Abstract
The competitive enzyme-linked immunosorbent assay (cELISA) format has proven to be an accurate, reliable, easily standardized, and high-throughput method for detecting hemoparasite infections. In the present study, a species-specific, broadly conserved, and tandemly repeated B-cell epitope within the C terminus of the rhoptry-associated protein 1 of the hemoparasite Babesia bovis was cloned and expressed as a histidine-tagged thioredoxin fusion peptide and used as antigen in a cELISA. The assay was optimized with defined negative and positive bovine sera, where positive sera inhibited the binding of the epitope-specific monoclonal antibody BABB75A4. The cELISA accurately differentiated animals with B. bovis-specific antibodies from uninfected animals and from animals with antibodies against other tick-borne hemoparasites (98.7% specificity). In addition, B. bovis-specific sera from Australia, Argentina, Bolivia, Puerto Rico, and Morocco inhibited the binding of BABB75A4, confirming conservation of the epitope. The assay first detected experimentally infected animals between 13 and 17 days postinfection, and with sera from naturally infected carrier cattle, was comparable to indirect immunofluorescence (98.3% concordance). The assay appears to have the characteristics necessary for an epidemiologic and disease surveillance tool.
Bovine babesiosis is a tick-borne, hemoprotozoan disease responsible for substantial morbidity and mortality in cattle throughout the world (16). Definitive diagnosis can be made by microscopic detection of parasites within stained blood films. However, parasitemias are often too low in subclinical and chronic infections for microscopic diagnosis. For this reason, several serologic assays, including complement fixation (14), indirect hemagglutination (7), rapid card agglutination (23), latex bead agglutination (12), and indirect immunofluorescence (IIF) (4) have been used to detect antibody in infected cattle. Although these assays allow for the detection of persistently infected cattle, they have limitations in specificity and/or sensitivity. The IIF assay has been the most sensitive assay, but cross-reactivity among babesial species, subjective interpretation, and low throughput have limited its usefulness.
Enzyme-linked immunosorbent assays (ELISAs) have found wide application in infectious disease diagnosis. The assay is nonsubjective, can be automated for high throughput, and has the potential for improved specificity depending on the efforts made in antigen preparation and characterization. A number of ELISAs have been designed for the diagnosis of Babesia bovis infection (1, 2, 13, 17, 26). Although these studies report an improvement in sensitivity over other assays, problems with specificity remain due to the fact that antigen preparations are at best only partially purified.
The cELISA format can provide an additional level of specificity since antibody against a single epitope known to be organism specific is detected. Several cELISA assays have been developed for diagnosis of hemoparasites, including B. equi and B. caballi in horses (9, 10) and Anaplasma in cattle (24). The cELISA for B. caballi relies on inhibition of a monoclonal antibody (MAb) against a repetitive epitope of rhoptry-associated protein 1 (RAP-1). Since the B. bovis RAP-1 homologue is structurally similar to B. caballi RAP-1 and contains a similar region of C-terminal tandem repeats encoding a B-cell epitope (20), we reasoned that a cELISA-based on this epitope would prove equally effective in detecting B. bovis-infected cattle.
A 1.9-kb sequence encodes the entire B. bovis RAP-1 protein and a recombinant product is a faithful antigenic replica of the native protein. Seven tandem 23-amino-acid degenerate repeats comprise the C-terminal third of the protein and, together with the secondary structure, contribute to its antigenicity and immunogenicity (20). The species-specific continuous B-cell epitope encoded by at least two of these repeats is recognized by MAb BABB75A4 and is conserved among geographic isolates (19, 21, 22). It is the basis for the development of a cELISA described here, where we define: (i) the conditions for optimal differentiation of defined positive and negative sera, (ii) the specificity of the cELISA, (iii) the kinetics of seroconversion, and (iv) the concordance of the cELISA with IIF.
MATERIALS AND METHODS
Recombinant antigen constructs.
