Abstract
To differentiate infectious endocarditis (IE) from other Bartonella infections and to identify infecting Bartonella bacteria at the species level on a serological basis, we used Western immunoblotting to test sera from 51 patients with Bartonella IE (of which 27 had previously benefited from species identification by molecular techniques), 11 patients with chronic Bartonella quintana bacteremia, and 10 patients with cat scratch disease. Patients with IE were Western blot positive in 49 of 51 cases, and significant cross-reactivity with three heterologous Bartonella antigens was found in 45 of 49 cases. Sera from bacteremic patients did not react with more than one heterologous antigen, and sera from patients with cat scratch disease gave negative results. Sera reacted only with B. henselae in four cases of IE, including one with a positive PCR result for valve tissue. Western blot and cross-adsorption performed on serum samples from patients with IE (the identity of the causative species having been determined by PCR) were demonstrated to identify efficiently the causative species in all cases. When applied to patients diagnosed on the basis of serological tests only, this technique allowed identification of the causative species in 20 of 22 cases. The results were in accordance with epidemiological features. Six reactive bands of B. quintana (of molecular sizes from 10 to 83 kDa) demonstrated significant association with sera from patients with B. quintana endocarditis. Overall, Western blotting and cross-adsorption made it possible to identify the causative species in 49 of 51 (96%) IE cases.
Bartonella spp. are gram-negative, short-rod bacteria belonging to the α2 subclass of Proteobacteria. Common features of Bartonella include transmission by an arthropod vector and persistence within mammalian reservoir hosts (24). Seven species have been implicated in human diseases (15, 24), and four have been associated with infectious endocarditis (IE) in people: Bartonella quintana, B. henselae, B. elizabethae, and B. vinsonii subsp. berkhoffii. B. quintana, the agent of trench fever, and B. henselae, the main agent of cat scratch disease, are responsible for most reported cases of Bartonella IE (29), although they have also been implicated in persistent asymptomatic bacteremia and in bacillary angiomatosis (24). There are only single reports of IE caused by B. elizabethae and B. vinsonii subsp. berkhoffii (5, 32). The variety of Bartonella spp. that can cause IE means that diagnostic tools for the identification of the agents to the species level are required. Culturing of these fastidious organisms is difficult, however, especially for those found in samples from patients already being treated with antimicrobials (18). Molecular identification by PCR amplification and sequencing of the 16S rRNA or the citrate synthase-encoding genes is best performed on surgically excised infected valves and is less sensitive when performed on peripheral blood (24, 28). Serological testing, especially the indirect immunofluorescent antibody (IFA) assay, remains the most commonly used diagnostic test and is frequently the only available means for the laboratory diagnosis of Bartonella endocarditis. An immunoglobulin G (IgG) titer of ≥1:800 for either B. quintana or B. henselae has been shown to have a positive predictive value (PPV) of 95.5% for detection of Bartonella etiology in patients with IE (9).
Serological testing avoids many of the problems associated with other methods, such as lengthy incubation periods, collection of samples by invasive means, or the requirement of specialized equipment (2). Nevertheless, it is hampered considerably by cross-reactivity among Bartonella species and also between Bartonella spp. and Chlamydia spp. or Coxiella burnetii (17, 25). As suggested by Maurin et al. (25), who diagnosed Bartonella infections in 10 patients incorrectly diagnosed as having chlamydial endocarditis, cross-adsorption and Western immunoblotting may be useful in making etiological diagnoses and overcoming confusing cross-reactivity. Cross-adsorption is performed by incubating serum from a patient with the bacterium known to cross-react in serological tests. Cross-adsorption results in the disappearance of homologous and heterologous antibodies when adsorption is performed with the bacterium causing the disease. When it is performed with the bacterium that did not cause the disease but that was responsible for the cross-reaction, antibodies reactive to this bacterium disappear but other antibodies, reactive with the bacterium causing the disease, remain detectable. Antigenic cross-reactivity is confirmed by Western immunoblotting after adsorption of sera with cross-reacting antigens.
The aim of our study was to compare the serological responses to B. quintana and B. henselae in patients with IE and the other diseases caused by these organisms. Also, we attempted to identify species-specific epitopes which would enable us to differentiate B. quintana infections from B. henselae infections in patients with endocarditis. We established our identification criteria in a series of 27 patients with IE and an identified Bartonella sp. and applied these criteria to 24 cases of IE diagnosed by serological tests.
MATERIALS AND METHODS
Patients and sera.
Based on Duke criteria (19), we selected patients with definite IE. Of these cases, the infecting agents in 27 were identified to the species level by culture or PCR (8), including those of 22 patients with B. quintana infections and 5 patients with B. henselae. Twenty-four patients with definite IE showed serum Bartonella IgG titers of ≥1:800 as the only etiologic evidence, as previously reported (9). As negative controls, we selected 11 homeless patients presenting with chronic B. quintana bacteremia and 10 patients with cat scratch disease; the members of both groups were free of IE but had high Bartonella IgG titers (≥1:200). A diagnosis of B. quintana bacteremia was made on the basis of a positive blood culture and transesophageal echocardiography results showing no signs of IE or valve lesions (4). The diagnosis of cat scratch disease was confirmed by the molecular detection by a seminested PCR technique of B. henselae in lymph node biopsy specimens (34). Clinical manifestations were obtained according to the results of a questionnaire as previously reported (10). Ten healthy blood donors were also tested.
