Abstract
Human β defensin 2 (hβD-2) is thought to play an important role in cutaneous immune defense. We hypothesized that (i) keratinocyte expression of hβD-2, measured by reverse transcription-PCR, would be upregulated in response to challenge with pathogenic bacteria, particularly highly adherent strains of Streptococcus pyogenes and Staphylococcus aureus, and (ii) hβD-2 would have potent antimicrobial activity against pathogenic but not commensal organisms. Expression of hβD-2 was induced consistently by S. aureus, Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa, whereas strains of S. pyogenes were poor and variable inducers of hβD-2. No correlation was found between levels of bacterial adherence and keratinocyte expression of hβD-2. S. pyogenes was significantly more sensitive to killing by hβD-2 than S. epidermidis. We conclude that the ability to induce hβD-2 expression in combination with sensitivity to its antimicrobial effects may contribute to the rarity of skin infections with the gram-negative bacterial organisms, whereas lack of stimulation of hβD-2 expression by S. pyogenes may be important in its ability to evade innate defenses and cause skin disease. Induction of expression of hβD-2 but relative tolerance to it may enable S. epidermidis to survive on the skin surface and modulate hβD-2 expression when the stratum corneum barrier is disrupted.
Human β defensin 2 (hβD-2) is a cationic antimicrobial peptide (CAP) found in the skin and is thought to play important roles in cutaneous host immune defense and numerous disease states. Premature infants and patients with burns and skin wounds have a compromised skin barrier, putting them at high risk for disseminated infection and delayed skin healing. CAPs may provide protection from widespread bacterial invasion and promote wound healing (13, 17). They may also be involved in the pathogenesis of common inflammatory cutaneous disorders such as psoriasis and acne (3, 21). Thus, defining their in vivo role could lead to novel therapeutic strategies. CAPs assert direct antimicrobial activity on the bacterial cytoplasmic membrane (12). In addition, hβD-2 links innate and adaptive immune responses; it is chemotactic for immature dendritic cells and memory T cells by interacting with chemokine receptor CCR6 (25), and the hβD-2 gene responds to nuclear transcription factor NF-κB (16), which in turn is activated in response to lipopolysaccharide and proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β) (9). On a molecular level, however, regulation of hβD-2 expression in epithelial keratinocytes, including the pathway whereby lipopolysaccharide stimulates hβD-2 expression (20), is not well understood, nor is it clear whether stimulation of hβD-2 expression occurs independently of or via CD14-Toll-like receptor interactions that trigger innate immune responses in other tissues.
Recent reports demonstrate the functional importance of the cathelicidin family of antimicrobial peptides, including human LL-37, in innate cutaneous immunity against group A Streptococcus (17). There is no evidence that directly links hβD-2 expression with antimicrobial defense in human skin. Indirect evidence suggests it has an antibacterial role, however, as expression of hβD-2 is variable in healthy, noninflamed keratinized neonatal or adult skin (1, 15) but is consistently upregulated in noninflamed gingival tissue (15) and in neonatal mucosal epithelium, which is constantly subject to injury and challenge with microorganisms (20). Moreover, a variety of stimuli, including inflammatory skin diseases (e.g., psoriasis, atopic dermatitis), chemical mediators of skin inflammation (e.g., phorbol esters, sodium lauryl sulfate) and exposure to bacteria and yeasts, have been shown to induce keratinocyte expression of hβD-2 (13, 22). Expression of hβD-2 appears to be localized to the upper Malpighian layer of the epidermis and the stratum corneum, as one would expect for a compound that is active in initial defense against pathogenic challenge (15). Murine β defensin 3, a close homologue to hβD-2, is induced by Pseudomonas aeruginosa (2). Another β defensin for which a role in host defense against bacterial infection at epithelial surfaces has been suggested but unproven is lingual antimicrobial peptide, which is increased in tissues constantly exposed to or colonized by microorganisms (15).
Previous models utilizing bacterial extracts and/or heat-killed organisms to study hβD-2 induction (13, 14) may not adequately depict the interactions between keratinocytes and live bacteria during initiation of skin infection. We have developed techniques utilizing cultured, early-passage human epidermal keratinocytes to examine initiating events in the pathogenesis of skin infections (5-8). In vivo, the skin contains an outer layer (stratum corneum) which is important in cutaneous host defense. Our model does not examine the role of the stratum corneum but should closely resemble in vivo circumstances when the stratum corneum may not be fully functional as in premature infant skin and wounds.