Recombinant antigens spanning RAP-1, including (i) full-length rRAP-1, (ii) the N terminus (amino acids 1 to 316; rRNT), and (iii) the C terminus (amino acids 317 to 565; rRCT) (18), were prepared from a Mexican isolate (5). Recombinant antigens were expressed as histidine-tagged thioredoxin fusion proteins (HTP). Each RAP-1 insert was amplified by reverse transcription-PCR with specific primer sets and ligated into pBAD/Thio-TOPO vector (Invitrogen, Carlsbad, Calif.) as recommended by the manufacturer. The primers included RAP-1 (forward, 5′-ATGAGAATCATTAGCGGCGTTG-3′; reverse, 5′-GAGGTATCCGGCGGTGTCTTCAC-3′), RNT (forward, 5′-TGAGAATCATTAGCGGCGTTG-3′; reverse, 5′-TTGGGTAACGTTTTTAGAG-3′), and RCT (forward, 5′-CCTACAAAGAAGTTCATCGAGG-3′; reverse, 5′-GAGGTATCCGGCGGTGTCTTCAC-3′). The insert orientation and frame of each clone was confirmed by sequencing. The expression and purification of each recombinant antigen was accomplished as recently described (18). Briefly, single recombinant Escherichia coli colonies were inoculated into Luria broth containing 50 μg of ampicillin per ml and incubated overnight at 37°C with shaking. Protein expression was induced with 0.2% l-(+)-arabinose for 4 h. E. coli were pelleted, lysed, and sonicated, followed by purification of the soluble protein on a ProBond resin column (Invitrogen). Protein was eluted with PNB (50 mM K2HPO4, 400 mM NaCl, 100 mM KCl, 10% glycerol) containing a series of imidazole solutions (50, 100, 200, and 500 mM and 1 M imidazole), and eluates were collected into separate tubes. For sodium dodecyl sulfate (SDS)-treated rRCT, the purified protein was stored in PNB containing 1% (wt/vol) SDS. The yield and expected size of the products were determined by SDS-polyacrylamide gel electrophoresis (Coomasie blue-stained dilutions of antigen compared to a diluted protein standard).
MAbs and antigen assessment by Western blotting.
Equivalent amounts of rRAP-1, rRCT, and rRNT were electrophoresed according to the method of Laemmli (11) by using 4 to 20% continuous gradient resolving gels. Proteins were transferred to nitrocellulose (Bio-Rad, Richmond, Calif.) (25) at 250 mA for 45 min and probed with the MAbs BABB75A4 and 23.53.156, previously described as specific for rRCT and rRNT, respectively (21). Bound MAb was detected with peroxidase-labeled affinity-purified goat anti-mouse immunoglobulin G (IgG; H+L; KPL, Gaithersburg, Md.) and ECL-Plus (Amersham Pharmacia Biotech, Piscataway, N.J.).
Bovine sera.
Bovine serum samples were from four sources: (i) Washington Animal Disease Diagnostic Laboratory (227 randomly selected samples); (ii) experimentally infected animals maintained at the USDA-ARS-ADRU research facility (32 preinoculation sera and 103 postinoculation and confirmed infected sera); (iii) United Nations Food and Agriculture Organization reference diagnostic serum from Argentina (1 sample); and (iv) cattle representing field samples from Bolivia (20 samples), Puerto Rico (38 samples), and Morocco (72 samples). Some samples were irradiated prior to use, and all were stored at −20°C until used. Samples from experimental animals were collected prior to and after inoculation with blood stabilates of B. bovis isolates from Mexico (19 animals and 76 sera), Texas (13 animals and 27 sera), or Australia (1 animal and 1 serum). All experimental animals were maintained according to the American Association for Laboratory Animal Care procedures and were provided an acceptable bovine ration, with water and mineral block provided ad libitum.
IIF.
Blood stabilates of the Texas isolate (T2Bo) of B. bovis (6) were used for antigen. Vials of stabilate with 50% packed erythrocytes (5% infected erythrocytes) in phosphate-buffered saline (PBS) containing 4 M dimethyl sulfoxide were thawed, washed in PBS three times, and adjusted to 20% packed cells in PBS containing 1% bovine serum albumin. Uniform thin films were made on microscope slides and air dried. Slides were fixed in cold acetone, and wells were formed on each slide by using fingernail polish. Each slide included three wells of control sera: a strong positive serum diluted 1/100, a negative serum diluted 1/100, and a weak positive serum (the strong positive serum diluted 1/6,400). Then, 10 μl of a 1/100 dilution of each test serum was applied to appropriate wells, followed by incubation in a humidity chamber at 37°C for 30 min. Slides were washed three times for 10 min each in cold PBS. Next, 10 μl of a 1/80 dilution of fluoresceinated goat anti-bovine IgG (H+L; KPL) was applied to each well, and the slides were incubated and washed as described above. Reactions were observed by using epifluorescence microscopy. A positive test serum was defined as a reaction equal to or greater than the weak positive control reaction.