Antigen preparation.
Bacteria of the four Bartonella species that have been associated with human endocarditis were used as antigens. The reference strains B. quintana Oklahoma (ATCC VR-51-694) (3), B. henselae Houston-1 (ATCC 49882) (31), B. vinsonii subsp. berkhoffii 93-CO1 (ATCC 51672) (16), and B. elizabethae F9251 (ATCC 49927) (5) were grown on blood agar (Biomérieux, Marcy l'Etoile, France) at 37°C in a 5% carbon dioxide incubator. After a 7-day incubation, bacteria were harvested and suspended in sterile distilled water prior to being frozen at −20°C. They were unlikely to possess pili.
Serum cross-adsorption.
Tested sera (20 ml) were diluted 1:50 with B. quintana and B. henselae antigen suspensions adjusted to contain 2 mg of protein/ml in a buffered saline solution (TBS; 20 mM Tris-HCl [pH 7.5], 500 mM NaCl, 0.1% merthiolate). The mixtures were shaken for 24 h at 4°C and centrifuged at 10,000 × g for 10 min. B. quintana and B. henselae serological tests were performed on all supernatants. Microimmunofluorescence (MIF) assays were performed with total Ig (Fluoline H; Biomérieux).
Western blot analysis.
B. quintana, B. henselae, B. vinsonii subsp. berkhoffii, and B. elizabethae cells were suspended in sterile distilled water and adjusted to 2 mg of protein/ml spectrophotometrically. A total of 2 volumes of antigen was mixed with 1 volume of 3× Laemmli solubilizer as previously reported (25), and the mixture was boiled for 15 min. Twenty microliters of the preparation was electrophoresed at 100 V for 2 h through 12% polyacrylamide separating gels with 4% polyacrylamide stacking gels with a Mini-Protean II cell apparatus (Bio-Rad, Hercules, Calif.). A mixture of prestained molecular mass standards (Kaleidoscope; Bio-Rad) was used to estimate the molecular masses of the separated antigens. Resolved antigens were then transferred to a 0.45-μm-pore-size nitrocellulose membrane (Bio-Rad) for 1 h at 4°C and 100 V. The blots were blocked overnight at 4°C with 5% nonfat milk powder in TBS buffer and washed with distilled water. Sera (diluted 1:200 in TBS-3% nonfat milk powder) were applied to the blots for 1 h at room temperature. After three 10-min washes in TBS-3% nonfat milk powder, the blots were incubated for 1 h with peroxidase-conjugated goat anti-human IgG and IgM (Southern Biotechnology Associates Inc., Birmingham, Ala.) diluted 1:750 in TBS-3% nonfat milk powder. The blots were washed three times in TBS, and bound conjugate was revealed by incubation with a solution consisting of 0.015% 4-chloro-1-naphthol (Sigma, St. Louis, Mo.) and 0.015% hydrogen peroxide in TBS-16.7% methanol for 15 min. Western blot analysis was performed both before and after cross-adsorption. The blots were assessed blindly and were always assessed by the same individual to minimize any variation in the interpretation. For 13 randomly selected serum samples (8 serum samples from patients with IE, 3 from bacteremic patients, and 2 from cat scratch disease patients), Western immunoblotting was performed twice on separate occasions to assess reproducibility.
Statistical analysis.
Using an Epi Info program (6), qualitative data were compared by the Mantel-Haenszel method or the Fisher exact test. To validate the results from the serological tests, epidemiological features of patients with B. quintana endocarditis were compared with those of patients with B. henselae endocarditis. Comparisons were performed separately for the 27 cases diagnosed by culture and/or PCR analyses of valve biopsy specimens and for the cases diagnosed by serological tests only. A difference was considered significant when the P value was <0.05. The reaction profiles obtained by Western blotting with sera from patients with B. quintana and B. henselae endocarditis were compared with those obtained with sera from patients with persistent B. quintana bacteremia and cat scratch disease to determine specific profiles for IE. Also, the profiles obtained with sera from B. quintanta endocarditis patients were compared with those obtained with sera from B. henselae endocarditis patients to identify species-specific patterns in patients with IE. For the 51 patients with IE, PPVs for species-specific bands were calculated as the proportion of patients infected with a Bartonella species (true positive) whose sera reacted with a specified band (true and false positive) in Western blots.
RESULTS
Western blot analysis results for two or more proteins.