In this study, we utilized our model of bacterial skin infection to test the hypothesis that if hβD-2 plays a role in vivo in host defense against skin infections, especially against the common skin pathogens Streptococcus pyogenes and Staphylococcus aureus, keratinocyte expression of hβD-2 would be upregulated in response to challenge with these live pathogenic bacteria. Moreover, we hypothesized that if hβD-2 is active in innate defense, it would display potent antimicrobial activity against skin pathogens such as S. pyogenes, but a commensal organism would be relatively tolerant.
(This work was presented in part at the Annual Meeting of the Society for Pediatric Dermatology in Banff, Alberta, Canada, 28 June 2001.)
MATERIALS AND METHODS
Bacterial strains and culture conditions. Strains of S. pyogenes, S. aureus, and Staphylococcus epidermidis were grown in Todd-Hewitt broth (THB) (Difco Laboratories, Detroit, Mich.) or on Todd-Hewitt agar (1.5% agar) (THA) (Difco) at 37°C in a 5% CO2 atmosphere. All strains of S. pyogenes studied are skin pathogens (8). S. pyogenes M49 serotype strain CS101 was originally provided and characterized by Patrick Cleary and colleagues at the University of Minnesota and has been used as a model strain for studies of skin infection pathogenesis (11). Capsule production was inactivated in the recombinant mutant CS101 has strain, as described previously (8). The CS101 has mutant was grown on selective THA containing 60 mg of spectinomycin. S. pyogenes serotype M52 protein strain 3732, M49 protein strain 5569, and strain Alabama 48 were provided by Susan K. Hollingshead, University of Alabama-Birmingham; these isolates were from patients with impetigo and have M-protein gene chromosomal pattern D or E, characteristic of impetigo strains (8). The clinically invasive serotype M1 protein strain 970771 isolated from blood of a patient with necrotizing fasciitis, and serotype M12 protein strain 950333 isolated from a varicella lesion of a patient with toxic shock syndrome, were provided by Edward Kaplan from the World Health Organization Collaborating Center for Reference and Research on Streptococci at the University of Minnesota. S. aureus strain FDA is a skin pathogen provided by George Murakawa at Wayne State University. S. epidermidis strain UW3 was provided by Seymour Klebanoff at the University of Washington (UW), Seattle.
Escherichia coli strain RS218 (K1 encapsulated clinical isolate) and P. aeruginosa strain PAO1 were obtained from Jane Burns at UW, and E. coli strains B171 (K-12 encapsulated laboratory strain) and ML35p were provided by Phil Tarr at UW and Tomas Ganz at the University of California-Los Angeles, respectively. These strains were grown at 37°C in an ambient atmosphere in shaken Luria-Bertani broth (Sigma Chemical Co., St. Louis, Mo.).
Keratinocyte culture.
Keratinocytes were cultured from fresh human neonatal foreskins in complete keratinocyte growth medium (cKGM) (0.15 mM calcium) (Clonetics, San Diego, Calif.), stored frozen, and reseeded for experiments into six-well tissue culture plates (Corning Glass Works, Corning, N.Y.) in cKGM as described previously (5, 18). Unless indicated otherwise, keratinocytes were grown to 80% confluence and induced to terminally differentiate by increasing the extracellular calcium concentration to 1.0 mM 2 days prior to exposure to bacteria.
Assay for bacterial adherence of S. pyogenes to keratinocytes.
Strains of S. pyogenes were inoculated from a culture grown overnight to an optical density at 600 nm of 0.05 in THB and incubated for 2 h at 37°C to achieve logarithmic growth. Bacterial suspensions were pelleted, washed twice in phosphate-buffered saline (PBS) (pH 7.0), and resuspended in an equal volume of RPMI 1640 (Mediatech Inc., Herndon, Va.).
