Abstract
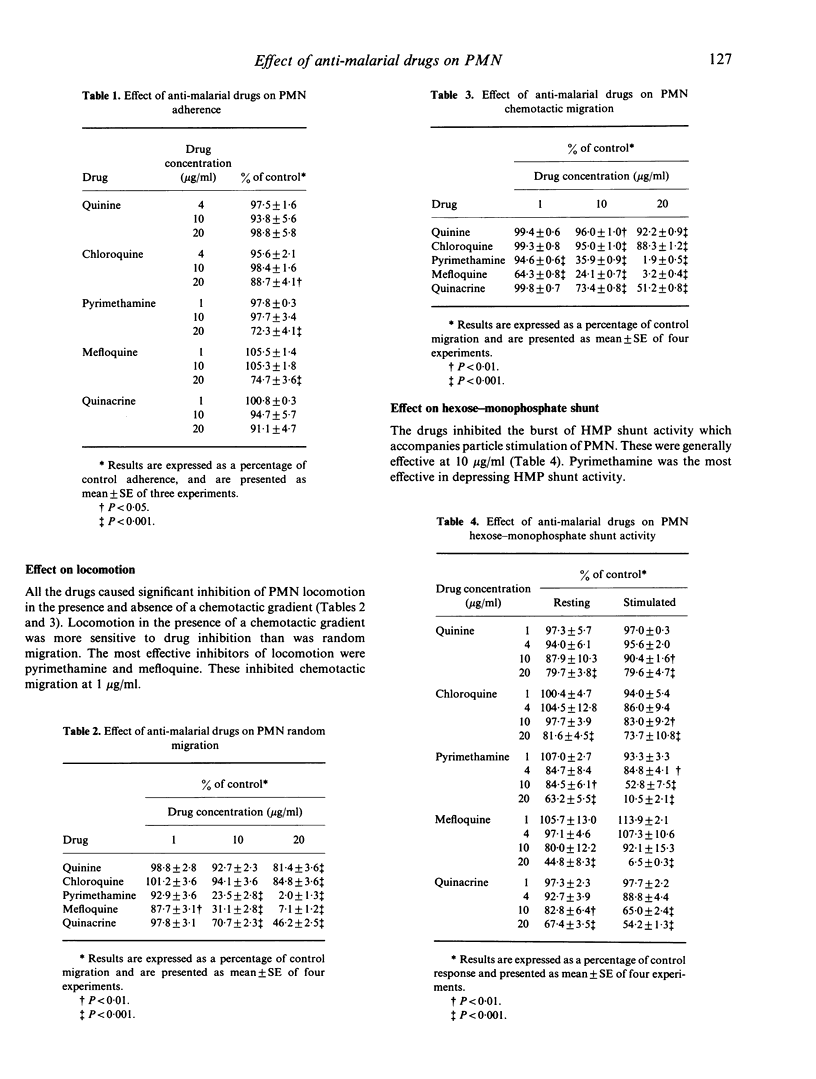
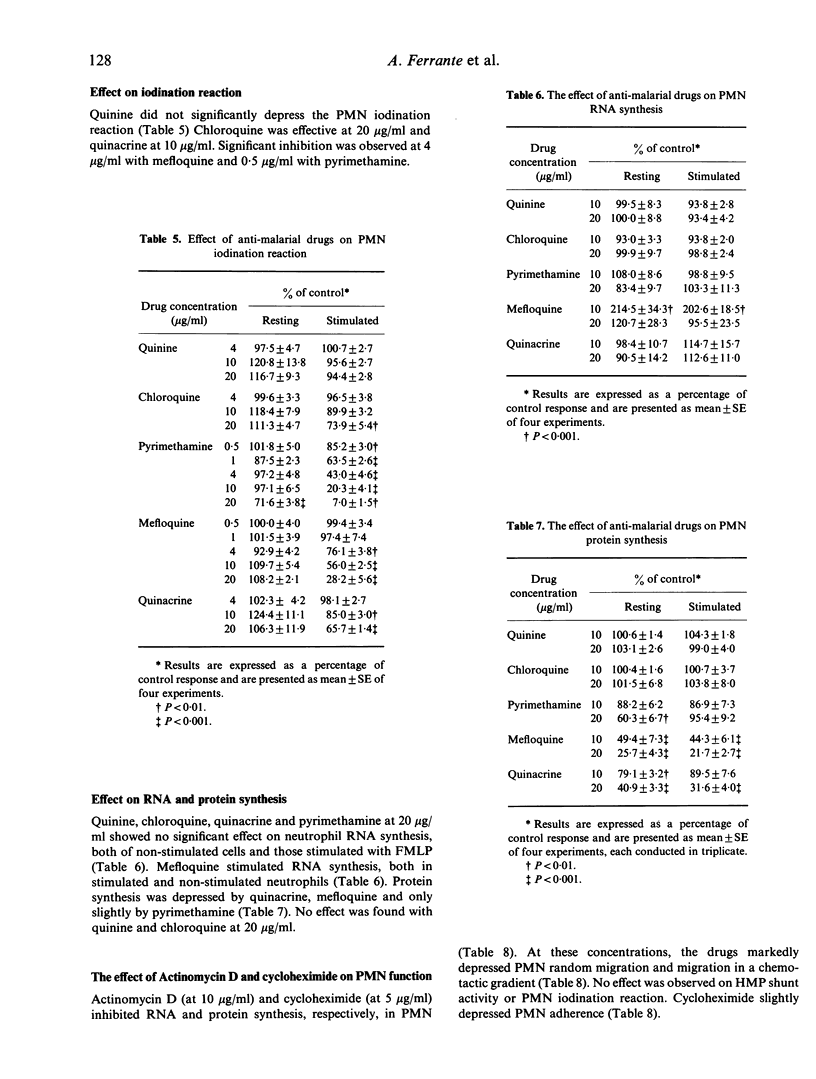
The effect of the anti-malarial drugs quinine, chloroquine, pyrimethamine, mefloquine and quinacrine on human polymorphonuclear leucocyte (PMN) function was examined in vitro. In general, all drugs had their greatest effect on PMN iodination reaction and locomotion, intermediate effects on PMN hexose-monophosphate shunt activity, and least effect on PMN adherence. The most potent of these were pyrimethamine and mefloquine. The PMN iodination reaction and locomotion were inhibited between 0.5-1 microgram/ml (congruent to 2-4 X 10(-6) M) pyrimethamine and 1-4 micrograms/ml (congruent to 0.25-1 X 10(-5) M) mefloquine. The study demonstrates that anti-malarial drugs depress PMN functions associated with antimicrobial activity of the cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. M., Yocum D. E., Bell C. L. Hydroxychloroquine in the treatment of rheumatoid arthritis. Am J Med. 1983 Aug;75(2):321–326. doi: 10.1016/0002-9343(83)91211-1. [DOI] [PubMed] [Google Scholar]
- Brown R. E., Stancato F. A., Wolfe A. D. The effects of mefloquine on Escherichia coli. Life Sci. 1979 Nov 19;25(21):1857–1864. doi: 10.1016/0024-3205(79)90434-x. [DOI] [PubMed] [Google Scholar]
- Buus S., Werdelin O. Chloroquine inhibits accessory cell presentation of soluble natural and synthetic protein antigens. Acta Pathol Microbiol Immunol Scand C. 1984 Oct;92(5):285–291. doi: 10.1111/j.1699-0463.1984.tb00089.x. [DOI] [PubMed] [Google Scholar]
- Chevli R., Fitch C. D. The antimalarial drug mefloquine binds to membrane phospholipids. Antimicrob Agents Chemother. 1982 Apr;21(4):581–586. doi: 10.1128/aac.21.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Beard L. J., Thong Y. H. Early decay of human neutrophil chemotactic responsiveness following isolation from peripheral blood. Clin Exp Immunol. 1980 Feb;39(2):532–537. [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Goh D. H. The effect of anti-malarial drugs on human natural killer cells in vitro. Parasite Immunol. 1984 Nov;6(6):571–580. doi: 10.1111/j.1365-3024.1984.tb00826.x. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Rencis V. O. Enhancement of base hexose-monophosphate shunt activity of human polymorphonuclear leucocytes by human beta-interferon. Immunol Lett. 1984;8(4):215–217. doi: 10.1016/0165-2478(84)90081-6. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Rowan-Kelly B., Thong Y. H. Suppression of immunological responses in mice by treatment with amphotericin B. Clin Exp Immunol. 1979 Oct;38(1):70–76. [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Separation of mononuclear and polymorphonuclear leucocytes from human blood by the one-step Hypaque-Ficoll method is dependent on blood column height. J Immunol Methods. 1982;48(1):81–85. doi: 10.1016/0022-1759(82)90212-5. [DOI] [PubMed] [Google Scholar]
- Grindel J. M., Tilton P. F., Shaffer R. D. Quantitation of the antimalarial agent, mefloquine, in blood, plasma, and urine using high-pressure liquid chromatography. J Pharm Sci. 1977 Jun;66(6):834–837. doi: 10.1002/jps.2600660625. [DOI] [PubMed] [Google Scholar]
- Hanson W. L. Chemotherapy and the immune response in protozoal infections. J Protozool. 1981 Feb;28(1):27–30. doi: 10.1111/j.1550-7408.1981.tb02799.x. [DOI] [PubMed] [Google Scholar]
- Isaacson D., Elgart M., Turner M. L. Anti-malarials in dermatology. Int J Dermatol. 1982 Sep;21(7):379–395. doi: 10.1111/j.1365-4362.1982.tb03155.x. [DOI] [PubMed] [Google Scholar]
- Kharazmi A., Valerius N. H., Høiby N. Effect of antimalarial drugs on human neutrophil chemotaxis in vitro. Acta Pathol Microbiol Immunol Scand C. 1983 Aug;91(4):293–298. [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Pincus S. H., Klebanoff S. J. Quantitative leukocyte iodination. N Engl J Med. 1971 Apr 8;284(14):744–750. doi: 10.1056/NEJM197104082841402. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Currell J. M. Development of a microassay technique for neutrophil adherence. J Immunol Methods. 1983 Oct 14;63(2):229–236. doi: 10.1016/0022-1759(83)90427-1. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Ferrante A. Inhibition of mitogen-induced lymphocyte proliferative responses by quinine. Am J Trop Med Hyg. 1978 Mar;27(2 Pt 1):354–356. doi: 10.4269/ajtmh.1978.27.354. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Ferrante A., Rowan-Kelly B., O'Keefe D. E. Effect of mefloquine on the immune response in mice. Trans R Soc Trop Med Hyg. 1979;73(4):388–390. doi: 10.1016/0035-9203(79)90160-3. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Ferrante A., Rowan-Kelly B. Primaquine inhibits mitogen-induced human lymphocyte proliferative responses. Trans R Soc Trop Med Hyg. 1978;72(5):537–539. doi: 10.1016/0035-9203(78)90181-5. [DOI] [PubMed] [Google Scholar]


