Abstract
Several different methods have been developed to quantitate neutralizing antibody responses to human papillomaviruses (HPVs), including in vivo neutralization assays, in vitro pseudoneutralization assays, competitive radioimmunoassays (cRIAs), and enzyme-linked immunosorbent assays. However, each of these techniques possesses one or more limitations that preclude testing large numbers of patient sera for use in natural history studies and large vaccine clinical trials. We describe here a new multiplexed assay, by using the Luminex Laboratory MultiAnalyte Profiling (LabMAP3) assay system, that can simultaneously quantitate neutralizing antibodies to human papillomavirus types 6, 11, 16, and 18 in 50 μl of serum. The HPV-Luminex competitive immunoassay measures titers of polyclonal antibodies in serum capable of displacing phycoerythrin-labeled detection monoclonal antibodies binding to conformationally sensitive, neutralizing epitopes on the respective virus-like particles. This competitive Luminex immunoassay was found to be as sensitive, accurate, and precise as the currently used cRIAs. An effective HPV vaccine will most likely require several distinct genotypes to protect against multiple cancer causing papillomaviruses. The HPV-Luminex immunoassay should prove to be a useful tool in simultaneously quantitating antibody immune responses to multiple HPV genotypes for natural history infection studies and for monitoring the efficacy of prospective vaccines.
Human papillomaviruses (HPVs) are double-stranded DNA viruses that infect epithelial cells and are significantly associated with low-grade cervical intraepithelial neoplasia, genital condyloma, and cervical cancer (23). Cervical cancer is the second most common cause of cancer deaths in women worldwide (31), resulting in approximately 400,000 deaths per year (46). HPVs are the primary cause of cervical cancer (46) and are the most common sexually transmitted viral pathogens in the United States (29). To date, at least 100 different HPV types have been described (3). “Low-risk” HPVs such as HPV-6 and -11 are associated with the production of benign genital warts, whereas “high-risk” types such as HPV-16 and -18 are associated with the development of cervical cancer. Strong epidemiological evidence that HPVs cause cervical carcinoma is suggested by the fact that HPV DNA is detected in more than 99.7% of cervical cancers (41). HPV-16 is the most prevalent oncogenic HPV, being present in more than 50% of all cervical tumor specimens worldwide (3). HPV-16 and -18, plus the less prevalent oncogenic types such as HPV-31, -33, -45, -52, and -58, contribute to more than 90% of cervical carcinomas (19, 32, 37). Vaccines for both the low-risk and high-risk HPV genotypes are currently being tested in clinical trials.
An effective vaccine against HPV is needed to prevent the development of cervical dysplasias and carcinomas and their associated morbidity and mortality (4). The L1 capsid protein of papillomaviruses (45) expressed in yeast (15, 36), insect cells (14, 18), or mammalian cells (45) self assembles into virus-like particles (VLPs) that are composed of 72 pentamers of L1 in a T=7 icosahedral structure (1). Several studies have shown that immunization with VLPs induces neutralizing antibodies and protects against experimental papillomavirus infection in rabbits (5), dogs (39), and cows (17). Conformationally sensitive epitopes on the VLPs are essential for inducing neutralizing antibodies since denatured L1 protein does not stimulate neutralizing antibodies or protect against experimental infection (39). For HPV-6, -11, -16, and -18, type-specific antibodies have been identified that neutralize viruses in in vivo neutralization assays and in in vitro pseudoneutralization assays (8, 10, 11, 42, 44). For an HPV vaccine to effectively prevent more than 80% of cervical carcinomas, it will most likely need to include multiple VLP genotypes to protect against the different cancer-causing viruses in the field (3). Specifically, a vaccine composed of HPV types 16, 18, 31, 33, and 45 would theoretically protect against more than 80% of cervical cancers (3).
An immunoassay that measures HPV type-specific antibodies to several HPV genotypes simultaneously would be preferred to running multiple separate tests. Luminex Laboratory MultiAnalyte Profiling (LabMAP3) technology was used to develop a competitive immunoassay that measures HPV type 6, 11, 16, and 18 specific antibodies to neutralizing epitopes from a single serum sample. The assay uses yeast-derived VLPs that have been coupled to a set of four distinct fluorescent Luminex microspheres. The type-specific HPV-VLP antibody responses are associated with specific Luminex microspheres that are identified by their distinct red and orange fluorescent dye spectral properties on the Luminex100 (13). Antibody titers are determined in a competitive format, where known, type-specific, phycoerythrin (PE)-labeled, neutralizing antibodies compete with patient serum antibodies for binding to conformationally sensitive, neutralizing epitopes on the VLPs. We describe here this sensitive, multiplexed Luminex immunoassay that simultaneously measures HPV type-specific antibodies to neutralizing epitopes on VLPs in human serum.
MATERIALS AND METHODS
VLPs.
VLPs for HPV types 6, 11, 16, and 18 formed by the expression of the L1 gene in yeast Saccharomyces cerevisiae were purified from lysates of S. cerevisiae as previously described (15, 16, 25, 35) with modifications (12).
Antibodies.
The antibodies chosen for the assay—HPV-6 (H6.B10.5) (11), HPV-11 (MAb 8740 or H11.B2; Chemicon, Temecula, Calif.) (10), HPV-16 (H16.V5) (8), and HPV-18 (H18.J4) (8)—were all previously shown to be HPV type-specific and to bind to neutralizing epitopes (44). The H6.B10.5, H11.B2, H16.V5, and H18.J4 antibodies were tagged with PE (Chromaprobe, Aptos, Calif.). For use in the assay, the four PE-tagged MAbs were combined so that the final concentrations of each MAb were 2.5 μg/ml for H6.B10.5, 1.0 μg/ml for H11.B2, 1.0 μg/ml for H16.V5, and 1.25 μg/ml for H18.J4.