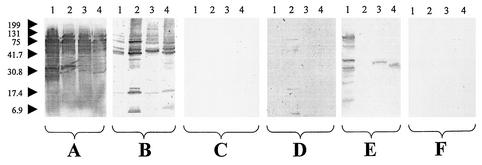
Unique profiles were found when the results of reactions of the sera of the 51 Bartonella endocarditis patients with B. quintana, B. henselae, B. vinsonii subsp. berkhoffii, and B. elizabethae antigens were examined, apart from those of two serum samples that were unreactive. Four reacted only with B. henselae antigens and therefore enabled the diagnosis of B. henselae endocarditis to be confirmed. One of these was from a patient diagnosed by DNA amplification of B. henselae from a valve sample. The other three sera came from patients infected with an unknown Bartonella sp., and in each case the results indicated four reactive bands with B. henselae. The other 45 sera reacted with a wide range of antigens of the four Bartonella spp. used in our study, confirming a high level of antigenic cross-reactivity between the organisms and preventing us from using the results to determine the species causing the infection (Fig. 1A and B). With each antigen tested, the sera recognized 1 to 10 major reactive bands, generally between 5 kDa and 83 kDa. Reaction patterns were also very variable in the 11 patients with chronic B. quintana bacteremia; while 1 serum sample was unreactive, the remaining 10 samples showed 1 to 6 reactive bands with the homologous antigen but no more than one band with the heterologous antigens (Fig. 2A). Immunoblotting of the 10 sera from cat scratch disease patients showed no reactivity in this study. Negative Western blot results were also obtained with the control sera from the 10 blood donors. The Western blot results were found to be reproducible, with the same profiles being found in the 13 sera that were analyzed twice on separate occasions.
FIG. 1.
Western immunoblotting of sera from patients with Bartonella endocarditis. Sera were analyzed using B. quintana (lanes 1), B. henselae (lanes 2), B. vinsonii subsp. berkhoffii (lanes 3), and B. elizabethae (lanes 4) antigens. (A and B) Untreated sera from a patient with B. quintana endocarditis (A) and a patient with B. henselae endocarditis (B). (C and D) B. quintana-adsorbed sera from a patient with B. quintana endocarditis (C) and a patient with B. henselae endocarditis (D). (E and F) B. henselae-adsorbed sera from a patient with B. quintana endocarditis (E) and a patient with B. henselae endocarditis (F). Molecular masses (in kilodaltons) are given to the left of the panels.
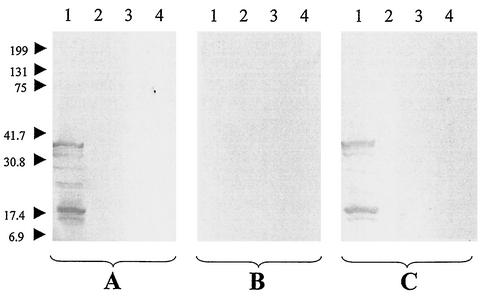
FIG. 2.
Western immunoblotting of serum from a patient with chronic B. quintana bacteremia. The serum was analyzed using B. quintana (lanes 1), B. henselae (lanes 2), B. vinsonii subsp. berkhoffii (lanes 3), and B. elizabethae (lanes 4) antigens. Experiments were performed with untreated serum (A), serum adsorbed with B. quintana (B), and serum adsorbed with B. henselae (C). Molecular masses (in kilodaltons) are given to the left of the panels.
Cross-adsorption studies.
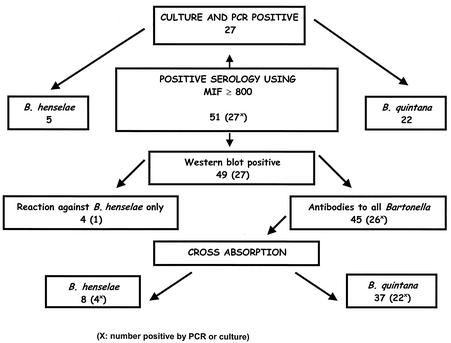
We adsorbed the 45 serum samples of endocarditis patients with cross-reactive antibodies; the results for all of these samples were negative after adsorption when tested by MIF with any antigen. Cross-adsorption was evaluated first in those patients whose infective agents had been identified to the species level. Of the 22 samples from patients with B. quintana IE, all exhibited reactions against B. quintana after B. henselae adsorption but no B. henselae reaction after B. quintana adsorption. The samples from the four patients with B. henselae exhibited reactions to B. henselae after B. quintana adsorption, and none exhibited reactions after B. henselae adsorption. After these results showed that this test could be considered efficient, cross-adsorption was applied to 24 IE cases without previous diagnosis of the infective species and enabled us to determine that 15 patients were infected with B. quintana and 4 patients were infected with B. henselae. Finally, 5 of the 51 sera from patients with Bartonella endocarditis did not react with either B. quintana or B. henselae after being adsorbed against either organism, 2 returning negative results before adsorption and 3 reacting only with B. henselae (Fig. 3). Furthermore, the epidemiological features of the patients we identified by cross-adsorption and Western immunoblotting with B. quintana and B. henselae endocarditis were similar to those found in other studies. Homelessness, contact with body lice, and chronic alcoholism were significantly more frequent for patients with B. quintana infections than for B. henselae-infected patients. Contact with cats, cat fleas, and previous valve disease was significantly more frequent for patients with B. henselae endocarditis (8) (Table 1). In total, by testing one serum sample from each patient we diagnosed 37 IE cases due to B. quintana and 12 due to B. henselae (Table 1).
FIG. 3.
Results for the 51 tested sera of patients with IE.
TABLE 1.
