts, L. Wu, C. Greineder, E. C. Ebert, and C. M. Parker. 2000. Human intestinal lamina propria and intraepithelial lymphocytes express receptors specific for chemokines induced by inflammation. Eur. J. Immunol. 30:819-826. [DOI] [PubMed] [Google Scholar]
- 2.Ando, T., K. Kusugami, M. Ohsuga, K. Ina, S. Ichiyama, T. Nada, and M. Ohta. 1999. Mucosal macrophage inflammatory protein-1 alpha levels are increased in Helicobacter pylori infection. J. Clin. Gastroenterol. 27:S144-S149. [DOI] [PubMed]
- 3.Andres, P. G., P. L. Beck, E. Mizoguchi, A. Mizoguchi, A. K. Bhan, T. Dawson, W. A. Kuziel, N. Maeda, R. P. MacDermott, D. K. Podolsky, and H. C. Reinecker. 2000. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J. Immunol. 164:6303-6312. [DOI] [PubMed] [Google Scholar]
- 4.Annunziato, F., L. Cosmi, G. Galli, C. Beltrame, P. Romagnani, R. Manetti, S. Romagnani, and E. Maggi. 1999. Assessment of chemokine receptor expression by human Th1 and Th2 cells in vitro and in vivo. J. Leukoc. Biol. 65:691-699. [DOI] [PubMed] [Google Scholar]
- 5.Baggiolini, M., B. Dewald, and B. Moser. 1994. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv. Immunol. 55:97-179. [PubMed] [Google Scholar]
- 6.Balashov, K. E., J. B. Rottman, H. L. Weiner, and W. W. Hancock. 1999. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc. Natl. Acad. Sci. USA 96:6873-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bamford, K. B., X. Fan, S. E. Crowe, J. F. Leary, W. K. Gourley, G. K. Luthra, E. G. Brooks, D. Y. Graham, V. E. Reyes, and P. B. Ernst. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114:482-492. [DOI] [PubMed] [Google Scholar]
- 8.Blanpain, C., B. Lee, M. Tackoen, B. Puffer, A. Boom, F. Libert, M. Sharron, V. Wittamer, G. Vassart, R. W. Doms, and M. Parmentier. 2000. Multiple nonfunctional alleles of CCR5 are frequent in various human populations. Blood 96:1638-1645. [PubMed] [Google Scholar]
- 9.Bonecchi, R., G. Bianchi, P. P. Bordignon, D. D'Ambrosio, R. Lang, A. Borsatti, S. Sozzani, P. Allavena, P. A. Gray, A. Mantovani, and F. Sinigaglia. 1998. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 187:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Ambrosio, D., A. Iellem, R. Bonecchi, D. Mazzeo, S. Sozzani, A. Mantovani, and F. Sinigaglia. 1998. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J. Immunol. 161:5111-5115. [PubMed] [Google Scholar]
- 11.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, S. J. O'Brien, et al. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 273:1856-1862. [DOI] [PubMed] [Google Scholar]
- 12.D'Elios, M. M., M. Manghetti, F. Almerigogna, A. Amedei, F. Costa, D. Burroni, C. T. Baldari, S. Romagnani, J. L. Telford, and G. Del Prete. 1997. Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer. Eur. J. Immunol. 27:1751-1755. [DOI] [PubMed]
- 13.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston, Tex., 1994. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed] [Google Scholar]
- 14.Eaton, K. A., M. Mefford, and T. Thevenot. 2001. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J. Immunol. 166:7456-7461. [DOI] [PubMed] [Google Scholar]
- 15.Fischereder, M., B. Luckow, B. Hocher, R. P. Wuthrich, U. Rothenpieler, H. Schneeberger, U. Panzer, R. A. Stahl, I. A. Hauser, K. Budde, H. Neumayer, B. K. Kramer, W. Land, and D. Schlöndorff. 2001. CC chemokine receptor 5 and renal-transplant survival. Lancet 357:1758-1761. [DOI] [PubMed] [Google Scholar]
- 16.Gottrand, F., D. Turck, and P. Vincent. 1992. Helicobacter pylori infection in early infancy. Lancet 340:495. [DOI] [PubMed] [Google Scholar]
- 17.Granstrom, M., Y. Tindberg, and M. Blennow. 1997. Seroepidemiology of Helicobacter pylori infection in a cohort of children monitored from 6 months to 11 years of age. J. Clin. Microbiol. 35:468-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruber, R., C. Reiter, and G. Riethmüller. 1993. Triple immunofluorescence flow cytometry, using whole blood, of CD4+ and CD8+ lymphocytes expressing CD45RO and CD45RA. J. Immunol. Methods 163:173-179. [DOI] [PubMed] [Google Scholar]