Patient population.
Serum samples were from women, 18 to 25 years in age, enrolled in phase I HPV VLP vaccine clinical trials. Eligibility criteria required that volunteers be in good health and have no history of genital warts or abnormal cervical cytology. In addition, at screening women were considered ineligible for the vaccine study if they demonstrated positive results for HPV-6, -11, -16, or -18 DNA at any of four anogenital sites or if they tested positive for HPV-6, -11, -16, or -18 antibodies in the competitive radioimmnuoassay (cRIA) (6). Informed written consent was obtained from all women before participation in the study. Laboratory assays were performed with no knowledge of the placebo or vaccine status of the individual participants.
Covalent coupling of HPV VLPs to Luminex microspheres.
The HPV-VLPs were coupled to the Luminex microspheres by using an N-hydroxysulfosuccinimide enhanced carbodiimide-mediated coupling reaction (38). Luminex microspheres are fluorescent polystyrene beads approximately 5,600 nm in diameter with functional carboxyl groups for covalently attaching proteins. Microspheres were stored at 4°C, in the dark, at a concentration of 1.25 × 107 microspheres/ml. The microspheres were brought to room temperature, sonicated for 2 min to obtain an equal distribution of microspheres, and divided into aliquots in 1.5-ml vials (VWR, West Chester, Pa.) at 2.5 × 106 microspheres per vial. Microspheres were pelleted and resuspended in 400 μl of 0.1 M sodium phosphate buffer (pH 6.2). The carboxylated sites on the surface of the microspheres were activated by adding 50 μl of a 50-mg/ml solution of N-hydroxysulfosuccinimide and 50 μl of a 50-mg/ml solution of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride. The tubes were sonicated for 2 min, wrapped in foil and incubated for 20 min at room temperature. After the activation step, the microspheres were washed once in 500 μl of phosphate-buffered saline (PBS; pH 7.4) before the addition of the VLPs.
Coupling of the HPV-VLPs to their respective microspheres was performed after the carboxyl sites on the microspheres were activated. HPV-VLPs for types 6, 11, 16, and 18 were diluted in PBS to a concentration of 12 μg/ml. A total 500 μl of the VLPs (12 μg/ml) was added to the activated microspheres and vortexed on a low setting for 10 to 20 s to resuspend the microspheres. The VLPs were coupled to the microspheres as follows: VLP-6 to microsphere 32, VLP-11 to microsphere 53, VLP-16 to microsphere 38, and VLP-18 to microsphere 18. The different microspheres were chosen because of the good spectral resolution between the different microsphere sets. After addition of the VLPs, vials were wrapped in foil and placed on a rotator for 2 h at room temperature to allow VLPs to covalently bind to the microspheres by forming amide bonds with the open carboxylate sites on the microspheres. VLPs coupled to microspheres were washed once with 1 ml of PBS containing 0.05% Tween 20 and resuspended in histidine buffer (20 mM histidine, 0.5 M NaCl; pH 6.2), Column A buffer (50 mM morpholinepropanesulfonic acid, 0.5 M NaCl mM; pH 7.0), or PBS with or without 1% bovine serum albumin (BSA) to block any remaining open carboxyl sites on the microspheres. VLP-microspheres (1.0 × 106 microspheres/ml) were stored in 1-ml aliquots at 4°C in light-resistant vials.
Competitive Luminex assay.
To perform the competitive immunoassay, VLP-microspheres of each of the four HPV VLP types were pooled in equal volumes and diluted with histidine buffer to a final concentration of 8.0 × 105 microspheres/ml. VLP-microspheres were added to each well of a 96-well, black opaque, microtiter plate (Costar, Corning, N.Y.) in a volume of 25 μl (20,000 VLP-microspheres total, 5,000 VLP-microspheres of each HPV type) per well. An HPV standard reference serum was generated by pooling sera from individual African green monkeys that had been immunized with either HPV-6, -11, or -18 VLPs and serum from a chimpanzee that had been immunized with HPV-16 VLPs. The titers for the four different sera had previously been determined in a pseudoneutralization assay (44). The stock concentrations of the pooled, standard, reference sera for the different HPV types were 250 milli-Merck units (mMU)/ml for HPV-6 and 1,000 mMU/ml for HPV-11, HPV-16, and HPV-18. An antibody titer of >200 mMU/ml for HPV-11 has been shown to neutralize ∼108 virions in the athymic mouse xenograft assay (7). To the 25 μl of microspheres, 50 μl of twofold serial dilutions of the stock, reference standard into HPV-negative normal human serum (NHS) was added in duplicate to generate a 12-point standard curve. A negative control was added in quadruplicate, and high and low controls were added in duplicate to each plate. Sera and VLP-microspheres were incubated at room temperature for 15 min in a foil-covered plate. The combined, PE-tagged, type-specific MAbs were added to each well of the plate in a volume of 25 μl and the sera, VLP-microspheres, and MAb-PE combinations were mixed three times by using a multichannel pipette. Plates were resealed with foil covers and allowed to incubate overnight at room temperature. After incubation, all samples were transferred to a filter plate (Millipore, Bedford, Mass.) prewet with PBS. The serum samples were washed three times with 200 μl of PBS buffer and the VLP-microspheres were resuspended in 200 μl of PBS plus 1% BSA for analysis. Samples were analyzed on a Luminex100 instrument with the XY plate handler platform in multiplexed acquisition mode with gates set to exclude microsphere multimers. Instrument analysis time was approximately 30 s per sample.
cRIA.
