Abstract
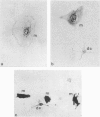
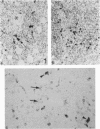
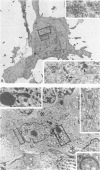
Cells with dendritic shape, the so-called dendritic cells (DCs), have been described in many tissues. In order to characterize one DCs population, normal human thymus specimens were obtained from children undergoing cardiovascular surgery. These specimens were either put in culture or fixed for in situ ultrastructural, immunocytochemical and cytochemical studies. In culture, DCs could be differentiated from other non-lymphoid cell populations. They presented long, fine processes and an irregular nucleus. Like interdigitating cells (IDCs) in situ, their cytoplasm contained many free ribosomes and mitochondria, and a well-developed endoplasmic reticulum and Golgi complex. They showed a variable number of tubulovesicular structures and membrane-bound dark homogeneous granules. They never displayed phagolysosomes, tonofilaments or desmosomes. They were Ia+, ATPase+, S-100 protein+, vimentin+, esterase-, lysozyme-, and cytokeratin- cells. Macrophages were easily identified by their numerous lysosomes and large phagolysosomes. They were esterase+, lysozyme+, vimentin+, ATPase +/-, S-100 protein- and cytokeratin-. Although they were Ia+, membrane labelling was not as important as on DC's membrane. In situ, S-100 protein-positive cells had a dendritic shape and were located mainly in medullary regions and at the cortico-medullary border. The staining was diffused both in the nucleus and in the cytoplasm. Lysozyme-positive cells were randomly distributed in the cortex, the medulla and the connective septa. They were round cells and the staining was intracytoplasmic. These observations demonstrate that DCs can be isolated in human thymic cultures, and they suggest that these cells correspond to IDCs in situ. They also provide evidence to suggest that DCs and macrophages are two distinct cellular populations.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bearman R. M., Levine G. D., Bensch K. G. The ultrastructure of the normal human thymus: a study of 36 cases. Anat Rec. 1978 Mar;190(3):755–781. doi: 10.1002/ar.1091900310. [DOI] [PubMed] [Google Scholar]
- Beller D. I., Unanue E. R. IA antigens and antigen-presenting function of thymic macrophages. J Immunol. 1980 Mar;124(3):1433–1440. [PubMed] [Google Scholar]
- Beller D. I., Unanue E. R. Thymic macrophages modulate one stage of T cell differentiation in vitro. J Immunol. 1978 Nov;121(5):1861–1864. [PubMed] [Google Scholar]
- Bolton W. K., Mesnard R. M. New technique of kidney tissue processing for immunofluorescence microscopy: formol sucrose/gum sucrose/paraffin. Lab Invest. 1982 Aug;47(2):206–213. [PubMed] [Google Scholar]
- Cowing C., Schwartz B. D., Dickler H. B. Macrophage Ia antigens. I. macrophage populations differ in their expression of Ia antigens. J Immunol. 1978 Feb;120(2):378–384. [PubMed] [Google Scholar]
- Duijvestijn A. M., Hoefsmit E. C. Ultrastructure of the rat thymus: the micro-environment of T-lymphocyte maturation. Cell Tissue Res. 1981;218(2):279–292. doi: 10.1007/BF00210344. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A. M., Schutte R., Köhler Y. G., Korn C., Hoefsmit E. C. Characterization of the population of phagocytic cells in thymic cell suspensions. A morphological and cytochemical study. Cell Tissue Res. 1983;231(2):313–323. doi: 10.1007/BF00222183. [DOI] [PubMed] [Google Scholar]
- Harper C. M., Jr, Sharp J. G. Evaluation of the morphological and functional characteristics of murine thymic non-lymphoid cells grown in vitro. J Anat. 1981 Jun;132(Pt 4):607–625. [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F. The human thymic microenvironment. Adv Immunol. 1984;36:87–142. doi: 10.1016/s0065-2776(08)60900-1. [DOI] [PubMed] [Google Scholar]
- Heusermann U., Stutte H. J., Müller-Hermelink H. K. Interdigitating cells in the white pulp of the human spleen. Cell Tissue Res. 1974;153(3):415–417. [PubMed] [Google Scholar]
- Higley H. R., Rowden G. Thymic interdigitating reticulum cells demonstrated by immunocytochemistry. Thymus. 1984;6(4):243–253. [PubMed] [Google Scholar]
- Hoshino T., Kukita A., Sato S. Cells containing Birbeck granules (Langerhans cell granules) in the human thymus. J Electron Microsc (Tokyo) 1970;19(3):271–276. [PubMed] [Google Scholar]
- Jordan R. K., Crouse D. A. Studies on the thymic microenvironment: morphologic and functional characterization of thymic nonlymphoid cells grown in tissue culture. J Reticuloendothel Soc. 1979 Oct;26(4):385–399. [PubMed] [Google Scholar]