Comparison of the epidemiological characteristics of patients with B. quintana endocarditis and of those with B. henselae endocarditisa
| Epidemiological characteristic(s) | Value for group with species-level diagnosis on the basis of:
|
|||||
|---|---|---|---|---|---|---|
| Culture and/or PCR results
|
Serological testing
|
|||||
| B. quintana endocarditis (n = 22) | B. henselae endocarditis (n = 5) | P | B. quintana endocarditis (n = 15) | B. henselae endocarditis (n = 7) | P | |
| Mean age (yr) | 46 | 56 | NSb | 57 | 53 | NS |
| Male | 18 | 3 | NS | 13 | 5 | NS |
| Homelessness and/or chronic alcoholism and/or contact with body lice | 17 | 0 | <0.005 | 9/14c | 0/6c | <0.05 |
| Contact with cats and/or cat fleas and previous valvular disease | 3 | 5 | <0.001 | 3/14c | 4/5c | <0.05 |
Comparisons among the 27 patients with culture- and/or PCR-proven endocarditis were performed separately from those performed among the 22 patients with serology-based etiologic diagnoses. Except where otherwise indicated, all non-P values are numbers of patients.
NS, not significant.
Value is the number of patients with the characteristic(s)/total number of patients tested.
For 8 of the 11 sera from patients with B. quintana bacteremia, Western immunoblotting was negative when performed with the sera adsorbed with B. quintana (Fig. 2B) but 1 to 6 bands were seen with B. quintana antigen after the sera were adsorbed with B. henselae (Fig. 2C). Three sera from bacteremic patients were unreactive after adsorption with either B. quintana or B. henselae. Sera from cat scratch disease patients were unreactive in Western immunoblots.
Differentiation of IE from other B. quintana and B. henselae infections.
A pattern of Western blot reactivity (consisting of at least one reactive band with B. quintana and at least two reactive bands with any of the other Bartonella spp.) was observed with 36 of 37 (97%) of the sera from patients with B. quintana IE but with only 1 of 11 (9%) of the sera from bacteremic patients (P < 0.000005). While none of the sera from patients with B. henselae IE were unreactive in Western blotting, all the sera from cat scratch disease patients were unreactive (P < 0.000005). In Western blotting tests with B. quintana, six protein bands (Bq83, Bq65, Bq45, Bq30, Bq20, and Bq10) were significantly associated with sera from patients with IE (Table 2). Similarly, the Bh65, Bh45, Bh35, and Bh23 antigens of B. henselae were significantly associated with sera from patients with B. henselae IE (Table 3). Two heterologous antigens, a 33-kDa antigen of B. vinsonii subsp. berkhoffii and a 30-kDa antigen of B. elizabethae, were reactive with 15 of 37 (41%) and 18 of 37 (49%) of the sera from B. quintana endocarditis patients (Fig. 1A, lanes 3 and 4), respectively. They were not, however, detected with sera from bacteremic patients (P < 0.01).
TABLE 2.
Frequency of recognition of proteins from B. quintana Fuller
| Protein banda | % Sera with Western blot reactivity to protein bandb
|
P value (Fisher's exact test)
|
|||
|---|---|---|---|---|---|
| B. quintana endocarditis (n = 37) | B. quintana bacteremia (n = 11) | B. henselae endocarditis (n = 12) | B. quintana endocarditis vs B. quintana bacteremia | B. quintana endocarditis vs B. henselae endocarditis | |
| Bq130 | 3 | NSc | NS | ||
| Bq83 | 78 | <0.000005 | <0.000005 | ||
| Bq65 | 100 | 27 | 8 | <0.0000005 | <0.005 |
| Bq50 | 9 | NS | NS | ||
| Bq45 | 95 | 27 | 67 | <0.00005 | <0.05 |
| Bq40 | 22 | 18 | 8 | NS | NS |
| Bq34 | 86 | 64 | 25 | NS | <0.0005 |
| Bq33 | 19 | NS | NS | ||
| Bq30 | 49 | 9 | 8 | <0.05 | <0.05 |
| Bq28 | 16 | 18 | NS | NS | |
| Bq25 | 57 | 18 | 42 | NS | NS |
| Bq23 | 8 | NS | NS | ||
| Bq20 | 78 | 36 | 25 | <0.05 | <0.005 |
| Bq15 | 14 | NS | NS | ||
| Bq10 | 65 | 17 | <0.001 | <0.05 | |
| Bq5 | 11 | 18 | NS | NS | |
Bands with boldface designations demonstrated significant association with B. quintana endocarditis compared to that with B. quintana bacteremia and B. henselae endocarditis (P < 0.05). The number in each protein band designation represents the apparent molecular mass in kilodaltons.
Proportion of sera recognizing each band. Results equal to zeros are omitted to improve clarity.
NS, not significant.
TABLE 3.
Frequency of recognition of proteins from B. henselae Houston-1
| Protein banda | % Sera with Western blot reactivity to protein bandb
|
P value (Fisher's exact test)
|
|||
|---|---|---|---|---|---|
| B. henselae endocarditis (n = 12) | Cat scratch disease (n = 10) | B. quintana endocarditis (n = 37) | B. henselae endocarditis vs cat scratch disease | B. henselae endocarditis vs B. quintanaendocarditis | |
| Bh200 | 8 | NSc | NS | ||
| Bh160 | 8 | NS | NS | ||
| Bh140 | 8 | 3 | NS | NS | |
| Bh65 | 100 | 100 | <0.000005 | NS | |
| Bh60 | 25 | NS | NS | ||
| Bh45 | 75 | 84 | <0.0005 | NS | |
| Bh40 | 25 | 19 | NS | NS | |
| Bh34 | 58 | 59 | <0.005 | NS | |
| Bh33 | 16 | NS | NS | ||
| Bh30 | 17 | 24 | NS | NS | |
| Bh28 | 8 | 11 | NS | NS | |
| Bh25 | 17 | 11 | NS | NS | |
| Bh23 | 42 | 30 | <0.05 | NS | |
| Bh20 | 33 | 65 | NS | NS | |
| Bh10 | 33 | 65 | NS | NS | |
Bands with boldface designations demonstrated significant association with B. henselae endocarditis compared to that with cat scratch disease (P < 0.05).