HPV type-specific cRIAs were used to evaluate HPV type-specific antibody titers to native HPV-6, HPV-11, HPV-16, and HPV-18 VLPs as previously described (28). Briefly, HPV L1 VLP antigens at 100 ng/ml (HPV-6 and HPV-11), 50 ng/ml (HPV-16), and 175 ng/ml (HPV-18) were coated onto solid-phase polystyrene beads (1/4 in. with specular finish, Precision Plastic Ball Co., Franklin Park, Ill.) for 1 h at room temperature (five beads/ml) with mild agitation. The beads were washed with 50 mM morpholinepropanesulfonic acid-0.5 M NaCl (pH 7.0) and stored submerged at 4°C in morpholinepropanesulfonic acid buffer until further use. Equal volumes of sera (100 μl) and diluted MAb sera (1:12,500 for H6.B10.5, 1:160,000 for H11.B2, 1:800,000 for H16.V5, or 1:200,000 for H18.J4) in PBS containing 1% BSA, 0.1% Tween 20, and 0.1% sodium azide were mixed in a well of a 20-well Abbott assay plate. A single HPV VLP-coated bead was added to each well, incubated overnight at room temperature, washed with deionized water, incubated with 125I-labeled goat anti-mouse immunoglobulin G (IgG; NEN Life Sciences, Boston, Mass.) at 37°C for 2.5 h, washed with deionized water, and counted in a gamma-counter (Wallac, Turku, Finland).
Relative inhibition of MAb binding was compared to a standard reference serum by using a four-parameter logistic curve fit. The standard reference sera was from individual African green monkeys that had been immunized with either HPV-6, -11, or -18 VLPs and a chimpanzee that had been immunized with HPV-16 VLPs and had been assigned arbitrary values expressed in mMU/milliliter. An antibody titer of >200 mMU/ml for HPV-11 has been shown to neutralize ∼108 virions in the athymic mouse xenograft assay (7).
Luminex data analysis.
Relative inhibition of MAb-PE binding was compared to a standard curve by using a four-parameter logistic curve fit (26). The standard reference sera used for the standard curve were assigned arbitrary values expressed in mMU/milliliter. The cutoff values were determined relative to the standard curve by using multiple assays of preimmune African green monkey or chimpanzee serum from monkeys or from uninfected women with a low risk of virus exposure (18- to 25-year-old women with no history of abnormal results of cervical cytological analysis or genital warts). A subset of these HPV-negative serum donors were also shown to be negative for HPV-6, -11, -16, and -18 DNA at four anogenital sites). Data, in median fluorescent intensity (MFI) units, was processed in a Microsoft Excel spreadsheet (Microsoft, Redmond, Wash.). Error bars represent the standard deviation of duplicate measurements. The Pearson correlation coefficient was used to compare the Luminex antibody titer results with antibody titers obtained in the HPV-cRIA.
RESULTS
HPV-Luminex immunoassay.
A quantitative, competitive immunoassay was developed by using LabMAP3 technology to evaluate HPV type-specific antibody titers to native HPV-6, -11, -16, and -18 VLPs. The principle of the immunoassay is that HPV type-specific antibodies in patient sera prevent binding of fluorescently labeled, HPV type-specific, neutralizing detection MAbs (Fig. 1). The fluorescent signal from the bound HPV-specific, detection MAbs is inversely proportional to the patients' neutralizing antibody titer (Fig. 1). The results reflect titers of antibody capable of preventing binding of MAbs to conformationally sensitive, type-specific, neutralizing epitopes on the respective VLPs. HPV type-specific antibody responses are detected simultaneously by analyzing the reported PE fluorescence signal associated with the different microspheres that have distinct spectral properties. The type-specific MAbs had all previously been shown to neutralize the respective HPVs in either the athymic mouse xenograft system or in pseudoneutralization assays and do not cross-react with the other HPV genotypes (10, 11, 33, 42). The specific spectral addresses of the Luminex microspheres and the HPV type specificity of the detection MAbs allowed us to develop a competitive immunoassay that simultaneously measured type-specific antibodies to neutralizing epitopes on HPV-6, -11, -16, and -18.
FIG. 1.
HPV-Luminex multiplex competitive immunoassay. The immunoassay quantitatively measures the ability of HPV type-specific antibodies in patient serum to prevent the binding of fluorescently labeled (with PE), HPV type-specific detection MAbs. The fluorescent signal from bound HPV-specific, detection MAbs is inversely proportional to the patient’s neutralizing antibody titer.
Covalent coupling of HPV VLPs to Luminex microspheres.
HPV VLP vaccines have been shown to induce type-specific, neutralizing antibodies in both humans and animals. However, denatured VLPs fail to elicit neutralizing antibodies, demonstrating that conformationally sensitive epitopes on the VLPs are critical for inducing an effective immune response. Additionally, the conformational integrity of the VLPs is sensitive to reducing conditions, pH, and ionic conditions (22). We therefore examined the effect of covalently coupling the four different VLPs to the Luminex microspheres and the effect of storing the VLP-microspheres in three different buffers on the conformational integrity of the VLPs. To measure the conformational integrity of the VLPs, we monitored the ability of the type-specific MAbs to bind to the respective VLPs over a period of 6 months. The VLP-microspheres were stored in the dark at 4°C in either PBS, Column A buffer, or histidine buffer in the presence or absence of 1% BSA. Representative data for H16.V5 binding to VLP-16 is shown in Fig. 2. Analysis of the average MFI values (Fig. 2A) and the coefficient of variation (Fig. 2B) of 32 replicate samples measured over a 6-month period revealed that binding of the H16.V5 MAb to VLP-16 was not adversely affected by the coupling reaction and that the conformational integrity of the VLPs attached to the Luminex microspheres was intact for at least 6 months when stored at 4°C in buffers containing 1% BSA (Fig. 2A). However, at the month 0 and month 1 time points, there was decreased binding of H16.V5 to VLP-16 when VLP-16 microspheres were stored in buffers in the absence of BSA (Fig. 2A). Interestingly, H16.V5 binding to VLP-16 microspheres stored in the absence of 1% BSA improved with time (Fig. 2A). Importantly, the percent coefficient of variation (%CV) values for the VLP-16 microspheres (Fig. 2B) and the other HPV-types (data not shown) stored in 1% BSA were all <10% over the 6-month period. These results demonstrated that L1 VLPs expressed from S. cerevisiae could safely be coupled to Luminex microspheres without affecting the type-specific, neutralizing epitopes on L1.