- 19.Hatz, R. A., G. Meimarakis, E. Bayerdorffer, M. Stolte, T. Kirchner, and G. Enders. 1996. Characterization of lymphocytic infiltrates in Helicobacter pylori-associated gastritis. Scand. J. Gastroenterol. 31:222-228. [DOI] [PubMed] [Google Scholar]
- 20.Hood, C. J., and M. Lesna. 1993. Immunocytochemical quantitation of inflammatory cells associated with Helicobacter pylori infection. Br. J. Biomed. Sci. 50:82-88. [PubMed] [Google Scholar]
- 21.Karttunen, R., T. Karttunen, H. P. Ekre, and T. T. MacDonald. 1995. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut 36:341-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kikuchi, T., K. Kato, S. Ohara, H. Sekine, T. Arikawa, T. Suzuki, K. Noguchi, M. Saito, Y. Saito, H. Nagura, T. Toyota, and T. Shimosegawa. 2000. The relationship between persistent secretion of RANTES and residual infiltration of eosinophils and memory T lymphocytes after Helicobacter pylori eradication. J. Pathol. 192:243-250. [DOI] [PubMed] [Google Scholar]
- 23.Kindermann, A., H. Demmelmair, B. Koletzko, S. Krauss-Etschmann, B. Wiebecke, and S. Koletzko. 2000. Influence of age on 13C-urea breath test results in children. J. Pediatr. Gastroenterol. Nutr. 30:85-91. [DOI] [PubMed] [Google Scholar]
- 24.Kirchner, T., A. Melber, W. Fischbach, K. L. Heilmann, and H. K. Müller-Hermelink. 1990. Immunohistological patterns of the local immune response in Helicobacter pylori gastritis, p. 213-222. In P. Malfertheiner (ed.), Helicobacter pylori gastritis and peptic ulcer. Springer Verlag KG, Berlin, Germany.
- 25.Kountouras, J., P. Boura, and N. J. Lygidakis. 2000. Omeprazole and regulation of cytokine profile in Helicobacter pylori-infected patients with duodenal ulcer disease. Hepatogastroenterology 47:1301-1304. [PubMed] [Google Scholar]
- 26.Lindholm, C., M. Quiding-Jarbrink, H. Lonroth, and A. M. Svennerholm. 2001. Induction of chemokine and cytokine responses by Helicobacter pylori in human stomach explants. Scand. J. Gastroenterol. 36:1022-1029. [DOI] [PubMed] [Google Scholar]
- 27.Loetscher, P., M. Uguccioni, L. Bordoli, M. Baggiolini, B. Moser, C. Chizzolini, and J. M. Dayer. 1998. CCR5 is characteristic of Th1 lymphocytes. Nature 391:344-345. [DOI] [PubMed] [Google Scholar]
- 28.Luster, A. D. 1998. Chemokines—chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338:436-445. [DOI] [PubMed] [Google Scholar]
- 29.Mack, M., H. Bruhl, R. Gruber, C. Jaeger, J. Cihak, V. Eiter, J. Plachy, M. Stangassinger, K. Uhlig, M. Schattenkirchner, and D. Schlöndorff. 1999. Predominance of mononuclear cells expressing the chemokine receptor CCR5 in synovial effusions of patients with different forms of arthritis. Arthritis Rheum. 42:981-988. [DOI] [PubMed] [Google Scholar]
- 30.Meining, A., R. Behrens, N. Lehn, E. Bayerdorffer, and M. Stolte. 1996. Different expression of Helicobacter pylori gastritis in children: evidence for a specific pediatric disease? Helicobacter 1:92-97. [DOI] [PubMed] [Google Scholar]
- 31.Meng, G., M. T. Sellers, B. M. Mosteller, T. S. Rogers, G. M. Shaw, and P. D. Smith. 2000. Lamina propria lymphocytes, not macrophages, express CCR5 and CXCR4 and are the likely target cell for human immunodeficiency virus type 1 in the intestinal mucosa. J. Infect. Dis. 182:785-791. [DOI] [PubMed] [Google Scholar]
- 32.Misu, T., H. Onodera, K. Fujihara, K. Matsushima, O. Yoshie, N. Okita, S. Takase, and Y. Itoyama. 2000. Chemokine receptor expression on T cells in blood and cerebrospinal fluid at relapse and remission of multiple sclerosis: imbalance of Th1/Th2-associated chemokine signaling. J. Neuroimmunol. 114:207-212. [DOI] [PubMed] [Google Scholar]
- 33.Mohammadi, M., S. Czinn, R. Redline, and J. Nedrud. 1996. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J. Immunol. 156:4729-4738. [PubMed] [Google Scholar]
- 34.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 35.Rossi, D., and A. Zlotnik. 2000. The biology of chemokines and their receptors. Annu. Rev. Immunol. 18:217-242. [DOI] [PubMed] [Google Scholar]
