Abstract
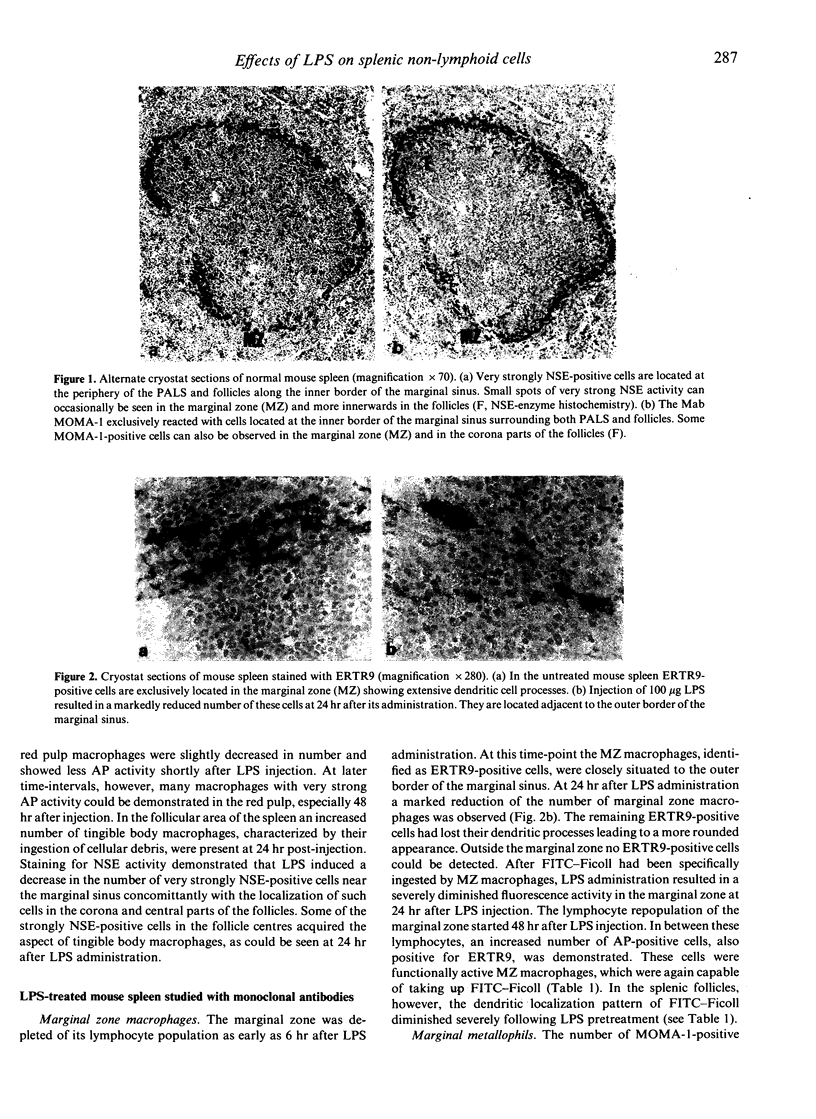
In the present study, the effect of LPS on different splenic non-lymphoid cells was investigated. Marginal zone (MZ) macrophages, marginal metallophils and interdigitating cells (IDC) were demonstrated using specific monoclonal antibodies in a two-step immunoperoxidase procedure in combination with enzyme histochemistry. The results indicate that the number of marginal zone macrophages decreases markedly after LPS treatment, but is followed by a rapid repopulation as observed by monoclonal antibody staining and selective uptake of FITC-Ficoll. Marginal metallophils are normally located at the inner border of the marginal sinus and can specifically be identified by the monoclonal antibody MOMA-1. Following LPS stimulation, many MOMA-1-positive cells were present in the corona and central parts of the follicles, with decreasing numbers near the marginal sinus. These findings strongly suggest that LPS induces a migration of marginal metallophils towards the follicle centres. Most of the tangible body macrophages in the follicle centres appeared to be slightly MOMA-1-positive, which indicates that marginal metallophils may, at least under certain circumstances, differentiate into tangible body macrophages. In the inner PALS, many interdigitating cells, NLDC-145-positive cells, can be found. The number of NLDC-145-positive cells was shown to be severely decreased at later time-intervals after LPS administration, resulting in an almost unstained inner PALS at 2 days. In contrast to the above-mentioned splenic non-lymphoid cells, the red pulp macrophages are only minimally affected by LPS.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Ito T. Early events in the splenic lymphoid tissue and mesenteric lymph node after a single typhoid-paratyphoid vaccine injection in the mouse, with special reference to the topographic cellular changes in the early immune response. Arch Histol Jpn. 1972 Sep;34(5):471–489. doi: 10.1679/aohc1950.34.471. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P. Characterization of non-lymphoid cells in the white pulp of the mouse spleen: an in vivo and in vitro study. Cell Tissue Res. 1978 Dec 29;195(3):445–460. doi: 10.1007/BF00233888. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P., Dijkstra C. D., Boorsma D. M., van Rooijen N. Characterization of lymphoid and nonlymphoid cells in the white pulp of the spleen using immunohistoperoxidase techniques and enzyme-histochemistry. Experientia. 1985 Feb 15;41(2):209–215. doi: 10.1007/BF02002615. [DOI] [PubMed] [Google Scholar]
- Filkins J. P. Monokines and the metabolic pathophysiology of septic shock. Fed Proc. 1985 Feb;44(2):300–304. [PubMed] [Google Scholar]
- Freudenberg N., Freudenberg M. A., Bandara K., Galanos C. Distribution and localization of endotoxin in the reticulo-endothelial system (RES) and in the main vessels of the rat during shock. Pathol Res Pract. 1985 Mar;179(4-5):517–527. doi: 10.1016/S0344-0338(85)80193-X. [DOI] [PubMed] [Google Scholar]
- Gray D., Kumararatne D. S., Lortan J., Khan M., MacLennan I. C. Relation of intra-splenic migration of marginal zone B cells to antigen localization on follicular dendritic cells. Immunology. 1984 Aug;52(4):659–669. [PMC free article] [PubMed] [Google Scholar]
- Groeneveld P. H., Erich T., Kraal G. In vivo effects of LPS on B lymphocyte subpopulations. Migration of marginal zone-lymphocytes and IgD-blast formation in the mouse spleen. Immunobiology. 1985 Dec;170(5):402–411. doi: 10.1016/S0171-2985(85)80064-4. [DOI] [PubMed] [Google Scholar]