FIG. 2.
Stability of HPV-VLP-16 coupled to Luminex microspheres. The stability of HPV-6, -11, -16, and -18 VLPs coupled to Luminex microspheres was examined for a period of 6 months. VLP-microspheres were stored at 4°C in the dark in PBS, Column A buffer (COL.A), or histidine buffer (HIST) in the presence or absence of 1% BSA. MFI values for H16.V5-PE binding to VLP-16 (A) and %CV values for MFI values of 32 replicate samples (B) measured at 1-month intervals. Open symbols represent storage in the absence of 1% BSA, and closed symbols represent storage conditions in the presence of 1% BSA, respectively. Representative data for VLP-16 coupled to microsphere 38 is shown. The MFI for 32 replicate samples was averaged, and the %CV determined. The %CV was <10% for all four HPV-VLP types stored in buffers containing 1% BSA.
Simplex and multiplex HPV standard curves.
To determine whether the individual HPV immunoassays could be multiplexed, we examined the four HPV standard sera singly or pooled together in the immunoassay in single and multiplex format (Fig. 3). A 12-point standard curve was run in duplicate in both the single (simplex) and the multiplex formats. As mentioned, the four MAbs used for detecting HPV antibody responses bind to HPV type-specific epitopes and do not cross-react with the other genotypes (8, 10, 11, 44). Pooling the four standards together had a minimal effect on the HPV-11, HPV-16, and HPV-18 standard curves (Fig. 3B, C, and D, respectively). However, multiplexing the assay slightly affected the HPV-6 standard curve (Fig. 3A). The analytical limits of quantitation for the different standard curves in the multiplex assay were determined for HPV-6 (1.2 to 54.8 mMU/ml), HPV-11 (9.8 to 365.6 mMU/ml), HPV-16 (4.5 to 476.5 mMU/ml), and HPV-18 (11.3 to 203.0 mMU/ml), which were comparable to the limits of quantitation determined for the cRIA (Table 1). The precision of the HPV-Luminex immunoassay was determined with eight replicate low-, medium-, and high-titer serum samples (data not shown). For within-run precision, the %CV values ranged from 4.3 to 4.5% for HPV-6, 3.6 to 7.4% for HPV-11, 7.4 to 22.0% for HPV-16, and 2.1 to 23.9% for HPV-18. These studies indicated that the Luminex assay could serve as a sensitive and precise multiplex assay to quantitate antibodies to neutralization epitopes on HPV-6, -11, -16, and -18.
FIG. 3.
Simplex and multiplex standard curves for HPV-6, -11, -16, and -18. Representative standard curves for a 12-point dilution series of different African green monkey sera are presented for HPV-VLP-6 (A), HPV-VLP-11 (B), and HPV-VLP-18 (D) and of chimpanzee sera for HPV-VLP-16 (C). A simplex curve depicts a single standard serum run in the assay with only one VLP-microsphere type present, and multiplex curves depict a quadrivalent standard with all four VLP-microspheres present in a single well. Error bars represent the standard deviation of duplicate samples.
TABLE 1.
Limits of detection and limits of quantitation for the competitive HPV RIAs and multiplex Luminex immunoassays
| Limit | Limits of detection or quantitation (m MU/ml) of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| HPV-6
|
HPV-11
|
HPV-16
|
HPV-18
|
|||||
| RIA | Luminex assay | RIA | Luminex assay | RIA | Luminex assay | RIA | Luminex assay | |
| Limit of detection | 2.7 | 0.8 | 2.7 | 1.0 | 0.4 | 1.6 | 3.9 | 5.3 |
| Lower limit of quantitation | 8.0 | 1.2 | 13.0 | 9.8 | 6.0 | 4.5 | 13.0 | 11.3 |
| Upper limit of quantitation | 83.0 | 54.8 | 130.0 | 365.6 | 130.0 | 476.5 | 130.0 | 203.0 |
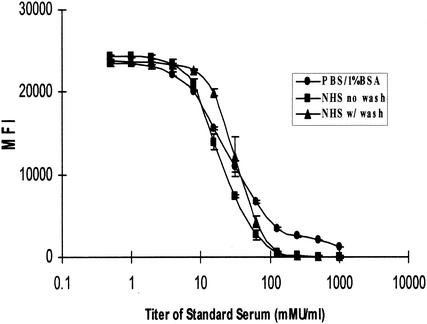
We also examined the effect of several different assay diluents on the immunoassay and the effect of adding a filtration-wash step to the procedure. Performing the assay in either a serum or a PBS-1% BSA matrix gave similar results (Fig. 4), suggesting that patient serum could be diluted into a PBS-1% BSA sample diluent for high-titer samples. The addition of a filtration-wash step did not significantly affect the standard curves (Fig. 4) but did significantly improve read times from 80 to 100 min per plate to 30 to 40 min per plate. The optimized assay was performed in a serum NHS matrix, and the samples were transferred to a filter plate that was washed three times in PBS-1% BSA before being placed on the Luminex100.