cELISA conditions.
rRAP-1, rRCT, or SDS-treated rRCT antigen was added to wells of Immulon II (Dynatech, Chantilly, Va.) plates in PBS containing 20 mM MgCl2 and incubated overnight. Antigen-coated plates were blocked with PBS plus 0.2% Tween 20 containing 20% nonfat dry milk for 1 h at room temperature on a rotator, followed by 100 μl of test sera in duplicate wells for 15 min. The sera were discarded, and 100 μl of diluted BABB-75A4 was added to each well, followed by incubation for 15 min as described above. Bound BABB-75A4 was detected by the addition of avidin-alkaline phosphatase complex (VectaStain ABC-AP; Vector Laboratories) and p-nitrophenyl phosphate substrate. Each plate contained a panel of five known negative samples (experimental calves confirmed negative by IIF and PCR [3]) that were used to compute a mean optical density (OD) at 414 nm for the plate. The percent inhibition of the mean of test sample wells was computed as follows: 100 − [(the OD of the test sample/the mean OD of the normal control serum panel) × 100].
RESULTS
MAb recognition of RAP-1 epitope in the C terminus.
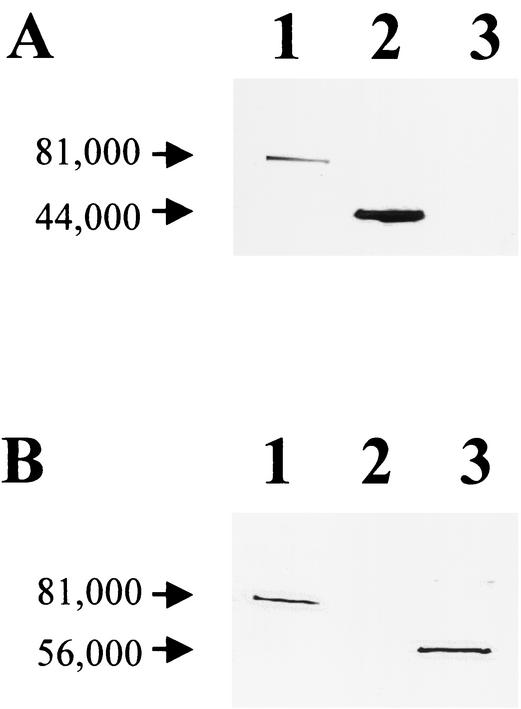
Full-length rRAP-1, rRCT, and rRNT constructs were purified, and products of the predicted size for each fusion protein were identified. The products were subjected to Western blotting with the epitope-specific MAbs BABB75A4 and 23.53.156. Earlier studies located the epitope recognized by BABB75A4 in the C terminus and the epitope recognized by 23.53.156 in the N terminus of RAP-1 (21). This was confirmed, as demonstrated in Fig. 1. BABB75A4 recognized rRAP-1 and rRCT but not rRNT (Fig. 1A), whereas 23.53.156 recognized only rRAP-1 and rRNT (Fig. 1B).
FIG. 1.
Western blot confirming the presence of the BABB75A4 epitope within the C-terminal RAP-1 recombinant construct rRCT. (A) MAb BABB75A4 was used as the probe. (B) MAb 23.53.156 was used as the probe. Lanes: 1, full-length rRAP-1 fusion protein; 2, rRCT fusion protein; 3, rRNT fusion protein.
Optimal conditions for cELISA.