- Groeneveld P. H., van Rooijen N., Eikelenboom P. In-vivo effects of lipopolysaccharide on lymphoid and non-lymphoid cells in the mouse spleen. Migration of marginal metallophils towards the follicle centres. Cell Tissue Res. 1983;234(1):201–208. doi: 10.1007/BF00217413. [DOI] [PubMed] [Google Scholar]
- Groeneveld P. H., van Rooijen N. In vivo effects of lipopolysaccharide on lymphoid and non-lymphoid cells in the mouse spleen. Reduction of T-lymphocytes and phagocytosis in the inner parts of the periarteriolar lymphocyte sheath. Cell Tissue Res. 1984;236(3):637–642. doi: 10.1007/BF00217233. [DOI] [PubMed] [Google Scholar]
- Groeneveld P. H., van Rooijen N. Localization of intravenously injected lipopolysaccharide (LPS) in the spleen of the mouse. An immunoperoxidase and histochemical study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;48(3):237–245. doi: 10.1007/BF02890132. [DOI] [PubMed] [Google Scholar]
- Humphrey J. H., Grennan D. Different macrophage populations distinguished by means of fluorescent polysaccharides. Recognition and properties of marginal-zone macrophages. Eur J Immunol. 1981 Mar;11(3):221–228. doi: 10.1002/eji.1830110311. [DOI] [PubMed] [Google Scholar]
- Kumararatne D. S., MacLennan I. C. Cells of the marginal zone of the spleen are lymphocytes derived from recirculating precursors. Eur J Immunol. 1981 Nov;11(11):865–869. doi: 10.1002/eji.1830111104. [DOI] [PubMed] [Google Scholar]
- Maier R. V., Ulevitch R. J. The response of isolated rabbit hepatic macrophages (H-M macrophage) to lipopolysaccharide (LPS). Circ Shock. 1981;8(2):165–181. [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979 Nov;123(5):2133–2143. [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Shands J. W., Jr, Peavy D. L., Gormus B. J., McGraw J. In vitro and in vivo effects of endotoxin on mouse peritoneal cells. Infect Immun. 1974 Jan;9(1):106–112. doi: 10.1128/iai.9.1.106-112.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streefkerk J. G., Veerman A. J. Histochemistry and electron microscopy of follicle lining reticular cells in the rat spleen. Z Zellforsch Mikrosk Anat. 1971;115(4):524–542. doi: 10.1007/BF00335718. [DOI] [PubMed] [Google Scholar]
- TANAKA N., NISHIMURA T., YOSHIYUKI T. Histochemical studies on the cellular distribution of endotoxin of Salmonella enteritidis in mouse tissues. Jpn J Microbiol. 1959 Apr;3:191–201. doi: 10.1111/j.1348-0421.1959.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J., Tobias P. S., Mathison J. C. Regulation of the host response to bacterial lipopolysaccharides. Fed Proc. 1984 Sep;43(12):2755–2759. [PubMed] [Google Scholar]
- Veerman A. J. On the interdigitating cells in the thymus-dependent area of the rat spleen: a relation between the mononuclear phagocyte system and T-lymphocytes. Cell Tissue Res. 1974 Apr 11;148(2):247–257. doi: 10.1007/BF00224586. [DOI] [PubMed] [Google Scholar]
- Veerman A. J., Vries H. D. T- and B-areas in immune reactions. Volume changes in T and B cell compartments of the rat spleen following intravenous administration of a thymus-dependent (SRBC) and a thymus-independent (paratyphoid vaccin-endotoxin) antigen. A histometric study. Z Immunitatsforsch Exp Klin Immunol. 1976 Apr;151(3):202–218. [PubMed] [Google Scholar]
- van Vliet E., Melis M., van Ewijk W. Marginal zone macrophages in the mouse spleen identified by a monoclonal antibody. Anatomical correlation with a B cell subpopulation. J Histochem Cytochem. 1985 Jan;33(1):40–44. doi: 10.1177/33.1.3880783. [DOI] [PubMed] [Google Scholar]