FIG. 4.
Effect of serum and different assay buffers on the HPV-Luminex assay. A standard curve serum from a VLP-11-immunized African green monkey was analyzed in various assay diluent buffers. Twofold serial dilutions of a stock standard into HPV-negative NHS was performed to create a 12-point standard curve. The standard serum was analyzed in PBS-1% BSA or HPV-negative NHS. Standard serum in an NHS diluent was analyzed in both a wash format and a no-wash format. Error bars represent the standard deviation of duplicate samples.
HPV-Luminex immunoassay shows concordance to the cRIAs.
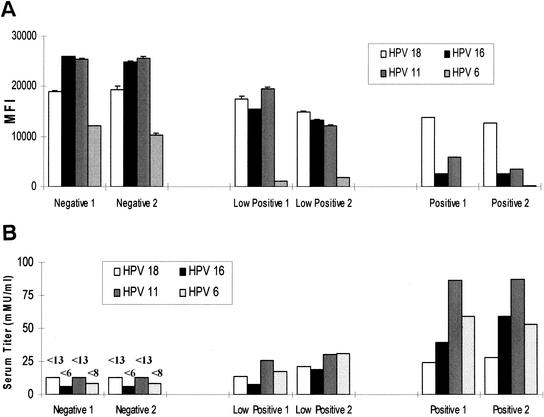
To determine the accuracy of the Luminex assay to the currently employed individual cRIAs, we tested a panel of 45 human sera twice in both assay formats. The sera were from HPV-negative individuals enrolled in a quadrivalent HPV-VLP (types 6, 11, 16, and 18) vaccine clinical trial pre- and postvaccination. A total of 15 negative and 15 low-, 10 medium-, and 5 high-titer samples to all four genotypes were run in duplicate in both assay formats, the titers were measured, and the relative concordance between the two assays was determined. A representative set of two samples that were negative, low positive, or positive for antibodies to HPV-6, -11, -16, and -18 shows the inverse relationship between the MFI (Fig. 5A) and the calculated serum titer in mMU/milliliter (Fig. 5B) in the HPV-Luminex assay. Representative titers in mMU/milliliter for the HPV-16 cRIA and Luminex assays are shown (Fig. 5C). Comparing the titers determined in both assay formats revealed that the two assays showed good concordance. Specifically, the Pearson correlation coefficients (R2) between the two assay formats were greater than 0.75 (HPV-6, 0.837; HPV-11, 0.751; HPV-16, 0.768; and HPV-18, 0.775), suggesting good agreement between the two assays. In addition, the HPV-Luminex assay had a 0% false-positive rate and a 0% false-negative rate compared to the cRIAs. In summary, there was good agreement between the Luminex and cRIA assay formats, suggesting that the multiplex Luminex assays could be used in addition to or as a replacement for cRIAs.
FIG. 5.
Calculated HPV type-specific, antibody titers. (A) MFI values for two representative HPV-negative, -low-positive, or -seropositive samples. (B) mMU/milliliter values for the same two representative samples shown in panel A. Error bars represent the standard deviation of duplicate samples. (C) Comparison of titers determined in the HPV-16 cRIA with HPV-16 titers from a multiplex Luminex competitive immunoassay. HPV type-specific antibody titers for 45 samples were determined by cRIA and Luminex immunoassays. Antibody titers were determined for 15 HPV-negative and 15 low-, 10 medium-, and 5 high-titer serum samples. The cRIA and Luminex assays were both performed in duplicate, and the average of the duplicate runs were compared for intra-assay precision and interassay accuracy. Average mMU/milliliter values for the HPV-16 cRIA and Luminex assays are shown. Titers were calculated from standard curves by using a four-parameter logistic curve fit.
DISCUSSION
A common goal for all vaccine programs is to identify clinically relevant correlates of protection including, specifically, laboratory parameter(s) that have been shown to be associated with protection from clinical disease. For many infectious diseases, neutralization antibody assays are used to assess the immunogenicity of prophylactic vaccines. For HPVs, the “gold standard” for measuring neutralizing antibody titers is the athymic mouse xenograft system (7, 20). In this assay, sera from individuals is mixed with infectious HPV and added to foreskin tissue, which is then implanted under the renal capsule of athymic mice. The implants are monitored for histological changes and for HPV DNA by in situ hybridization, which can take several months to develop. Because of the technical difficulties in testing a large number of sera in the athymic mouse xenograft system (7, 20), several complementary assays have been developed to measure neutralizing and non-neutralizing antibody titers. These include in vitro pseudoneutralization assays (7, 34), cRIAs (28), and VLP-based enzyme-linked immunosorbent assays (43). However, studies have shown that the pseudoneutralization assay can be 20- to 30-fold less sensitive than HPV-VLP enzyme-linked immunosorbent assays (24) and up to a 100-fold less sensitive than a cRIA (28). The pseudoneutralization assay is also a complex cell-based assay that requires dilution of the serum sample to avoid interference (44). Since an immunoassay that measures HPV type-specific antibodies simultaneously is preferred to running multiple separate tests, we employed the Luminex LabMAP3 technology to develop a competitive immunoassay that simultaneously measures type-specific antibodies to neutralizing epitopes on HPV-6, -11, -16, and -18.