In preparation for adherence experiments, keratinocytes were washed three times in PBS and aspirated to dryness, and 1.5 ml (each) of bacterial suspension (multiplicity of infection of 50 to 100 bacteria per keratinocyte) was added to three wells. Bacteria were incubated with the keratinocytes for 3 h at 37°C in 5% CO2. To determine the percentage of bacteria in the suspension that was adherent to the keratinocyte monolayer after 3 h of incubation, the plate was first vortexed to dislodge nonadherent bacteria from the monolayer, an aliquot of the supernatant was removed, and the number of CFU in the supernatant was determined. Nonspecifically attached bacteria were removed from the monolayer by washing and vortexing three times in PBS. Total cell-associated bacteria were quantified after dislodging keratinocytes from the tissue culture plate by incubation with 950 μl of 0.25% trypsin (Gibco BRL, Grand Island, N.Y) at 37°C for 8 min, followed by addition of 50 μl of 0.025% Triton X-100 (Sigma) to lyse the keratinocytes. The number of viable, cell-associated bacteria was determined by plating aliquots of the final suspension on THA. The percentage of bacterial adherence was determined by dividing the number of total cell-associated bacteria per well by the number of CFU in the supernatant after the plate was vortexed plus the number of CFU associated with the keratinocytes. Level of adherence in experiments involving 16 h of incubation was estimated visually on the basis of the density of bacteria adherent to the monolayer relative to the number that remained in suspension. All experiments were repeated at least three times.
Measurement of keratinocyte CAP mRNA.
Strains of bacteria were inoculated from a culture grown overnight to an optical density at 600 nm of 0.05 in their respective media and grown to logarithmic phase, yielding 106 to 107 CFU/ml. Bacterial suspensions were washed twice in PBS and resuspended in an equal volume of RPMI 1640. Heat-killed bacteria were placed in a 60°C water bath for 15 min; an aliquot was plated on appropriate agar to confirm lack of growth.
Cultured keratinocytes were challenged for various time intervals with live and heat-killed bacteria, as described above (see “Assay for bacterial adherence of S. pyogenes to keratinocytes”). Keratinocytes exposed to the proinflammatory cytokines TNF-α (10 ng/ml) and IL-6 (10 ng/ml) served as positive controls for upregulation of hβD-2, and keratinocytes exposed to media alone (KGM or RPMI) served as negative controls for induction of CAP expression. Expression of antileukoprotease (ALP) was known to be constitutive (5, 18).
After 16 h of challenge with bacteria, keratinocytes were washed three times in 2 ml of PBS. Total keratinocyte RNA was isolated utilizing the DNase digest protocol in the RNeasy minikit (Qiagen Inc., Valencia, Calif.). One microgram of total RNA was reverse transcribed and amplified utilizing a thermostable reverse transcriptase kit (Thermo-RT; PGC Scientifics Corp., Frederick, Md.). The housekeeping gene encoding ribosomal phosphoprotein (RPO) was amplified as a control for equal loading of samples. One micromolar concentrations of primers for hβD-2 (5′-CCAGCCATCAGCCATGAGGGT-3′ and 5′-GGAGCCCTTTCTGAATCCGCA-3′), ALP (5′-AAACCCAACAAGGAGGAAGC-3′ and 5′-GGACCACACAGAGCAGGACT-3′), and RPO (5′-AGCAGGTGTTCGACAATGGCA-3′ and 5′-ACTCTTCTTTGGCTTCAACC-3′) were amplified for 30 cycles at an annealing temperature of 63°C with display TAQ FL DNA polymerase, according to the protocol of the manufacturer (PGC Scientifics Corp.). The DNA products were separated by electrophoresis on a 2% agarose gel.
Bactericidal assays.
Assays measuring the antibacterial activity of hβD-2 were modified from the methods of Valore et al. (23) and Porter et al. (19). Synthetic hβD-2 was obtained from the Peptide Institute, Inc. (Minoh-shi, Osaka, Japan). The antimicrobial activity of this peptide against E. coli was similar to that previously shown for native hβD-2 (50% lethal dose of approximately 10 μg/ml [14]), suggesting proper formation of disulfide bonds and proper folding. Briefly, single-cell colonies were cultured in THB in a 5% CO2 incubator at 35°C (S. pyogenes) or in Trypticase soy broth (TSB) in a 37°C shaking water bath (S. epidermidis UW3 and E. coli ML35p) to optimize bacterial growth. Bacteria were washed by centrifugation at 12,000 × g, and S. pyogenes was resuspended in 10% THB in 10 mM phosphate buffer (pH 7.3), while S. epidermidis was resuspended in 10% TSB in 10 mM PO4 buffer (pH 7.3). Bacterial concentrations were estimated and adjusted as necessary using a 0.5 McFarland standard to an initial concentration of 108 CFU/ml. To confirm the concentration, aliquots of the bacterial suspension were diluted to 102, plated on blood agar in four wells, and counted.