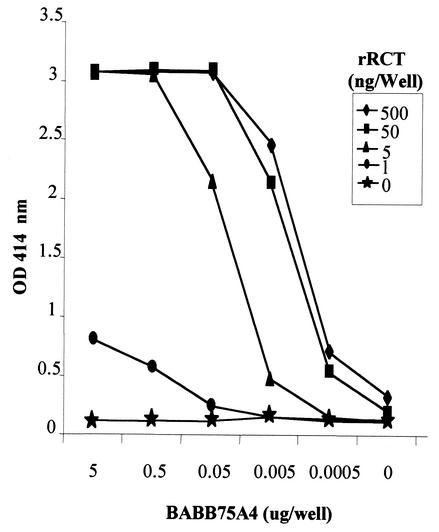
Appropriate concentrations of rRCT and BABB75A4 for the cELISA were determined by an ELISA block titration (Fig. 2.). An OD of between 2.0 and 2.5 was selected as the optimal reactivity from which inhibition could be determined. BABB75A4 at 50 ng/well with 5 ng of rRCT/well consistently produced an OD at the target level and at a point on the titration curve (linear range) allowing optimal inhibition, and so these concentrations were used in the cELISA thereafter.
FIG. 2.
ELISA block titration with the indicated concentrations of rRCT and MAb BABB75A4.
In order to facilitate the storage and shipment of plates and increase the utility of the assay, rRCT was dried onto plates and compared to fresh rRCT. Thirty different sera representing both known positive and negative cattle were used to evaluate the two preparations. There was no significant difference in OD between the two antigen preparations (data not shown), and dried antigen plates were used throughout the remainder of the study.
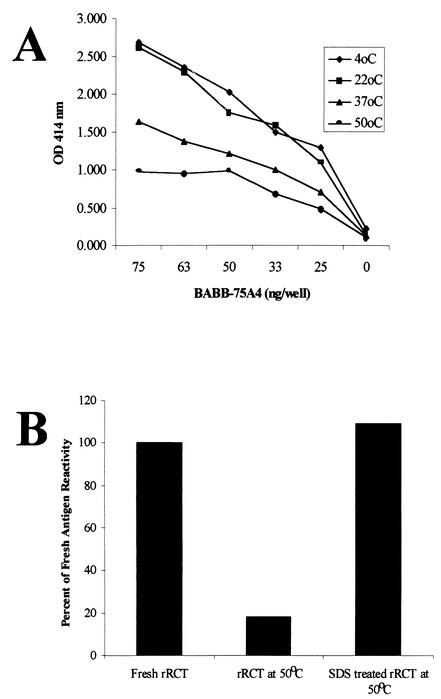
The rRCT antigen was temperature sensitive even when dried onto plates. Plates were sealed with acetate covers and stored at four different temperatures for 48 h. The effect of temperature on the binding of BABB75A4 is shown in Fig. 3. Although there was no difference between plates stored at 4°C and room temperature over this period of time, there was a pronounced decrease in MAb binding with increased temperature (Fig. 3A). However, the addition of 1% SDS to fresh rRCT overcame this heat degradation and stabilized the dried antigen for MAb binding at 50°C for at least 1 month (Fig. 3B).
FIG. 3.
Effects of temperature and SDS treatment on the stability of rRCT. (A) Purified rRCT dried onto plates and stored for 48 h at the indicated temperatures before reaction with MAb BABB75A4. (B) Purified rRCT dried onto plates and used in a competitive format with MAb BABB75A4 and a known positive control bovine serum immediately (Fresh Antigen), stored at 50°C for 1 month without SDS treatment, and stored at 50°C for 1 month after SDS treatment.
Inhibition levels.
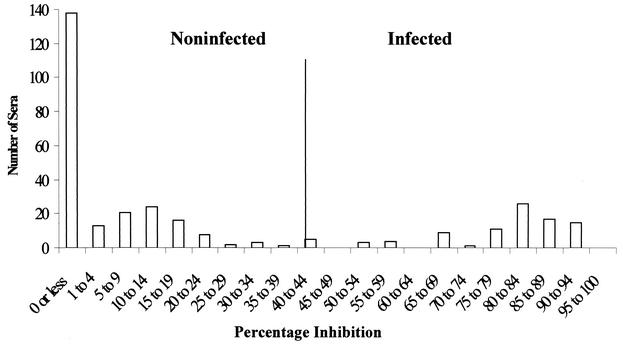
Initially, 30 sera from uninfected calves that were subsequently used for experimental infections in this and other studies were assessed with the cELISA to determine a percent inhibition that would differentiate positive and negative reactions. The mean percent inhibition plus three standard deviations (41.4%) was chosen as the level above which samples were considered positive on this initial assessment. To refine this level, 318 sera representing 227 sera from disease-free areas of the northern United States acquired from the Washington Animal Disease Diagnostic Laboratory (defined negative samples) and 91 additional known positive samples from experimental infections were assessed. Figure 4 shows the frequency distribution of the percent inhibition for these samples. An inhibition of 40% represented the point between the lowest percent inhibition from the positive group and the highest percent inhibition from the negative group and was used as the threshold level above which samples would be considered positive thereafter. Of 227 negative samples, 86.3% (196 of 227) inhibited BABB75A4 binding <15%, whereas 75.8% (69 of 91) of the positive samples inhibited BABB75A4 binding by >75%.