The rationale for developing a multiplexed competitive immunoassay, rather than a capture assay, was that capture assays to VLPs measure a combination of antibodies to both neutralizing and non-neutralizing epitopes and, potentially, other antibodies to yeast-derived proteins. Other advantages of the competitive assay format are that the serum sample does not need to be diluted and the assay measures antibodies specific to HPV type-specific neutralizing epitopes. Multiplexing the assay had little to no effect on the standard curves for HPV-11, -16, and -18 but did affect the HPV-6 standard curve by shifting the curve to the left (Fig. 3A). This effect on the HPV-6 curve was most likely due to the cross-reactivity of the HPV-11 sera to the HPV-6 VLP. This cross-reactivity was not unexpected, since HPV-6 and HPV-11 have 93% amino acid identity in the L1 protein (9) and animals or individuals immunized with VLP-11 can neutralize HPV-6 (44). Nevertheless, the HPV-Luminex assay showed good correlation with the competitive HPV type-specific RIAs (Pearson correlation coefficients of between 0.751 and 0.837). It is also noted that changing the number of microspheres analyzed did not affect the MFI values (data not shown), which confirms the observation by Vignali (40). Since the HPV-Luminex assay correlated well with the currently used cRIAs, the Luminex assay should be a useful tool for analyzing antibody titers to different HPV genotypes.
The main advantage of the LabMAP3 technology is that it provides a sensitive and precise method to simultaneously measure antibodies to HPV type-specific, neutralizing epitopes against multiple HPV genotypes. Because multiple tests can be run on a single serum sample, LabMAP3 technology has the advantage of being ideal for use on pediatric samples for which serum volumes are often limiting. This has been shown previously by Pickering et al., who used the Luminex technology to simultaneously quantitate antibodies to 14 different pneumococcus polysaccharides in pneumococcal vaccine studies (30). Bellisario et al. have also used Luminex technology to simultaneously measure antibodies to human immunodeficiency virus type 1 p24, gp160, and gp120 eluted from dried blood-spot specimens from newborns (2). Luminex LabMAP3 technology has also been used to test serum samples for antibodies to a panel of seven respiratory viruses, including influenza A and B viruses; adenovirus; parainfluenza viruses 1, 2, and 3; and respiratory syncytial virus (21). In this respiratory panel Luminex assay, internal controls were also added to test for rheumatoid factor and sufficient quantities of IgG to assure sample addition (21). Because an effective HPV vaccine may include additional genotypes to cover more of the known cancer-causing viruses, LabMAP3 technology provides a platform for adding additional HPV genotypes to the assay. In addition to the flexibility of adding other VLP genotypes to the assay, Luminex platform technology provides the ability to add other assays, such as viral load measurements or cytokine assays, to the same test.
Although the assay described here included a wash step, a number of investigators have developed rapid, no-wash Luminex assays that can be performed more quickly (27). A no-wash assay would certainly be advantageous, especially since the sensitivity and the precision of the HPV-Luminex assay were not compromised (Fig. 4). However, the high concentrations of serum appeared to clog the Luminex100 and caused many of the VLP-microspheres to fall outside their specified calibration gates, lengthening the read times to greater than 1 h. An advantage of the 96-well plate Luminex assay format is that it avails itself to automation. We have used a Tecan Genesis liquid handler to automate the assay, which has saved time and has the added value of preventing accidental exposure of laboratory personal to potentially infectious serum. In summary, LabMAP3 technology provided a sensitive and robust system to develop a competitive immunoassay that accurately and precisely measures antibodies to neutralizing epitopes on HPV-6, -11, -16, and -18.
Acknowledgments
We thank James Drummond, Rocio Marchese, and Sonela Schlottmann for critical review of the manuscript; Robin Mogg for statistical analysis; and Kerry Oliver and Michael Sorial of Luminex for technical assistance.
REFERENCES
- 1.Baker, T. S., W. W. Newcomb, N. H. Olson, L. M. Cowsert, C. Olson, and J. C. Brown. 1991. Structures of bovine and human papillomaviruses: analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys. J. 60:1445-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellisario, R., R. J. Colinas, and K. A. Pass. 2001. Simultaneous measurement of antibodies to three HIV-1 antigens in newborn dried blood-spot specimens using a multiplexed microsphere-based immunoassay. Early Hum. Dev. 64:21-25. [DOI] [PubMed] [Google Scholar]
- 3.Bosch, F. X., M. M. Manos, N. Munoz, M. Sherman, A. M. Jansen, J. Peto, M. H. Schiffman, V. Moreno, R. Kurman, and K. V. Shah. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J. Natl. Cancer Inst. 87:796-802. [DOI] [PubMed] [Google Scholar]
- 4.Breitburd, F., and P. Coursaget. 1999. Human papillomavirus vaccines. Semin. Cancer Biol. 9:431-444. [DOI] [PubMed] [Google Scholar]