A 95-μl aliquot of the appropriate buffer was pipetted into each well of a 96-well microtiter plate, to which 5 μl of the 108-CFU/ml bacterial suspension was added to give a final bacterial concentration of 5 × 106 CFU/ml. A stock solution of hβD-2 in 0.01% acetic acid was serially diluted in buffer and added to appropriate wells containing bacterial solution. Buffer alone served as a positive control. After the plates were incubated for 5 (S. epidermidis and E. coli) or 8 (S. pyogenes) h, bacterial suspensions were diluted, plated on blood agar in four wells, and incubated for 24 or 48 h, respectively, and colonies were counted. All assays were repeated at least three times. Data were analyzed using analysis of variance and Dunnett's test (GraphPad Software, Inc., San Diego, Calif.).
RESULTS
Keratinocyte expression of hβD-2 following bacterial challenge is isolate specific.
In time course experiments in which keratinocytes were exposed to cytokines (TNF-α or IL-6) as positive controls for hβD-2 expression or to live bacteria for 3 to 16 h, expression of hβD-2 was first seen after exposure for 6 h, although only in terminally differentiated (i.e., completely developed) and not undifferentiated keratinocytes. The strength of the response as detected by reverse transcription-PCR (RT-PCR) analysis for hβD-2 transcripts increased with time of exposure (data not shown). At incubation times of >16 h, keratinocytes exposed to live bacteria began to show extensive cellular damage with rounding and detachment of keratinocytes, especially after prolonged exposure to S. aureus and invasive strains of S. pyogenes. In addition, under these circumstances, the amount of total RNA recovered was decreased, and detection of hβD-2 expression by RT-PCR analysis was inconsistent. Expression of hβD-2 was not detected in keratinocytes exposed to either cKGM, the medium in which the keratinocytes were cultured, or RPMI, the medium in which the bacterial adherence assay was performed.
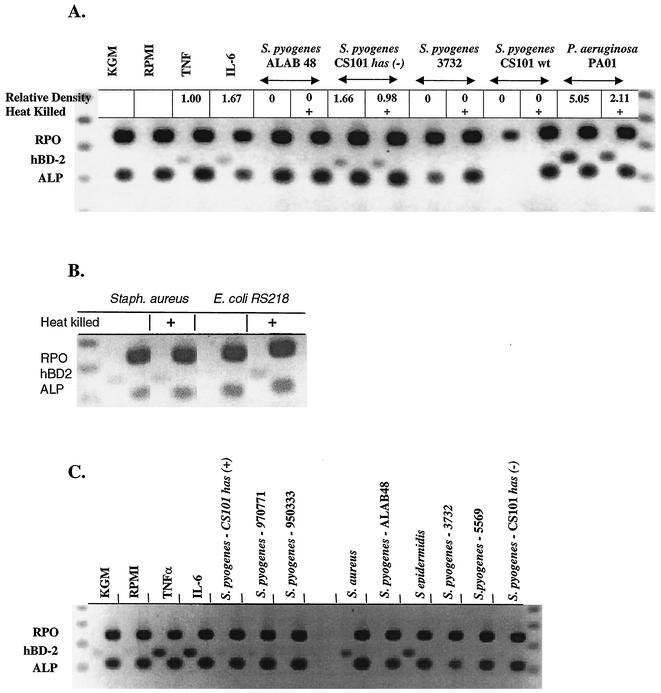
Expression of hβD-2 was induced consistently by the gram-positive organisms S. aureus and S. epidermidis and the gram-negative organisms E. coli and P. aeruginosa (Fig. 1). Strains of S. pyogenes were poor and variable inducers of hβD-2 (Fig. 1A and C). No correlation was found between the level of adherence of S. pyogenes to keratinocytes in standard 3-h adherence assays or after 16-h incubation times and keratinocyte expression of hβD-2 (Table 1 and Fig. 1). Similarly, no correlation was found between adherence to keratinocytes and level of hβD-2 expression in all bacterial strains examined in this study (Table 1 and Fig. 1). Little or no difference in hβD-2 mRNA expression was found following challenge with live compared to heat-killed bacteria. Expression of ALP was constitutive (Fig. 1), as expected (5, 24).
FIG. 1.
