Abstract
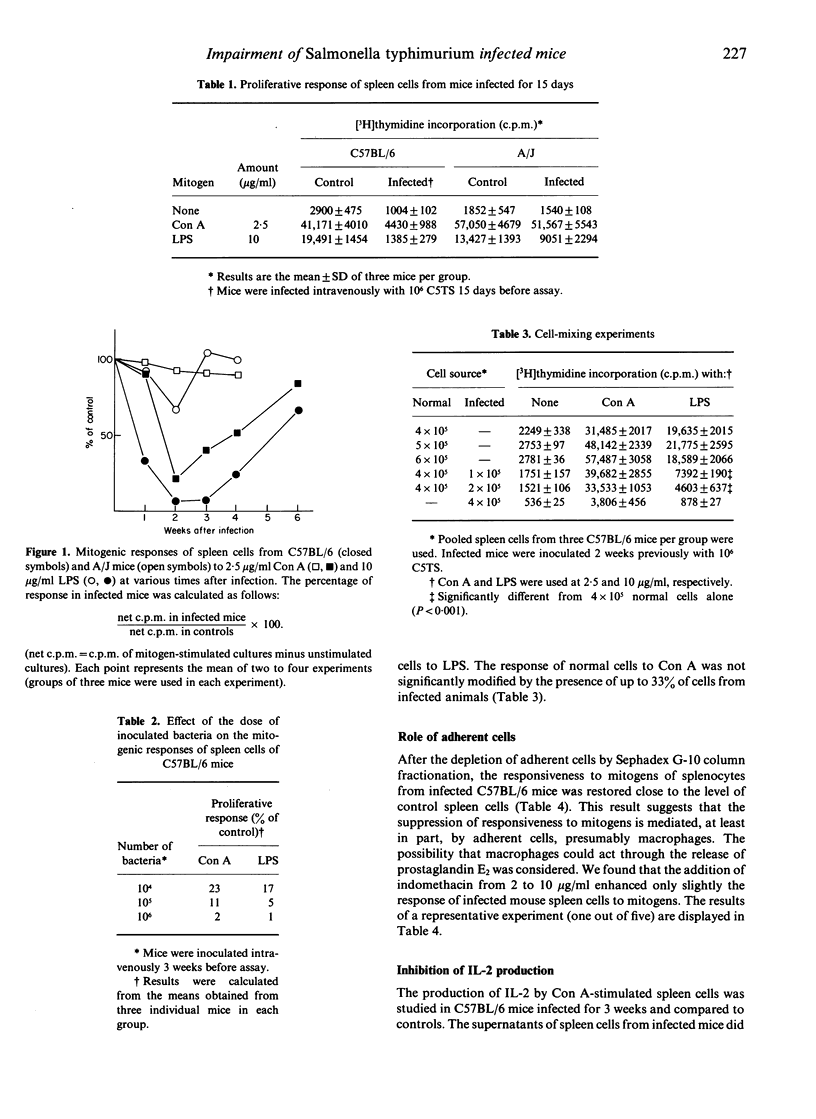
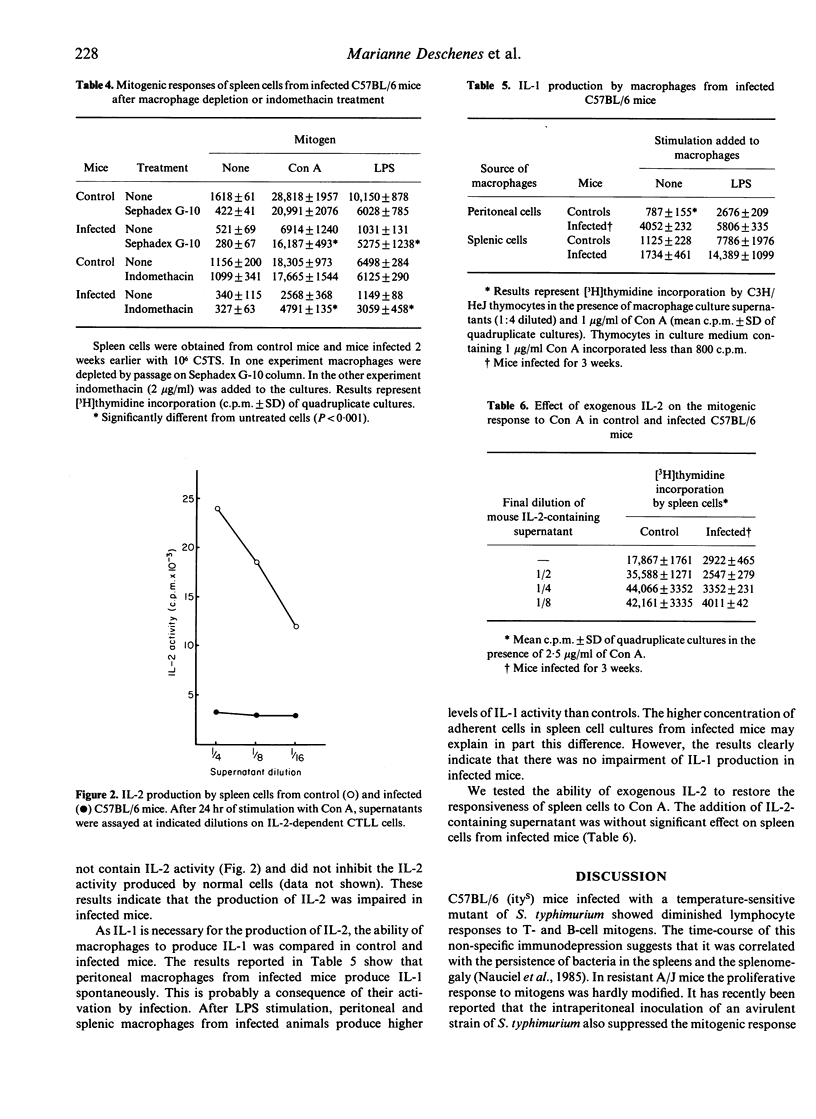
C57BL/6 (susceptible) and A/J (resistant) mice were infected intravenously with a temperature-sensitive mutant of Salmonella typhimurium. In C57BL/6 mice a marked depression of the proliferative response of spleen cells to B and T mitogens occurred and was maximal at 2-3 weeks post-infection, whereas only minor changes were found in A/J mice. This immunodepression was mediated, at least in part, by adherent cells. Moreover, spleen cells from infected C57BL/6 mice did not produce interleukin-2 (IL-2) after concanavalin A stimulation. The impairment of IL-2 production was not related to a defect in IL-1 release. The addition of IL-2 to spleen cells did not restore their ability to respond to mitogens. The depression of mitogenic responses in infected C57BL/6 mice occurred at a time when increased resistance was present.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. M., Moore V. L. Suppression of phytohemagglutinin and lipopolysaccharide responses in mouse spleen cells by Bacillus Calmette-Guerin. J Reticuloendothel Soc. 1979 Oct;26(4):349–356. [PubMed] [Google Scholar]
- Chouaib S., Fradelizi D. The mechanism of inhibition of human IL 2 production. J Immunol. 1982 Dec;129(6):2463–2468. [PubMed] [Google Scholar]
- Friedman R. M., Vogel S. N. Interferons with special emphasis on the immune system. Adv Immunol. 1983;34:97–140. doi: 10.1016/s0065-2776(08)60378-8. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Harel-Bellan A., Joskowicz M., Fradelizi D., Eisen H. Modification of T-cell proliferation and interleukin 2 production in mice infected with Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3466–3469. doi: 10.1073/pnas.80.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffenbach A., Lagrange P. H., Bach M. A. Deficit of interleukin 2 production associated with impaired T-cell proliferative responses in Mycobacterium lepraemurium infection. Infect Immun. 1983 Jan;39(1):109–116. doi: 10.1128/iai.39.1.109-116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormaeche C. E., Pettifor R. A., Brock J. The fate of temperature-sensitive salmonella mutants in vivo in naturally resistant and susceptible mice. Immunology. 1981 Apr;42(4):569–576. [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Gibson C. W., Eisenstein T. K. Macrophage-mediated mitogenic suppression induced in mice of the C3H lineage by a vaccine strain of Salmonella typhimurium. Cell Immunol. 1985 Mar;91(1):75–91. doi: 10.1016/0008-8749(85)90033-4. [DOI] [PubMed] [Google Scholar]