- 5.Breitburd, F., R. Kirnbauer, N. L. Hubbert, B. Nonnenmacher, C. Trin-Dinh-Desmarquet, G. Orth, J. T. Schiller, and D. R. Lowy. 1995. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69:3959-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, D. R., J. T. Bryan, J. M. Schroeder, T. S. Robinson, K. H. Fife, C. M. Wheeler, E. Barr, P. R. Smith, L. Chiacchierini, A. DiCello, and K. U. Jansen. 2001. Neutralization of human papillomavirus type 11 (HPV-11) by serum from women vaccinated with yeast-derived HPV-11 L1 virus-like particles: correlation with competitive radioimmunoassay titer. J. Infect. Dis. 184:1183-1186. [DOI] [PubMed] [Google Scholar]
- 7.Bryan, J. T., K. U. Jansen, R. S. Lowe, K. H. Fife, T. McClowry, D. Glass, and D. R. Brown. 1997. Human papillomavirus type 11 neutralization in the athymic mouse xenograft system: correlation with virus-like particle IgG concentration. J. Med. Virol. 53:185-188. [DOI] [PubMed] [Google Scholar]
- 8.Christensen, N. D., J. Dillner, C. Eklund, J. J. Carter, G. C. Wipf, C. A. Reed, N. M. Cladel, and D. A. Galloway. 1996. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 223:174-184. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, N. D., R. Kirnbauer, J. T. Schiller, S. J. Ghim, R. Schlegel, A. B. Jenson, and J. W. Kreider. 1994. Human papillomavirus types 6 and 11 have antigenically distinct strongly immunogenic conformationally dependent neutralizing epitopes. Virology 205:329-335. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, N. D., J. W. Kreider, N. M. Cladel, S. D. Patrick, and P. A. Welsh. 1990. Monoclonal antibody-mediated neutralization of infectious human papillomavirus type 11. J. Virol. 64:5678-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen, N. D., C. A. Reed, N. M. Cladel, K. Hall, and G. S. Leiserowitz. 1996. Monoclonal antibodies to HPV-6 L1 virus-like particles identify conformational and linear neutralizing epitopes on HPV-11 in addition to type-specific epitopes on HPV-6. Virology 224:477-486. [DOI] [PubMed] [Google Scholar]
- 12.Cook, J. C., J. G. Joyce, H. A. George, L. D. Schultz, W. M. Hurni, K. U. Jansen, R. W. Hepler, C. Ip, R. S. Lowe, P. M. Keller, and E. D. Lehman. 1999. Purification of virus-like particles of recombinant human papillomavirus type 11 major capsid protein L1 from Saccharomyces cerevisiae. Protein Expr. Purif. 17:477-484. [DOI] [PubMed] [Google Scholar]
- 13.Fulton, R. J., R. L. McDade, P. L. Smith, L. J. Kienker, and J. R. Kettman, Jr. 1997. Advanced multiplexed analysis with the FlowMetrix system. Clin. Chem. 43:1749-1756. [PubMed] [Google Scholar]
- 14.Hagensee, M. E., N. Yaegashi, and D. A. Galloway. 1993. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J. Virol. 67:315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann, K. J., J. C. Cook, J. G. Joyce, D. R. Brown, L. D. Schultz, H. A. George, M. Rosolowsky, K. H. Fife, and K. U. Jansen. 1995. Sequence determination of human papillomavirus type 6a and assembly of virus-like particles in Saccharomyces cerevisiae. Virology 209:506-518. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann, K. J., M. P. Neeper, H. Z. Markus, D. R. Brown, M. Muller, and K. U. Jansen. 1996. Sequence conservation within the major capsid protein of human papillomavirus (HPV) type 18 and formation of HPV-18 virus-like particles in Saccharomyces cerevisiae. J. Gen. Virol. 77:465-468. [DOI] [PubMed] [Google Scholar]
- 17.Jarrett, W. F., K. T. Smith, B. W. O'Neil, J. M. Gaukroger, L. M. Chandrachud, G. J. Grindlay, G. M. McGarvie, and M. S. Campo. 1991. Studies on vaccination against papillomaviruses: prophylactic and therapeutic vaccination with recombinant structural proteins. Virology 184:33-42. [DOI] [PubMed] [Google Scholar]
- 18.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 89:12180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutsky, L. A., K. K. Holmes, C. W. Critchlow, C. E. Stevens, J. Paavonen, A. M. Beckmann, T. A. DeRouen, D. A. Galloway, D. Vernon, and N. B. Kiviat. 1992. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N. Engl. J. Med. 327:1272-1278. [DOI] [PubMed] [Google Scholar]
- 20.Kreider, J. W., M. K. Howett, S. A. Wolfe, G. L. Bartlett, R. J. Zaino, T. Sedlacek, and R. Mortel. 1985. Morphological transformation in vivo of human uterine cervix with papillomavirus from condylomata acuminata. Nature 317:639-641. [DOI] [PubMed] [Google Scholar]
- 21.Martins, T. B. 2002. Development of internal controls for the Luminex instrument as part of a multiplex seven-analyte viral respiratory antibody profile. Clin. Diagn. Lab. Immunol. 9:41-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy, M. P., W. I. White, F. Palmer-Hill, S. Koenig, and J. A. Suzich. 1998. Quantitative disassembly and reassembly of human papillomavirus type 11 viruslike particles in vitro. J. Virol. 72:32-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGlennen, R. C. 2000. Human papillomavirus oncogenesis. Clin. Lab. Med. 20:383-406. [PubMed] [Google Scholar]
- 24.Nardelli-Haefliger, D., R. Roden, C. Balmelli, A. Potts, J. Schiller, and P. De Grandi. 1999. Mucosal but not parenteral immunization with purified human papillomavirus type 16 virus-like particles induces neutralizing titers of antibodies throughout the estrous cycle of mice. J. Virol. 73:9609-9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neeper, M. P., K. J. Hofmann, and K. U. Jansen. 1996. Expression of the major capsid protein of human papillomavirus type 11 in Saccharomyces cerevisiae. Gene 180:1-6. [DOI] [PubMed] [Google Scholar]