FIG. 4.
Frequency distribution of percent inhibition of MAb BABB75A4 binding in the cELISA by samples from known B. bovis-infected and uninfected animals.
Specificity.
An additional 12 sera representing both pre- and post-experimentally infected animals were added, for a total of 330 sera. Using the 40% inhibition threshold, the cELISA correctly identified 99% of the 330 samples (Table 1) . Three samples were falsely positive (all three were negative when evaluated with IIF and resulted in decreased inhibition levels when reevaluated by cELISA after adsorption with an irrelevant purified HTP). Therefore, under these conditions, the specificity was 98.7% (95% confidence interval of 98.75 to 100%).
TABLE 1.
cELISA comparison of B. bovis-infected and uninfected cattle sera
| RCT cELISAb result | No. of B. bovis samplesa
|
Total samples | |
|---|---|---|---|
| Infected | Uninfected | ||
| Positive | 103 | 3 | 106 |
| Negative | 0 | 224 | 224 |
| Total | 103 | 227 | 330 |
Samples were from infected animals confirmed microscopically or by nested PCR and from uninfected animals originating and maintained in a Babesia-free region of the northern United States.
Threshold inhibition of 40%.
Serum samples from cattle after experimental infection with B. bigemina (5 sera), Theileria orientalis (1 serum), Anaplasma marginale (4 sera), and B. bovis from Argentina (1 serum) and Australia (1 serum), along with field samples from Puerto Rico (38 sera), Bolivia (20 sera), and Morocco (72 sera) (all areas where B. bovis is endemic), were assayed by both cELISA and IIF. The cELISA detected antibody in the sera from animals infected with isolates of B. bovis from Argentina and Australia, whereas none of the samples from animals infected exclusively with other hemoparasite species reacted in either the cELISA or in the IIF analysis. Field sera reacted at a range of inhibition levels and, when the tests were repeated, similar levels of inhibition were recorded for each sample. The cELISA clearly identified positive animals from diverse geographic regions, and the mean percent inhibition of the positive samples varied from 56% in Morocco to 74% in Bolivia. The cELISA and IIF were compared by using the field sera from Puerto Rico, Bolivia, and Morocco. The cELISA and the IIF analysis were in 98.5% agreement, with only two discrepant samples (Kappa value = 0.97) (Table 2).
TABLE 2.
Concordance of the B. bovis cELISA with the B. bovis IIF analysis
| cELISAb result | IIF analysis
|
Total samplesa | |
|---|---|---|---|
| No. positive | No. negative | ||
| Positive | 44 | 1 | 45 |
| Negative | 1 | 84 | 85 |
| Total | 45 | 85 | 130 |
Field samples were obtained from Argentina, Bolivia, Morocco, and Puerto Rico.
Threshold inhibition of 40%.
Kinetics.
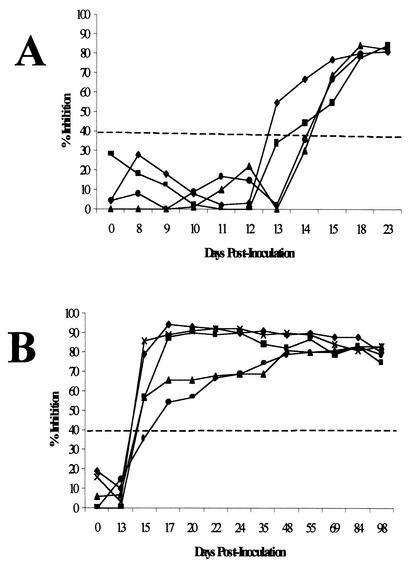
The kinetics of antibody induction against the RAP-1 repeat epitope was assessed in two groups of cattle experimentally infected with the T2Bo isolate. One group was sampled daily from the time of inoculation through 23 days postinoculation (p.i.) (Fig. 5A), while the second group was sampled once prior to inoculation and then every other day beginning at day 13 p.i. and continuing through 98 days p.i. (Fig. 5B). RCT-specific antibodies were detected in sera from experimentally inoculated animals at days 13 (one animal), 14 (one animal), 15 (six animals), and 17 (one animal) p.i. (mean of 14.89 ± 1.05 days p.i.). Inhibition levels of between 60 and 90% were maintained for at least 98 days p.i. without challenge exposure. Each serum sample was also assessed with IIF and, in general, IIF detected specific antibody 1 to 2 days earlier than did the cELISA.