- 36.Sallusto, F., D. Lenig, C. R. Mackay, and A. Lanzavecchia. 1998. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 187:875-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 38.Schulte, R., and I. B. Autenrieth. 1998. Yersinia enterocolitica-induced interleukin-8 secretion by human intestinal epithelial cells depends on cell differentiation. Infect. Immun. 66:1216-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segerer, S., M. Mack, H. Regele, D. Kerjaschki, and D. Schlondorff. 1999. Expression of the C-C chemokine receptor 5 in human kidney diseases. Kidney Int. 56:52-64. [DOI] [PubMed] [Google Scholar]
- 40.Seifarth, C., A. Funk, K. Reich, I. Dahne, M. Classen, and K. Deusch. 1995. Selective increase of CD4+ and CD25+ T cells but not of gamma delta T cells in H. pylori associated gastritis. Adv. Exp. Med. Biol. 371:1-4. [PubMed] [Google Scholar]
- 41.Shields, P. L., C. M. Morland, M. Salmon, S. Qin, S. G. Hubscher, and D. H. Adams. 1999. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J. Immunol. 163:6236-6243. [PubMed] [Google Scholar]
- 42.Shimizu, T., K. Kusugami, K. Ina, A. Imada, Y. Nishio, T. Hosokawa, M. Ohsuga, M. Shimada, M. Noshiro, H. Kaneko, and T. Ando. 2000. Helicobacter pylori-associated gastric ulcer exhibits enhanced mucosal chemokine activity at the ulcer site. Digestion 62:87-94. [DOI] [PubMed] [Google Scholar]
- 43.Shimoyama, T., S. M. Everett, M. F. Dixon, A. T. Axon, and J. E. Crabtree. 1998. Chemokine mRNA expression in gastric mucosa is associated with Helicobacter pylori cagA positivity and severity of gastritis. J. Clin. Pathol. 51:765-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sommer, F., G. Faller, P. Konturek, T. Kirchner, E. G. Hahn, J. Zeus, M. Rollinghoff, and M. Lohoff. 1998. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect. Immun. 66:5543-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki, N., A. Nakajima, S. Yoshino, K. Matsushima, H. Yagita, and K. Okumura. 1999. Selective accumulation of CCR5+ T lymphocytes into inflamed joints of rheumatoid arthritis. Int. Immunol. 11:553-559. [DOI] [PubMed] [Google Scholar]
- 46.Tamasauskas, D., V. Powell, K. Saksela, and K. Yazdanbakhsh. 2001. A homologous naturally occurring mutation in Duffy and CCR5 leading to reduced receptor expression. Blood 97:3651-3654. [DOI] [PubMed] [Google Scholar]
- 47.Thomas, J. E., A. Dale, M. Harding, W. A. Coward, T. J. Cole, and L. T. Weaver. 1999. Helicobacter pylori colonization in early life. Pediatr. Res. 45:218-223. [DOI] [PubMed] [Google Scholar]
- 48.Trejdosiewicz, L. K., A. Calabrese, C. J. Smart, D. J. Oakes, P. D. Howdle, J. E. Crabtree, M. S. Losowsky, F. Lancaster, and A. W. Boylston. 1991. Gamma delta T cell receptor-positive cells of the human gastrointestinal mucosa: occurrence and V region gene expression in Heliobacter pylori-associated gastritis, coeliac disease and inflammatory bowel disease. Clin. Exp. Immunol. 84:440-444. [PMC free article] [PubMed] [Google Scholar]
- 49.Wedderburn, L. R., N. Robinson, A. Patel, H. Varsani, and P. Woo. 2000. Selective recruitment of polarized T cells expressing CCR5 and CXCR3 to the inflamed joints of children with juvenile idiopathic arthritis. Arthritis Rheum. 43:765-774. [DOI] [PubMed] [Google Scholar]
- 50.Xia, H. H., and N. J. Talley. 1997. Natural acquisition and spontaneous elimination of Helicobacter pylori infection: clinical implications. Am. J. Gastroenterol. 92:1780-1787. [PubMed] [Google Scholar]
- 51.Yamaoka, Y., M. Kita, T. Kodama, N. Sawai, T. Tanahashi, K. Kashima, and J. Imanishi. 1998. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut 42:609-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zingoni, A., H. Soto, J. A. Hedrick, A. Stoppacciaro, C. T. Storlazzi, F. Sinigaglia, D. D'Ambrosio, A. O'Garra, D. Robinson, M. Rocchi, A. Santoni, A. Zlotnik, and M. Napolitano. 1998. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J. Immunol. 161:547-551. [PubMed] [Google Scholar]