- Kaiserling E., Stein H., Müller-Hermelink H. K. Interdigitating reticulum cells in the human thymus. Cell Tissue Res. 1974;155(1):47–55. doi: 10.1007/BF00220283. [DOI] [PubMed] [Google Scholar]
- Klinkert W. E., LaBadie J. H., O'Brien J. P., Beyer C. F., Bowers W. E. Rat dendritic cells function as accessory cells and control the production of a soluble factor required for mitogenic responses of T lymphocytes. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5414–5418. doi: 10.1073/pnas.77.9.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug H., Mager B. Ultrastructure and function of interdigitating cells in the guinea pig thymus. Acta Morphol Acad Sci Hung. 1979;27(1-2):11–19. [PubMed] [Google Scholar]
- Mueller J., Brun del Re G., Buerki H., Keller H. U., Hess M. W., Cottier H. Nonspecific acid esterase activity: a criterion for differentiation of T and B lymphocytes in mouse lymph nodes. Eur J Immunol. 1975 Apr;5(4):270–274. doi: 10.1002/eji.1830050411. [DOI] [PubMed] [Google Scholar]
- Oláh I., Dunay C., Röhlich P., Törö I. A special type of cells in the medulla of the rat thymus. Acta Biol Acad Sci Hung. 1968;19(1):97–113. [PubMed] [Google Scholar]
- Papiernik M., Nabarra B., Savino W., Pontoux C., Barbey S. Thymic reticulum in mice. II. Culture and characterization of nonepithelial phagocytic cells of the thymic reticulum: their role in the syngeneic stimulation of thymic medullary lymphocytes. Eur J Immunol. 1983 Feb;13(2):147–155. doi: 10.1002/eji.1830130211. [DOI] [PubMed] [Google Scholar]
- Pyke K. W., Gelfand E. W. Morphological and functional maturation of human thymic epithelium in culture. Nature. 1974 Oct 4;251(5474):421–423. doi: 10.1038/251421a0. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973 May 1;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974 Feb 1;139(2):380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Witmer M. D., Nussenzweig M. C., Chen L. L., Schlesinger S., Cohn Z. A. Dendritic cells of the mouse: identification and characterization. J Invest Dermatol. 1980 Jul;75(1):14–16. doi: 10.1111/1523-1747.ep12521052. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Isobe T., Ohtsuki Y., Akagi T., Sonobe H., Okuyama T. Immunohistochemical study on the distribution of alpha and beta subunits of S-100 protein in human neoplasm and normal tissues. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(4):385–396. doi: 10.1007/BF02889881. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamaguchi H., Ishizeki J., Nakajima T., Nakazato Y. Immunohistochemical and immunoelectron microscopic localization of S-100 protein in the interdigitating reticulum cells of the human lymph node. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;37(2):125–135. doi: 10.1007/BF02892562. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Hair L. S., Steinman R. M., Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982 Apr 1;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerman A. J. On the interdigitating cells in the thymus-dependent area of the rat spleen: a relation between the mononuclear phagocyte system and T-lymphocytes. Cell Tissue Res. 1974 Apr 11;148(2):247–257. doi: 10.1007/BF00224586. [DOI] [PubMed] [Google Scholar]
- Waksal S. D., Cohen I. R., Waksal H. W., Wekerle H., St Pierre R. L., Feldman M. Induction of T-cells differentiation in vitro by thymus epithelial cells. Ann N Y Acad Sci. 1975 Feb 28;249:492–498. doi: 10.1111/j.1749-6632.1975.tb29098.x. [DOI] [PubMed] [Google Scholar]
- Willis-Carr J. I., Ochs H. D., Wedgwood R. J. Induction of T-lymphocyte differentiation by thymic epithelial cell monolayers. Clin Immunol Immunopathol. 1978 Jul;10(3):315–324. doi: 10.1016/0090-1229(78)90187-3. [DOI] [PubMed] [Google Scholar]
- Wolff K., Winkelmann R. K. Quantitative studies on the Langerhans cell population of guinea pig epidermis. J Invest Dermatol. 1967 Jun;48(6):504–513. doi: 10.1038/jid.1967.82. [DOI] [PubMed] [Google Scholar]
- van Ewijk W., Verzijden J. H., van der Kwast T. H., Luijcx-Meijer S. W. Reconstitution of the thymus dependent area in the spleen of lethally irradiated mice. A light and electron microscopical study of the T-cell microenvironment. Cell Tissue Res. 1974;149(1):43–60. doi: 10.1007/BF00209049. [DOI] [PubMed] [Google Scholar]
- von Gaudecker B., Müller-Hermelink H. K. Ontogeny and organization of the stationary non-lymphoid cells in the human thymus. Cell Tissue Res. 1980;207(2):287–306. doi: 10.1007/BF00237813. [DOI] [PubMed] [Google Scholar]