Keratinocyte expression of hβD-2 is upregulated by S. aureus, S. epidermidis, E. coli, and P. aeruginosa but poorly and variably upregulated by S. pyogenes. hβD-2 (hBD-2) expression in epidermal keratinocytes was determined by RT-PCR in three representative experiments (each panel shows the results of one representative experiment). Healthy human cultured foreskin keratinocytes were stimulated for 16 h by TNF-α or IL-6 or challenged with 1.5 ml of a suspension (106 to 107 CFU per ml of RPMI 1640) of bacteria of various pathogenicities and levels of adherence. Total RNA was isolated from keratinocytes, and RT-PCR was performed to detect expression of hβD-2 by gel electrophoresis. Analysis for each sample is shown in two adjacent lanes, one for hβD-2 and the second for ALP and RPO. S. pyogenes ALAB 48, S. pyogenes Alabama 48; wt, wild type.
TABLE 1.
Adherence of bacterial strains to keratinocytes
| Bacterial strain | Adherencea (%)
|
|
|---|---|---|
| 3 h | 16 h | |
| S. pyogenes | ||
| M49 serotype CS101 | ||
| Wild-type | 1-10b | 95-100 |
| Capsule (has) knockoutb | 80-95b | ≈50 |
| M52 serotype 3732 | 20-50b,c | ≈50 |
| M49 serotype 5569 | 25 | ≈50 |
| Alabama 48 | 40-60 | ≈30 |
| M1 serotype 970771 | 18b | ≈30 |
| M12 serotype 950333 | 37b | —d |
| Staphylococcus spp. | ||
| S. epidermidis UW3 | 30 | ≈10 |
| S. aureus FDA | 30e | ≈90 |
| E. coli | ||
| RS218 | 1 | ≈50 |
| B171 | 20-30 | ≈90 |
| P. aeruginosa PAO1 | NAf | <5 |
In vitro hβD-2 sensitivity assay.
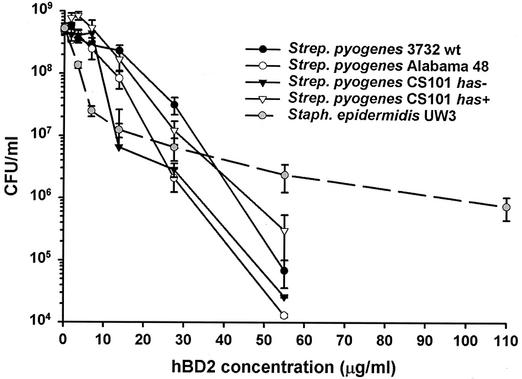
hβD-2 showed significant in vitro activity against all bacteria tested (P < 0.001 at concentrations of ≥7 μg/ml) (Fig. 2). The assay used allowed determination of bactericidal or bacteriostatic action. Under the conditions used here, hβD-2 activity was bactericidal against all bacterial strains tested. At concentrations above 35 μg/ml (9 μM), all S. pyogenes strains tested were more sensitive to hβD-2 than S. epidermidis UW3 (Fig. 2) or E. coli ML35p (not shown), although the S. pyogenes Alabama 48 and CS101 has mutant strains were more susceptible than the others (Fig. 2). Activity against S. epidermidis was significant (P < 0.001) at all concentrations tested; however, hβD-2 effectiveness did not increase noticeably when the peptide concentration was increased above 13.75 μg/ml (3.6 μM). With all other bacteria tested, a certain threshold hβD-2 concentration appeared necessary for biologically and statistically significant activity to occur, and then activity increased with increasing peptide concentration.
FIG. 2.
hβD-2 (hBD2) has greater bactericidal activity against S. pyogenes than S. epidermidis. Bacteria were incubated in 10% THB (S. pyogenes) or TSY (S. epidermidis) broth in 10 mM phosphate buffer in the presence of serially diluted concentrations of peptide. After several hours, aliquots were diluted and plated in four wells, and the number of colonies was compared to that in the control well (no peptide added). Data points are the means from a minimum of three experiments. Error bars represent the standard errors. S. epidermidis UW3 and three clinical strains and one capsule-negative strain of S. pyogenes are shown. Note that S. pyogenes strains are more sensitive than S. epidermidis. wt, wild type.