FIG. 5.
Kinetics of antibody detection by cELISA. (A) Samples obtained from a group of four cattle before and daily through 23 days after experimental intravenous inoculation of the T2Bo isolate of B. bovis. (B) Samples obtained from a group of five cattle before and every other day beginning on day 13 and continuing through day 98 after experimental intravenous inoculation of the T2Bo isolate of B. bovis. The horizontal dashed line indicates the threshold inhibition of 40%, above which samples are considered positive.
DISCUSSION
Bovine babesiosis remains an important disease impacting herd health management throughout much of the world. Animals that survive the acute initial infection become persistently infected; long-term carriers can serve as reservoirs for infection in the herd. One goal in developing improved diagnostics is to be able to identify these carrier animals. Another goal is differential diagnosis, a concern in countries where several hemoparasitic agents, particularly different babesial species, occur concomitantly. Since differences in pathogenicity, epidemiology, and treatment exist for each species, accurate diagnosis is important.
The B. bovis-specific rRAP-1 cELISA represents an improvement in diagnostic capability for this important agent of bovine babesiosis in several ways. First, the cELISA format employing MAb-based detection provides exceptional specificity. RAP-1 from the equine pathogen, B. caballi, is remarkably similar to that from B. bovis (with regard to size, structure, and repetitive epitopes in the C terminus). A MAb directed toward an epitope in the B. caballi RAP-1 C-terminal repeat region has also been used in a cELISA (9). Based on the success of the B. caballi cELISA and the similarities of B. caballi and B. bovis RAP-1 homologues, we hypothesized that a B. bovis RAP-1-associated rRCT cELISA would be effective. Previous work had identified a MAb that bound to an epitope in the C terminus of B. bovis RAP-1 (20). All RAP-1 repeat sequences are species specific, despite being remarkably conserved among isolates and strains (9, 19, 20, 21, 22). Conservation of the epitope allowed detection of specific antibody in cattle infected with geographically distinct isolates of B. bovis.
Second, it is relatively inexpensive and reproducible, lending itself to standardization. Although the stability of the antigen was an initial problem, SDS denaturation of the purified rRCT rendered the antigen stable over time and temperatures and did not affect antibody binding to the non-conformationally dependent epitope. As long as the SDS concentration is kept below its critical micelle concentration, it has been shown to improve ELISA responses in other systems (15). Bacterial lysate containing rRAP-1 from B. caballi is similarly heat sensitive but also is stabilized in the presence of SDS (8). Thus, low concentrations of SDS provide adequate stability of these B. caballi and B. bovis RAP-1-associated antigen epitopes in either bacterial lysates or as a purified preparation, and either as fresh or dried antigen, allowing for deployment in laboratories with various ambient temperatures.
Third, the cELISA detected specific antibody for at least 3 months without evidence of a loss of titer even though the animals were never reexposed. Kinetic differences between IIF and cELISA, with IIF detecting specific antibody about 2 days before cELISA, are probably a result of the ability of IIF to detect antibody binding to multiple epitopes, some of which may be earlier targets of the immune response. Despite this, it appears that the sensitivity of the cELISA is similar to that of IIF, with 98.5% concordance with field-collected samples. Additionally, a difference of 2 days in detection of acutely infected cattle is probably of little clinical or practical significance.
Finally, the cELISA is a high-throughput format. This is an advantage over that of IIF and other non-ELISA-based diagnostic assays.