- Lelchuk R., Rose G., Playfair J. H. Changes in the capacity of macrophages and T cells to produce interleukins during murine malaria infection. Cell Immunol. 1984 Apr 1;84(2):253–263. doi: 10.1016/0008-8749(84)90097-2. [DOI] [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Metzger Z., Hoffeld J. T., Oppenheim J. J. Macrophage-mediated suppression. I. Evidence for participation of both hdyrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J Immunol. 1980 Feb;124(2):983–988. [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Nauciel C., Vilde F., Ronco E. Host response to infection with a temperature-sensitive mutant of Salmonella typhimurium in a susceptible and a resistant strain of mice. Infect Immun. 1985 Sep;49(3):523–527. doi: 10.1128/iai.49.3.523-527.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Rosenstreich D. L. Genetic control of the susceptibility of C3HeB/FeJ mice to Salmonella typhimurium is regulated by a locus distinct from known salmonella response genes. J Immunol. 1983 Dec;131(6):2613–2615. [PubMed] [Google Scholar]
- Opitz H. G., Niethammer D., Jackson R. C., Lemke H., Huget R., Flad H. D. Biochemical characterization of a factor released by macrophages. Cell Immunol. 1975 Jul;18(1):70–75. doi: 10.1016/0008-8749(75)90037-4. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Immune response to atypical mycobacteria: immunocompetence of heavily infected mice measured in vivo fails to substantiate immunosuppression data obtained in vitro. Infect Immun. 1984 Jan;43(1):32–37. doi: 10.1128/iai.43.1.32-37.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Reiner N. E., Finke J. H. Interleukin 2 deficiency in murine Leishmaniasis donovani and its relationship to depressed spleen cell responses to phytohemagglutinin. J Immunol. 1983 Sep;131(3):1487–1491. [PubMed] [Google Scholar]
- Stuart A. E., Collee J. G. Selective lymphoid deletion and generalised lymphoid depletion in mouse typhoid. J Pathol Bacteriol. 1967 Oct;94(2):429–437. doi: 10.1002/path.1700940224. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