Proportion of sera recognizing each band. Results equal to zero were omitted to improve clarity.
NS, not significant.
Differentiation of Bartonella species causing IE.
Of the 37 sera from patients with B. quintana IE, 24 (65%) gave seven or more bands with B. quintana. Such reactivity was not found with any of the sera from patients with B. henselae endocarditis or chronic B. quintana bacteremia (P < 0.001, PPV = 1.0). Western blots with sera from 16 of 37 (43%) patients with B. quintana endocarditis, including 10 of the 24 sera producing seven or more bands with B. quintana, showed a significantly higher number of bands (i.e., a difference of at least two bands) with B. quintana than with B. henselae. This result was not found with any sera from patients with B. henselae endocarditis (P < 0.005, PPV = 1.0). Conversely, immunoblots with sera from 5 of 12 (42%) of the patients with B. henselae endocarditis had a significantly higher number of bands with B. henselae than with B. quintana. This was not found with sera from any of the other patients (P < 0.0005, PPV = 1.0). Proteins Bq83, Bq65, Bq45, Bq30, Bq20, and Bq10 of B. quintana were more frequently recognized by sera from patients with B. quintana endocarditis than by sera from B. henselae endocarditis patients (Table 2). Only 1 of 12 (P < 0.05) sera from B. henselae endocarditis patients reacted with the 33-kDa antigen of B. vinsonii subsp. berkhoffii, while none (P < 0.005) of the sera reacted with the 30-kDa antigen of B. elizabethae. In Western blotting tests with B. henselae as antigen, there were no bands that were significantly associated with sera from B. henselae endocarditis patients but not with sera from B. quintana endocarditis patients (Table 3).
DISCUSSION
Over the last decade, the spectrum of clinical conditions caused by the human pathogens in the genus Bartonella has expanded dramatically (14, 24). In particular, since the first definite case of Bartonella IE was described (33), a further 71 cases have now been reported in the international literature. These have mostly been cases due to B. quintana or B. henselae, but single cases of IE due to B. elizabethae and B. vinsonii subsp. berkhoffii have also been previously described (8). It has been shown that Bartonella spp. may cause 3% of all cases of IE (29). Endocarditis is a life-threatening disease, and it is important that rapid etiological diagnoses be made as early as possible. Isolation of Bartonella is rarely attempted for diagnosis of infections, as specialized culture methods and facilities are required. Furthermore, as specimens are often collected after antimicrobial treatment has been implemented, the success rate for isolation of Bartonella spp. remains low (17, 18). Molecular methods, although they are rapid and specific, are presently restricted to large laboratories and require specific equipment and expertise. Another limitation of PCR is the need to collect samples, usually cardiac valve tissue, by invasive means (2). Serological testing, by MIF in particular but also by enzyme-linked immunosorbent assays (12), has become the most practical means of confirming present or prior infection with Bartonella spp., although the technique has limitations. High IgG titers (≥1:800) are associated with chronic Bartonella infections but do not differentiate between IE and persistent bacteremia or even cat scratch disease with visceral involvement (4, 29, 30). Serological cross-reactivity between Bartonella spp. when using MIF precludes identification of the species causing the infection. On the other hand, Western immunoblotting may help to overcome some of these limitations in situations where sera are the only available samples. Our study shows that the technique is satisfactorily reproducible and may be used to differentiate endocarditis from other Bartonella infections such as chronic bacteremia and cat scratch disease. Also, infections with the two species principally responsible for endocarditis may be differentiated, based on the number and type of reactive bands of B. quintana and B. henselae and also on the reaction patterns obtained after cross-adsorption of sera.
Means for differentiating two B. quintana infections, chronic bacteremia and endocarditis, are of critical importance, as the latter should be treated immediately and for at least 6 weeks with a combination of antimicrobials (7, 29). The optimal management of chronic bacteremia is not yet clear but might require substantially shorter treatment (4, 33). As reported by Spach et al. (33), persistent bacteremia can result in subacute endocarditis in about 20% of cases. The only method for distinguishing between these two diseases to date has been echocardiography, but this method is limited by the fact that B. quintana endocarditis frequently occurs in the absence of preexisting valvulopathy and valve lesions may be difficult to detect in early stages of the disease (8, 29). IFA results showed that more-extensive cross-reactivity occurs with sera from patients with B. quintana IE than with sera from bacteremic patients (4, 29). Serological tools, then, may be useful for following up patients with chronic bacteremia and in the detection of IE. Indeed, our Western immunoblotting results confirm that the level of cross-reactivity is significantly higher in sera from patients with IE than in sera from patients with bacteremia. For the diagnosis of B. quintana IE, the demonstration of one B. quintana protein band of either 83 or 10 kDa has a sensitivity and specificity of 100% (Table 4). Similarly, immunoblot studies with sera to B. bacilliformis have demonstrated different reactivity patterns with sera collected from the septicemic and tissue forms of the disease (23). In patients with IE, the presence of close interactions between Bartonella and human tissues may explain the intense immunogenic stimulation. With B. henselae infections, Western immunoblotting enabled the differentiation of endocarditis and cat scratch disease patients, with a lack of reactivity characterizing the latter. Previous studies have identified antigens recognized by sera from patients with cat scratch disease (1, 22, 27), and our failure to confirm these findings may be a result of the concentration of antigens we used in our blots or the relatively high (1:200) dilution of the sera we used. However, we were able to detect reactive antigens in the Western blot analyses we performed on sera from patients with other Bartonella infections.