Additional validation studies with field sera from areas where B. bovis is endemic paired with whole blood for PCR analysis are under way to rigorously determine the sensitivity and predictive value of the assay and to verify its specificity in these areas. When complete, the B. bovis RAP-1 cELISA should lend itself to application in both epidemiologic and control programs in regions where this infection is endemic and to surveillance programs in regions, such as the United States, where B. bovis infection is not endemic.
Acknowledgments
This work was supported by USDA-ARS-CWU-5348-32000-010-00D and the National Institutes of Health grant R01-AI30136.
We thank Pete Steiner and Emma Karel for excellent animal care and Paul Lacy and Bev Hunter for technical assistance. We are indebted to Hamid Sahibi, Abdelkebir Rhalem, and Saadia Lasri of the Institut Agronomique et Veterinaire, Hassan II, Rabat, Morocco, for the Moroccan samples; Sharon L. Deem of the Wildlife Conservation Society, New York, N.Y., for the Bolivian samples; and Ignacio Pino (local practitioner) and David Jimenez of the Federacion de Asociaciones Pecuarias de Puerto Rico, Inc., Mayaguez, Puerto Rico, for the Puerto Rican samples. We thank also John VanderSchalie of the Washington Animal Disease Diagnostic Laboratory for providing serum samples from cattle in a region where B. bovis is not endemic.
REFERENCES
- 1.Araujo, F. R., C. R. Madruga, C. R. Leal, M. A. Schenk, R. H. Kessler, A. P. Marques, and D. C. Lemaire. 1998. Comparison between enzyme-linked immunosorbent assay, indirect fluorescent antibody and rapid conglutination test in detecting antibodies against Babesia bovis. Vet. Parasitol. 74:101-108. [DOI] [PubMed] [Google Scholar]
- 2.Barry, D. N., B. J. Rodwell, P. Timms, and W. McGregor. 1982. A microplate enzyme immunoassay for detecting and measuring antibodies to Babesia bovis in cattle serum. Aust. Vet. J. 59:136-140. [DOI] [PubMed] [Google Scholar]
- 3.Figueroa, J. V., L. P. Chieves, G. S. Johnson, W. L. Goff, and G. M. Buening. 1994. Polymerase chain reaction-based diagnostic assay to detect cattle chronically infected with Babesia bovis. Rev. Lat. Am. Microbiol. 36:47-55. [PubMed] [Google Scholar]
- 4.Goff, W. L., G. G. Wagner, T. M. Craig, and R. F. Long. 1982. The bovine immune response to tick-derived Babesia bovis infection: serological studies of isolated immunoglobulins. Vet. Parasitol. 11:109-120. [DOI] [PubMed] [Google Scholar]
- 5.Goff, W. L., W. C. Davis, G. H. Palmer, T. F. McElwain, W. C. Johnson, J. F. Bailey, and T. C. McGuire. 1988. Identification of Babesia bovis merozoite surface antigens by using immune bovine sera and monoclonal antibodies. Infect. Immun. 56:2363-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goff, W. L., W. C. Johnson, and C. W. Cluff. 1998. Babesia bovis immunity: in vitro and in vivo evidence for IL-10 regulation of IFN-γ and iNOS. Ann. N. Y. Acad. Sci. 849:161-180. [DOI] [PubMed] [Google Scholar]
- 7.Goodger, B. V. 1971. Preparation and preliminary assessment of purified antigens in the passive haemagglutination test for bovine babesiosis. Aust. Vet. J. 47:251-256. [DOI] [PubMed] [Google Scholar]
- 8.Johnson, W. C., S. Lasri, L. S. Kappmeyer, A. Rhalem, H. Sahibi, D. P. Knowles, and W. L. Goff. 2001. Factors influencing the deployment of a Babesia caballi cELISA in tropical areas, p. 322-323. In Proceedings of the 10th International Symposium of Veterinary Laboratory Diagnosticians. Veterinary Laboratory Diagnosticians, Parma, Italy.