DISCUSSION
Our data suggest that the role of hβD-2 in cutaneous host defense against skin infections is complex. Use of multiple species of (i) various propensities to adhere to and invade keratinocytes in our model of skin infection and (ii) various pathogenic potentials for causing clinically superficial and invasive skin infections revealed no relationship between adherence or invasion or clinical pathogenicity and hβD-2 expression. Specifically, hβD-2 expression was variably induced by challenge with S. aureus (consistent inducer) and S. pyogenes (poor inducer), suggesting that hβD-2 expression may not be targeted directly toward cutaneous host defense against major skin pathogens per se. hβD-2 has been shown previously to be bacteriostatic for S. aureus only at high concentrations above 100 μg/ml (13), which may enable S. aureus to survive on the skin surface in spite of upregulated β-defensin expression. On the other hand, we found that expression of hβD-2 was consistently induced by bacteria rarely implicated in skin disease: S. epidermidis, P. aeruginosa, and E. coli. The latter result is consistent with a previous report on a human skin equivalent challenged with E. coli K-12 (20). hβD-2 is known to be bactericidal for E. coli and P. aeruginosa at a 90% lethal dose of approximately 10 μg/ml (14, 22). Perhaps strong induction of hβD-2 expression in combination with sensitivity to being killed by the agent contributes to the rarity of skin infections with these gram-negative organisms. Conversely, lack of stimulation of hβD-2 expression by S. pyogenes may be important in its ability to evade innate defenses and cause disease, as it is highly sensitive to killing by hβD-2 (Table 2).
TABLE 2.
Summary of hβD-2 induction and sensitivity by bacterial strain
| Bacterial strain | hβD-2 induction | Reductionb |
|---|---|---|
| S. pyogenes | ||
| M49 serotype CS101 | ||
| Wild-type | Poor inducer | 3 |
| Capsule has knockouta | Poor inducer | 4 |
| M52 serotype 3732 | Poor inducer | 4 |
| M49 serotype 5569 | Poor inducer | NT |
| Alabama 48 | Poor inducer | 5 |
| M1 serotype 970771 | Poor inducer | NT |
| M12 serotype 950333 | Poor inducer | NT |
| Staphylococcus spp. | ||
| S. epidermidis UW3 | Consistent inducer | 2 |
| S. aureus FDA | Consistent inducer | NT |
| E. coli | ||
| RS218 | Consistent inducer | NT |
| B171 | Consistent inducer | 2 |
| P. aeruginosa PAO1 | Consistent inducer | NT |
See Darmstadt et al. (8).
Reduction in log CFU/milliliter with 55 μg of hβD-2 per ml. NT, not tested.
Consistent and strong induction of hβD-2 expression by S. epidermidis but relative resistance (versus that of other tested bacteria) to the antimicrobial activity of hβD-2 may reflect an ability of S. epidermidis to colonize the skin surface while regulating hβD-2 expression, possibly in concert with other stimuli (Table 2). Induction of hβD-2 has been reported previously when cultured human foreskin keratinocytes were challenged with heat-killed S. epidermidis (13). Similarly, in cultured oral keratinocytes, the oral commensal organism Fusobacterium nucleatum induced hβD-2 expression independent of IL-8, another component of the innate immune system, whereas the oral pathogen Porphyromonas gingivalis was a poor inducer of hβD-2 (15). In addition, hβD-2 is not expressed in healthy uninflamed epidermis, but it is expressed in healthy oral epithelium (4). This led to the conclusion that the oral epithelium is in a constantly activated state with respect to hβD-2 expression and that this activation by commensal organisms plays an important role in barrier defense (15). With regard to the skin, it may be that commensal organisms such as S. epidermidis are necessary to induce an activated state that helps to preserve the commensal flora while priming the epidermis for further response to challenge with pathogens, especially when the skin barrier is compromised, as is seen in cutaneous wounds and inflammatory skin conditions, such as psoriasis and atopic dermatitis.
There was no difference between live bacteria and heat-killed organisms in their ability to induce antimicrobial peptide expression. This suggests that active bacterial growth or metabolism is not necessary to induce hβD-2 responses and that heat-killed bacterial preparations contained the component(s) responsible for triggering hβD-2 signaling, as reported for induction of hβD-2 expression upon bacterial challenge of oral mucosal epithelium (15).