TABLE 4.
Evaluation of Western blot interpretation criteria for B. quintana endocarditisa
| Criterion(s) (bands required) |
B. quintana infectionb
|
Bartonella sp. endocarditisc
|
|||
|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | PPV (%) | |
| ≥1 of any B. quintana band and ≥2 of any B. henselae bands | 97.0 | 90.9 | 97.0 | 33.3 | 81.8 |
| ≥7 of any B. quintana bands or no. of B. quintana bands ≥ no. of B. henselae bands + 2 | 81.1 | 100.0 | 81.1 | 100.0 | 100.0 |
| ≥1 of Bq83 and/or Bq10 | 100.0 | 100.0 | 100.0 | 83.3 | 94.9 |
| ≥3 of Bq83, Bq45, Bq35, Bq30, and/or Bq20 | 97.3 | 90.9 | 97.3 | 83.3 | 94.7 |
Recommended criteria are in boldface.
Sensitivity and specificity of the various criteria were determined for all tested sera from patients with B. quintana infection (n = 48).
Sensitivity, specificity, and PPV of the various criteria were determined for all tested sera from patients with Bartonella endocarditis (n = 49).
It was not possible to determine by MIF the Bartonella species causing endocarditis, because of extensive cross-reactivity and the removal of all detectable antibodies by cross-adsorption (17). Western immunoblotting confirmed the extent of serological cross-reactivity among the four Bartonella species studied and the high variability of the immune responses that individuals may exhibit (7, 27). Most of the cross-reactions were with antigens of molecular sizes ranging from 5 to 83 kDa, a range similar to that reported by Maurin et al. for a patient with bacillary angiomatosis (26) and by Liang and Raoult in experimental studies using murine polyclonal antisera (20). The most prominent cross-reactivity occurred with a 60-kDa antigen, which may be a heat shock protein. These proteins are broadly conserved across many bacterial genera and species and were identified as antigens cross-reacting between Bartonella and Chlamydia species (25). As proposed for Lyme disease (13), interpretation of Western blots can be facilitated using standardized criteria. Western blots showing seven or more bands with B. quintana or a significantly higher number of bands (at least two bands more) with B. quintana than with B. henselae have a PPV of 100% and a sensitivity of 81% for B. quintana endocarditis (Table 4). Conversely, Western blots with significantly more bands with B. henselae than with B. quintana have a PPV of 100% and a sensitivity of 41.7% for B. henselae endocarditis (Table 5). Furthermore, the presence of protein bands Bq83, Bq65, Bq45, Bq30, Bq20, and Bq10 was recognized as possibly being diagnostic for B. quintana endocarditis (Table 2) and the presence of only Bq83 or Bq10 had a PPV of 94.9% for the disease (Table 4). Combining the above three criteria, we were able to determine retrospectively the Bartonella species causing endocarditis in 42 of 51 (82%) patients. Interpretation was easier, however, when Western immunoblotting was performed with adsorbed sera. In this way, differentiation between B. quintana and B. henselae endocarditis was possible in all five patients with cross-reactive antibodies. The Western blots for these 45 patients were unequivocal and easy to interpret (Fig. 1C to F) and were consistent with etiological data based on culture and PCR amplification. They were also consistent with the existing data on the epidemiology of the infections, with B. quintana endocarditis occurring more frequently (76% of cases) than B. henselae endocarditis (Table 1) (8). When combined, Western immunoblotting and cross-adsorption provide a highly sensitive (96%) diagnostic test. Interestingly, sera adsorbed against B. quintana and B. henselae gave completely negative results in IFAs while they still reacted with protein bands of B. henselae and B. quintana, respectively, in Western immunoblots. This suggests that immunogenic proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis were masked in vitro while they were expressed in vivo. As adsorption of the sera completely removed cross-reacting antibodies for other Bartonella spp., these proteins might contain species-specific epitopes. In particular, a 34-kDa protein band was recognized by about 60% of sera from B. quintana-infected patients, even after cross-adsorption. Several monoclonal antibodies have recently been produced against one of these B. quintana-specific epitopes located on a 34-kDa protein (21).
TABLE 5.