- 9.Kappmeyer, L. S., L. E. Perryman, S. A. Hines, T. V. Baszler, J. B. Katz, S. G. Hennager, and D. P. Knowles. 1999. Detection of equine antibodies to Babesia caballi by recombinant B. caballi rhoptry-associated protein-1 in a competitive-inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37:2285-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles, D. P., L. E. Perryman, L. S. Kappmeyer, and S. G. Hennager. 1991. Detection of equine antibody to Babesia equi merozoite proteins by a monoclonal antibody-based competitive inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 29:2056-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 12.Lopez, V. G., and R. A. Todorovic. 1978. Rapid latex agglutination (RLA) test for the diagnosis of Babesia argentina. Vet. Parasitol. 4:1-9. [Google Scholar]
- 13.Machado, R. Z., H. J. Montassier, A. A. Pinto, E. G. Lemos, M. R. Machado, I. F. Valadao, L. G. Barci, and E. B. Malheiros. 1997. An enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against Babesia bovis in cattle. Vet. Parasitol. 71:17-26. [DOI] [PubMed] [Google Scholar]
- 14.Mahoney, D. F. 1962. Bovine babesiosis: diagnosis of infection by a complement-fixation test. Aust. Vet. J. 38:48-52. [Google Scholar]
- 15.McCabe, J. P., S. M. Fletcher, and M. N. Jones. 1988. The effects of detergent on the enzyme-linked immunosorbent assay (ELISA) of blood group substances. J. Immunol. Methods 108:129-135. [DOI] [PubMed] [Google Scholar]
- 16.McCosker, P. J. 1981. The global importance of babesiosis, p. 1-24. In M. Ristic and J. P. Kreier (ed.), Babesiosis. Academic Press, New York, N.Y.
- 17.Molloy, J. B., P. M. Bowles, R. E. Bock, J. A. Turton, T. C. Katsande, J. M. Katende, L. G. Mabikacheche, S. J. Waldron, G. W. Blight, and R. J. Dalgliesh. 1998. Evaluation of an ELISA for detection of antibodies to Babesia bovis in cattle in Australia and Zimbabwe. Prev. Vet. Med. 33:59-67. [DOI] [PubMed] [Google Scholar]
- 18.Norimine, J., C. E. Suarez, T. F. McElwain, M. Florin-Christensen, and W. C. Brown. 2002. Immunodominant epitopes in Babesia bovis rhoptry-associated protein-1 that elicit memory CD4+-T-lymphocyte responses in B. bovis-immune individuals are located in the amino-terminal domain. Infect. Immun. 70:2039-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer, G. H., T. F. McElwain, L. E. Perryman, W. C. Davis, D. R. Reduker, D. P. Jasmer, V. Shkap, E. Pipano, W. L. Goff, and T. C. McGuire. 1991. Strain variation of Babesia bovis merozoite surface-exposed epitopes. Infect. Immun. 59:3340-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suarez, C. E., G. H. Palmer, D. P. Jasmer, S. A. Hines, L. E. Perryman, and T. F. McElwain. 1991. Characterization of the gene encoding a 60-kilodalton Babesia bovis merozoite protein with conserved and surface exposed epitopes. Mol. Biochem. Parasitol. 46:45-52. [DOI] [PubMed] [Google Scholar]
- 21.Suarez, C. E., G. H. Palmer, S. A. Hines, and T. F. McElwain. 1993. Immunogenic B-cell epitopes of Babesia bovis rhoptry-associated protein 1 are distinct from sequences conserved between species. Infect. Immun. 61:3511-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suarez, C. E., T. F. McElwain, I. Echaide, S. Torioni de Echaide, and G. H. Palmer. 1994. Interstrain conservation of babesial RAP-1 surface-exposed B-cell epitopes despite rap-1 genomic polymorphism. Infect. Immun. 62:3576-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Todorovic, R. A., and K. L. Kuttler. 1974. A babesiosis card agglutination test. Am. J. Vet. Res. 35:1347-1350. [PubMed] [Google Scholar]
- 24.Torioni de Echaide, S., D. P. Knowles, T. C. McGuire, G. H. Palmer, C. E. Suarez, and T. F. McElwain. 1998. Detection of cattle naturally infected with Anaplasma marginale in a region of endemicity by nested PCR and a competitive enzyme-linked immunosorbent assay using recombinant major surface protein 5. J. Clin. Microbiol. 36:777-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waltisbuhl, D. J., B. V. Goodger, I. G. Wright, M. A. Commins, and D. F. Mahoney. 1987. An enzyme-linked immunosorbent assay to diagnose Babesia bovis infection in cattle. Parasitol. Res. 73:126-131. [DOI] [PubMed] [Google Scholar]