Models such as ours that utilize cultured keratinocytes stratified to only a few cell layers and not fully differentiated (5) have limitations when conclusions about the role of CAPs in vivo are drawn. The differentiation state clearly impacts CAP expression, as we have reported here and previously (4). The stratum corneum provides an interface between bacteria and keratinocytes, and our data do not reflect the interaction of bacteria with an entirely intact skin barrier. Cell culture methods that utilize skin substitutes more closely model interactions between the skin and bacteria, but these models pose difficulties in isolating and measuring the responses of the population of cells of interest (i.e., those cells to which the bacteria have bound). A recent study utilized the skin substitute Apligraf, but common skin pathogens were not tested (20). Nizet et al. (17) and Dorschner et al. (10) recently utilized a mouse model and a molecular genetic approach to demonstrate that the cathelicidins protect against group A streptococcal skin infection.
In summary, our data suggest that the ability to induce hβD-2 expression in combination with sensitivity to its antimicrobial effects may contribute to the rarity of skin infections with the gram-negative organisms E. coli and P. aeruginosa, whereas the lack of stimulation of hβD-2 expression by S. pyogenes may be important in its ability to evade front-line defenses and cause skin infection. Induction of keratinocyte expression of hβD-2 but relative tolerance to it may enable S. aureus and S. epidermidis to survive for periods of time on the skin surface where they may modulate hβD-2 expression when the stratum corneum barrier is disrupted. The ubiquitous expression of CAPs in nature, their importance in mucosal and skin defense, and the variability in expression and patterns of upregulation in human skin in response to commensal and pathogenic bacteria suggest that further investigation of the role of CAPs in human skin immunity is warranted and may provide important insights into the regulation of skin flora in health and infection.
Acknowledgments
This research was funded in part by a National Research Service Award (NRSA) Institutional Training Grant (T32AR07019) from the National Institutes of Health (J.G.H.D.); National Institutes of Health grant P30 HD28834 through the University of Washington Child Health Research Center (G.L.D.) and grant R01DE13573 (B.A.D.), a Leaders Society Clinical Career Development Award from the Dermatology Foundation (G.L.D.), and the Bill and Melinda Gates Foundation.
We gratefully acknowledge the advice and help of Marilyn Roberts, Department of Pathobiology, University of Washington, for assistance in designing the antibacterial assays.
REFERENCES
- 1.Ali, R. S., A. Falconer, M. Ikram, C. E. Bissett, R. Cerio, and A. G. Quinn. 2001. Expression of the peptide antibiotics human beta defensin-1 and human beta defensin-2 in normal human skin. J. Investig. Dermatol. 117:106-111. [DOI] [PubMed] [Google Scholar]
- 2.Bals, R., X. Wang, R. L. Meegalla, S. Wattler, D. J. Weiner, M. C. Nehls, and J. M. Wilson. 1999. Mouse beta-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect. Immun. 67:3542-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chronnell, C. M., L. R. Ghali, R. S. Ali, A. G. Quinn, D. B. Holland, J. J. Bull, W. J. Cunliffe, I. A. McKay, M. P. Philpott, and S. Muller-Rover. 2001. Human beta defensin-1 and -2 expression in human pilosebaceous units: upregulation in acne vulgaris lesions. J. Investig. Dermatol. 117:1120-1125. [DOI] [PubMed] [Google Scholar]
- 4.Dale, B. A., J. R. Kimball, S. Krisanaprakornkit, F. Roberts, M. Robinovitch, R. O'Neal, E. V. Valore, T. Ganz, G. M. Anderson, and A. Weinberg. 2001. Localized antimicrobial peptide expression in human gingiva. J. Periodontal Res. 36:285-294. [DOI] [PubMed] [Google Scholar]
- 5.Darmstadt, G. L., P. Fleckman, M. Jonas, E. Chi, and C. E. Rubens. 1998. Differentiation of cultured keratinocytes promotes the adherence of Streptococcus pyogenes. J. Clin. Investig. 101:128-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darmstadt, G. L., P. Fleckman, and C. E. Rubens. 1999. Tumor necrosis factor-alpha and interleukin-1alpha decrease the adherence of Streptococcus pyogenes to cultured keratinocytes. J. Infect. Dis. 180:1718-1721. [DOI] [PubMed] [Google Scholar]