Evaluation of Western blot interpretation criteria for B. henselae endocarditisa
| Criterion(s) (bands required) |
B. henselae infectionb
|
Bartonella sp. endocarditisc
|
|||
|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | PPV (%) | |
| No. of B. henselae bands ≥ no. of B. quintana band(s) + 2 | 41.7 | 100.0 | 41.7 | 100.0 | 100.0 |
| ≥2 of Bh65, Bh45, and/or Bh35 | 100.0 | 100.0 | 100.0 | 10.8 | 26.7 |
Recommended criteria are in boldface.
Sensitivity and specificity of the various criteria were determined for all sera tested from patients with B. henselae infection (n = 22).
Sensitivity, specificity, and PPV of the various criteria were determined for all sera tested from patients with Bartonella endocarditis (n = 49).
In conclusion, our study has shown that serum and cross-adsorption and Western blot analysis are powerful tools not only for establishing an etiological diagnosis of Bartonella endocarditis to the species level in the absence of tissue samples but also for identifying genus-, species-, and even disease-specific immunodominant antigens recognized by the human immune system in Bartonella infection. These antigens are good candidates for species-specific diagnostic tests, and we are attempting to produce them as recombinant proteins to be used in simple enzyme-linked immunosorbent assays for the diagnosis of IE. Also, the generation and use of multiple polyclonal and monoclonal antibodies against these antigens would aid in our understanding the pathogenesis of Bartonella infections (11).
Acknowledgments
We thank Bernard Amphoux, Christine Lecam, and Ginette Garaizar for assistance with the experiments and Patrick Kelly for reviewing the manuscript.
REFERENCES
- 1.Anderson, B., E. Lu, D. Jones, and R. Regnery. 1995. Characterization of a 17-kilodalton antigen of Bartonella henselae reactive with sera from patients with cat scratch disease. J. Clin. Microbiol. 33:2358-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, B. E., and M. A. Neuman. 1997. Bartonella spp. as emerging human pathogens. Clin. Microbiol. Rev. 10:203-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner, D. J., S. O'Connor, H. H. Winkler, and A. G. Steigerwalt. 1993. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int. J. Syst. Bacteriol. 43:777-786. [DOI] [PubMed] [Google Scholar]
- 4.Brouqui, P., B. La Scola, V. Roux, and D. Raoult. 1999. Chronic Bartonella quintana bacteremia in homeless patients. N. Engl. J. Med. 340:184-189. [DOI] [PubMed] [Google Scholar]
- 5.Daly, J. S., M. G. Worthington, D. J. Brenner, W. C. Moss, D. G. Hollis, R. S. Weyant, A. G. Steigerwalt, R. E. Weaver, M. I. Daneshvar, and S. P. O'Connor. 1993. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J. Clin. Microbiol. 31:872-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean, A. G., J. A. Dean, A. H. Burton, and R. C. Dicker. 1990. Epi Info, version 5: a word processing, database, and statistics program for epidemiology on microcomputers. Centers for Disease Control and Prevention, Atlanta, Ga.
- 7.Drancourt, M., J. L. Mainardi, P. Brouqui, F. Vandenesch, A. Carta, F. Lehnert, J. Etienne, F. Goldstein, J. Acar, and D. Raoult. 1995. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N. Engl. J. Med. 332:419-423. [DOI] [PubMed] [Google Scholar]
- 8.Fournier, P. E., H. Lelievre, S. J. Eykyn, J. L. Mainardi, T. J. Marrie, F. Bruneel, C. Roure, J. Nash, D. Clave, E. James, C. Benoit-Lemercier, L. Deforges, H. Tissot-Dupont, and D. Raoult. 2001. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine (Baltimore) 80:245-251. [DOI] [PubMed] [Google Scholar]
- 9.Fournier, P. E., J. L. Mainardi, and D. Raoult. 2002. Value of microimmunofluorescence for the diagnosis and follow-up of Bartonella endocarditis. Clin. Diagn. Lab. Immunol. 9:795-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fournier, P.-E., M. F. Minnick, H. Lepidi, E. Salvo, and D. Raoult. 2001. Experimental model of human body louse infection using green fluorescent protein-expressing Bartonella quintana. Infect. Immun. 69:1876-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeland, R. L., D. T. Scholl, K. R. Rhode, L. J. Shelton, and K. L. O'Reilly. 1999. Identification of Bartonella-specific immunodominant antigens recognized by feline humoral immune system. Clin. Diagn. Lab. Immunol. 6:558-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giladi, M., Y. Kletter, B. Avidor, E. Metzkor-Cotter, M. Varon, Y. Golan, M. Weinberg, I. Riklis, M. Ephros, and L. Slater. 2001. Enzyme immunoassay for the diagnosis of cat-scratch disease defined by polymerase chain reaction. Clin. Infect. Dis. 33:1852-1858. [DOI] [PubMed] [Google Scholar]