- 26.O'Connell, M., B. Belanger, and P. Haaland. 1992. The four-parameter logistic model for calibration and assay development. Am. Stat. Assoc. Proc. Biopharm. Section 1992:180-185. [Google Scholar]
- 27.Oliver, K. G., J. R. Kettman, and R. J. Fulton. 1998. Multiplexed analysis of human cytokines by use of the FlowMetrix system. Clin. Chem. 44:2057-2060. [PubMed] [Google Scholar]
- 28.Palker, T. J., J. M. Monteiro, M. M. Martin, C. Kakareka, J. F. Smith, J. C. Cook, J. G. Joyce, and K. U. Jansen. 2001. Antibody, cytokine and cytotoxic T lymphocyte responses in chimpanzees immunized with human papillomavirus virus-like particles. Vaccine 19:3733-3743. [DOI] [PubMed] [Google Scholar]
- 29.Phelps, W. C., and K. A. Alexander. 1995. Antiviral therapy for human papillomaviruses: rational and prospects. Ann. Intern. Med. 123:368-382. [DOI] [PubMed] [Google Scholar]
- 30.Pickering, J. W., T. B. Martins, R. W. Greer, M. C. Schroder, M. E. Astill, C. M. Litwin, S. W. Hildreth, and H. R. Hill. 2002. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am. J. Clin. Pathol. 117:589-596. [DOI] [PubMed] [Google Scholar]
- 31.Pisani, P., D. M. Parkin, and J. Ferlay. 1993. Estimates of the worldwide mortality from eighteen major cancers in 1985. Implications for prevention and projections of future burden. Int. J. Cancer 55:891-903. [DOI] [PubMed] [Google Scholar]
- 32.Remmink, A. J., J. M. Walboomers, T. J. Helmerhorst, F. J. Voorhorst, L. Rozendaal, E. K. Risse, C. J. Meijer, and P. Kenemans. 1995. The presence of persistent high-risk HPV genotypes in dysplastic cervical lesions is associated with progressive disease: natural history up to 36 months. Int. J. Cancer 61:306-311. [DOI] [PubMed] [Google Scholar]
- 33.Roden, R. B., A. Armstrong, P. Haderer, N. D. Christensen, N. L. Hubbert, D. R. Lowy, J. T. Schiller, and R. Kirnbauer. 1997. Characterization of a human papillomavirus type 16 variant-dependent neutralizing epitope. J. Virol. 71:6247-6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roden, R. B., H. L. Greenstone, R. Kirnbauer, F. P. Booy, J. Jessie, D. R. Lowy, and J. T. Schiller. 1996. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J. Virol. 70:5875-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi, J. L., L. Gissmann, K. Jansen, and M. Muller. 2000. Assembly of human papillomavirus type 16 pseudovirions in Saccharomyces cerevisiae. Hum. Gene Ther. 11:1165-1176. [DOI] [PubMed] [Google Scholar]
- 36.Sasagawa, T., P. Pushko, G. Steers, S. E. Gschmeissner, M. A. Hajibagheri, J. Finch, L. Crawford, and M. Tommasino. 1995. Synthesis and assembly of virus-like particles of human papillomaviruses type 6 and type 16 in fission yeast Schizosaccharomyces pombe. Virology 206:126-135. [DOI] [PubMed] [Google Scholar]
- 37.Schiffman, M. H., H. M. Bauer, R. N. Hoover, A. G. Glass, D. M. Cadell, B. B. Rush, D. R. Scott, M. E. Sherman, R. J. Kurman, S. Wacholder, et al. 1993. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J. Natl. Cancer Inst. 85:958-964. [DOI] [PubMed] [Google Scholar]
- 38.Staros, J. V., R. W. Wright, and D. M. Swingle. 1986. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal. Biochem. 156:220-222. [DOI] [PubMed] [Google Scholar]
- 39.Suzich, J. A., S. J. Ghim, F. J. Palmer-Hill, W. I. White, J. K. Tamura, J. A. Bell, J. A. Newsome, A. B. Jenson, and R. Schlegel. 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. USA 92:11553-11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vignali, D. A. 2000. Multiplexed particle-based flow cytometric assays. J. Immunol. Methods 243:243-255. [DOI] [PubMed] [Google Scholar]
- 41.Walboomers, J. M., M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. Snijders, J. Peto, C. J. Meijer, and N. Munoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12-19. [DOI] [PubMed] [Google Scholar]
- 42.White, W. I., S. D. Wilson, F. J. Palmer-Hill, R. M. Woods, S. J. Ghim, L. A. Hewitt, D. M. Goldman, S. J. Burke, A. B. Jenson, S. Koenig, and J. A. Suzich. 1999. Characterization of a major neutralizing epitope on human papillomavirus type 16 L1. J. Virol. 73:4882-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wideroff, L., M. H. Schiffman, B. Nonnenmacher, N. Hubbert, R. Kirnbauer, C. E. Greer, D. Lowy, A. T. Lorincz, M. M. Manos, A. G. Glass, et al. 1995. Evaluation of seroreactivity to human papillomavirus type 16 virus-like particles in an incident case-control study of cervical neoplasia. J. Infect. Dis. 172:1425-1430. [DOI] [PubMed] [Google Scholar]
- 44.Yeager, M. D., M. Aste-Amezaga, D. R. Brown, M. M. Martin, M. J. Shah, J. C. Cook, N. D. Christensen, C. Ackerson, R. S. Lowe, J. F. Smith, P. Keller, and K. U. Jansen. 2000. Neutralization of human papillomavirus (HPV) pseudovirions: a novel and efficient approach to detect and characterize HPV neutralizing antibodies. Virology 278:570-577. [DOI] [PubMed] [Google Scholar]
- 45.Zhou, J., X. Y. Sun, D. J. Stenzel, and I. H. Frazer. 1991. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 185:251-257. [DOI] [PubMed] [Google Scholar]
- 46.zur Hausen, H. 1991. Viruses in human cancers. Science 254:1167-1173. [DOI] [PubMed] [Google Scholar]