- 7.Darmstadt, G. L., L. Mentele, P. Fleckman, and C. E. Rubens. 1999. Role of keratinocyte injury in adherence of Streptococcus pyogenes. Infect. Immun. 67:6707-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darmstadt, G. L., L. Mentele, A. Podbielski, and C. E. Rubens. 2000. Role of group A streptococcal virulence factors in adherence to keratinocytes. Infect. Immun. 68:1215-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond, G., D. E. Jones, and C. L. Bevins. 1993. Airway epithelial cells are the site of expression of a mammalian antimicrobial peptide gene. Proc. Natl. Acad. Sci. USA 90:4596-4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorschner, R. A., V. K. Pestonjamasp, S. Tamakuwala, T. Ohtake, J. Rudisill, V. Nizet, B. Agerberth, G. H. Gudmundsson, and R. L. Gallo. 2001. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A streptococcus. J. Investig. Dermatol. 117:91-97. [DOI] [PubMed] [Google Scholar]
- 11.Haanes, E. J., and P. P. Cleary. 1989. Identification of a divergent M protein gene and an M protein-related gene family in Streptococcus pyogenes serotype 49. J. Bacteriol. 171:6397-6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hancock, R. E. 1997. Peptide antibiotics. Lancet 349:418-422. [DOI] [PubMed] [Google Scholar]
- 13.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 1997. A peptide antibiotic from human skin. Nature 387:861. [DOI] [PubMed] [Google Scholar]
- 14.Harder, J., U. Meyer-Hoffert, L. M. Teran, L. Schwichtenberg, J. Bartels, S. Maune, and J. M. Schroder. 2000. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am. J. Respir. Cell Mol. Biol. 22:714-721. [DOI] [PubMed] [Google Scholar]
- 15.Krisanaprakornkit, S., J. R. Kimball, A. Weinberg, R. P. Darveau, B. W. Bainbridge, and B. A. Dale. 2000. Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect. Immun. 68:2907-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, L., L. Wang, H. P. Jia, C. Zhao, H. H. Heng, B. C. Schutte, P. B. McCray, Jr., and T. Ganz. 1998. Structure and mapping of the human beta-defensin HBD-2 gene and its expression at sites of inflammation. Gene 222:237-244. [DOI] [PubMed] [Google Scholar]
- 17.Nizet, V., T. Ohtake, X. Lauth, J. Trowbridge, J. Rudisill, R. A. Dorschner, V. Pestonjamasp, J. Piraino, K. Huttner, and R. L. Gallo. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454-457. [DOI] [PubMed] [Google Scholar]
- 18.Piepkorn, M., P. Hovingh, A. Dillberger, and A. Linker. 1995. Divergent regulation of proteoglycan and glycosaminoglycan free chain expression in human keratinocytes and melanocytes. In Vitro Cell. Dev. Biol. Anim. 31:536-541. [DOI] [PubMed] [Google Scholar]
- 19.Porter, E. M., E. van Dam, E. V. Valore, and T. Ganz. 1997. Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect. Immun. 65:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmid, P., O. Grenet, J. Medina, S. D. Chibout, C. Osborne, and D. A. Cox. 2001. An intrinsic antibiotic mechanism in wounds and tissue-engineered skin. J. Investig. Dermatol. 116:471-472. [DOI] [PubMed] [Google Scholar]
- 21.Schroder, J. M., and J. Harder. 1999. Human beta-defensin-2. Int. J. Biochem. Cell Biol. 31:645-651. [DOI] [PubMed] [Google Scholar]
- 22.Singh, P. K., H. P. Jia, K. Wiles, J. Hesselberth, L. Liu, B. A. Conway, E. P. Greenberg, E. V. Valore, M. J. Welsh, T. Ganz, B. F. Tack, and P. B. McCray, Jr. 1998. Production of beta-defensins by human airway epithelia. Proc. Natl. Acad. Sci. USA 95:14961-14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valore, E. V., C. H. Park, A. J. Quayle, K. R. Wiles, P. B. McCray, Jr., and T. Ganz. 1998. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J. Clin. Investig. 101:1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiedow, O., J. Harder, J. Bartels, V. Streit, and E. Christophers. 1998. Antileukoprotease in human skin: an antibiotic peptide constitutively produced by keratinocytes. Biochem. Biophys. Res. Commun. 248:904-909. [DOI] [PubMed] [Google Scholar]
- 25.Yang, D., O. Chertov, S. N. Bykovskaia, Q. Chen, M. J. Buffo, J. Shogan, M. Anderson, J. M. Schroder, J. M. Wang, O. M. Howard, and J. J. Oppenheim. 1999. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286:525-528. [DOI] [PubMed] [Google Scholar]