- 13.Hauser, U., G. Lehnert, and B. Wilske. 1999. Validity of interpretation criteria for standardized Western blots (immunoblots) for serodiagnosis of Lyme borreliosis based on sera collected throughout Europe. J. Clin. Microbiol. 37:2241-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacomo, V., P. J. Kelly, and D. Raoult. 2002. Natural history of Bartonella infections (an exception to Koch's postulate). Clin. Diagn. Lab. Immunol. 9:8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerkhoff, F., A. M. Bergmans, A. van der Zee, and A. Rothova. 1999. Demonstration of Bartonella grahamii DNA in ocular fluids of a patient with neuroretinitis. J. Clin. Microbiol. 37:4034-4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kordick, D. L., B. Swaminathan, C. E. Greene, K. H. Wilson, A. M. Whitney, S. O'Connor, D. G. Hollis, G. M. Matar, A. G. Steigerwalt, G. B. Malcolm, P. S. Hayes, T. L. Hadfield, E. B. Breitschwerdt, and D. J. Brenner. 1996. Bartonella vinsonii subsp. berkhofii subsp. nov., isolated from dogs; Bartonella vinsonii subsp. vinsonii; and emended description of Bartonella vinsonii. Int. J. Syst. Bacteriol. 46:704-709. [DOI] [PubMed] [Google Scholar]
- 17.La Scola, B., and D. Raoult. 1996. Serological cross-reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J. Clin. Microbiol. 34:2270-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.La Scola, B., and D. Raoult. 1999. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J. Clin. Microbiol. 37:1899-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, J. S., D. J. Sexton, N. Mick, R. Nettles, V. G. J. Fowler, T. Ryan, T. Bashore, and G. R. Corey. 2000. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 30:633-638. [DOI] [PubMed] [Google Scholar]
- 20.Liang, Z., and D. Raoult. 2000. Differentiation of Bartonella species by a microimmunofluorescence assay, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western immunoblotting. Clin. Diagn. Lab. Immunol. 7:617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang, Z., and D. Raoult. 2000. Species-specific monoclonal antibodies for rapid identification of Bartonella quintana. Clin. Diagn. Lab. Immunol. 7:40-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litwin, C. M., T. B. Martins, and H. R. Hill. 1997. Immunologic response to Bartonella henselae as determined by enzyme immunoassay and Western blot analysis. Am. J. Clin. Pathol. 108:202-209. [DOI] [PubMed] [Google Scholar]
- 23.Mallqui, V., E. C. Speelmon, M. Verastegui, C. Maguina-Vargas, P. Pinell-Salles, R. Lavarello, J. Delgado, M. Kosek, S. Romero, Y. Arana, and R. H. Gilman. 2000. Sonicated diagnostic immunoblot for bartonellosis. Clin. Diagn. Lab. Immunol. 7:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurin, M., R. Birtles, and D. Raoult. 1997. Current knowledge of Bartonella species. Eur. J. Clin. Microbiol. Infect. Dis. 16:487-506. [DOI] [PubMed] [Google Scholar]
- 25.Maurin, M., F. Eb, J. Etienne, and D. Raoult. 1997. Serological cross-reactions between Bartonella and Chlamydia species: implications for diagnosis. J. Clin. Microbiol. 35:2283-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurin, M., V. Roux, A. Stein, F. Ferrier, R. Viraben, and D. Raoult. 1994. Isolation and characterization by immunofluorescence, SDS-PAGE, Western blot, RFLP-PCR, 16S rRNA gene sequencing and pulsed-field gel electrophoresis of Rochalimaea quintana from a French patient with bacillary angiomatosis. J. Clin. Microbiol. 32:1166-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGill, S. L., R. L. Regnery, and K. L. Karem. 1998. Characterization of human immunoglobulin (Ig) isotype and IgG subclass response to Bartonella henselae infection. Infect. Immun. 66:5915-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel, R., J. O. Newell, G. W. Procop, and D. H. Persing. 1999. Use of polymerase chain reaction for citrate synthase gene to diagnose Bartonella quintana endocarditis. Am. J. Clin. Pathol. 112:36-40. [DOI] [PubMed] [Google Scholar]
- 29.Raoult, D., P. E. Fournier, M. Drancourt, T. J. Marrie, J. Etienne, J. Cosserat, P. Cacoub, Y. Poinsignon, P. Leclercq, and A. M. Sefton. 1996. Diagnosis of 22 new cases of Bartonella endocarditis. Ann. Intern. Med. 125:646-652. [DOI] [PubMed] [Google Scholar]
- 30.Raoult, D., H. Tissot-Dupont, and M. Enea-Mutillod. 1994. Positive predictive value of Rochalimaea henselae antibodies in the diagnostic of cat scratch disease (CSD). Clin. Infect. Dis. 19:355. [DOI] [PubMed] [Google Scholar]
- 31.Regnery, R. L., M. Martin, and J. G. Olson. 1992. Naturally occurring Rochalimaea henselae infection in domestic cat. Lancet 340:557-558. [DOI] [PubMed] [Google Scholar]
- 32.Roux, V., S. J. Eykyn, S. Wyllie, and D. Raoult. 2000. Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture-negative endocarditis in a human. J. Clin. Microbiol. 38:1698-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spach, D. H., A. S. Kanter, M. J. Dougherty, A. M. Larson, M. B. Coyle, D. J. Brenner, B. Swaminathan, G. M. Matar, D. F. Welch, R. K. Root, and W. E. Stamm. 1995. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N. Engl. J. Med. 332:424-428. [DOI] [PubMed] [Google Scholar]
- 34.Zeaiter, Z., P.-E. Fournier, and D. Raoult. 2002. Genomic variation of Bartonella henselae strains detected in lymph nodes from patients with cat scratch disease. J. Clin. Microbiol. 40:1023-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]