Abstract
Microbial growth in moisture-damaged buildings is associated with respiratory and other symptoms in the occupants. Streptomyces spp. are frequently isolated from such buildings. In the present study, we evaluated the responses of mice after repeated exposure to spores of Streptomyces californicus. Mice were exposed via intratracheal instillation to six doses (at 7-day intervals) of the spores of S. californicus, originally isolated from the indoor air of a moisture-damaged building, at three dose levels (2 × 103, 2 × 105, and 2 × 107 spores). Inflammation and toxicity, including changes in cell populations in the lungs, lymph nodes, and spleen, were evaluated 24 h after the last dosage. The exposure provoked a dose-dependent inflammatory cell response, as detected by the intense recruitment of neutrophils, but the numbers of macrophages and lymphocytes in the airways also increased. The cellular responses corresponded to the dose-dependent increases in inflammation- and cytotoxicity-associated biochemical markers (i.e., levels of albumin, total protein, and lactate dehydrogenase) in bronchoalveolar lavage fluid. The spore exposure increased the number of both activated and nonactivated T lymphocytes. Also, the amounts of CD3− CD4− and unconventional CD3− CD4+ lymphocytes in the lung tissue were augmented. Interestingly, the spore exposure decreased cells in the spleen. This effect was strongest at the dose of 2 × 105 spores. These results indicate that the spores of S. californicus are capable of provoking both immunostimulation in lungs (inflammation) and systemic immunotoxicity, especially in the spleen. The immunotoxic effect resembled that caused by chemotherapeutic agents, originally isolated from Streptomyces spp. Thus, S. californicus must be considered a microbial species with potential to cause systemic adverse health effects in occupants of moisture-damaged buildings.
Microbial growth subsequent to moisture damage in buildings has been connected to increased adverse respiratory and other harmful health effects (7, 32, 33, 37, 38, 44). Increased numbers of microbial spores are present in the indoor air of moisture-damaged buildings (15, 16, 27). Moreover, several of the microbial species that are frequently detected in moisture-damaged buildings are rarely observed in reference buildings (34). The spores are inhaled deeply into the lungs due to their relatively small particle size (23). Since the spores may also deliver nonvolatile microbial toxins into the lungs (36), they presumably play a role in provoking adverse health effects.
Streptomyces californicus is a sporulating gram-positive bacterium that is used as a moisture indicator and that is frequently isolated from the indoor air of moisture-damaged buildings (27, 34). Streptomycetes have the genetic capability to produce many bioactive secondary metabolites, such as antibiotics, immunosuppressive agents, antitumor substances, and inhibitors of Ca2+- and calmodulin-dependent cyclic nucleotide phosphodiesterases, which are important enzymes involved in intracellular signaling (1-3, 17, 25, 30, 43). Moreover, streptomycetes can produce genotoxic substances, such as dihydroabikoviromycin (4, 13, 40).
We have previously shown that a single intratracheal dose of the spores of S. californicus provokes acute inflammation in mouse lungs, indicated by the increased production of proinflammatory cytokines (tumor necrosis factor alpha [TNF-α] and interleukin-6 [IL-6]) and nitric oxide (NO) and a strong inflammatory cell response (20). These results corroborated our in vitro findings, which demonstrated that the spores of streptomycetes effectively induced mouse RAW264.7 macrophages to produce proinflammatory mediators (TNF-α, IL-6, IL-1, and NO) (10-12). Individuals in moisture-damaged buildings usually experience repeated exposures to relatively low doses of the spores, and thus, it seemed of interest to study the responses caused by repeated exposures to the spores over a wide dose range in vivo.
In the present study, we investigated the responses in the lungs, lymph nodes, and spleens of mice to repeated exposures to the spores of S. californicus originally isolated from a moisture-damaged building. The cell profiles in bronchoalveolar lavage fluid (BALF) and the lymphocyte subpopulations in lung and lymphoid tissues were analyzed. TNF-α and IL-6 levels in BALF and serum were determined. Other biochemical markers of inflammation and cytotoxicity in BALF were analyzed. Moreover, since some secondary metabolites produced by streptomycetes are genotoxic, damage to the DNA in blood leukocytes was evaluated.
MATERIALS AND METHODS
Exposures.
A strain of S. californicus, a mesophilic gram-positive bacterium, was isolated from the indoor air of a moisture-damaged building, as described by Hyvärinen and coworkers (16). The strain, encoded strain A4 in our previous in vitro studies (10-12), was identified by the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany) identification service. The microbe was cultured on Tryptone yeast-glucose agar (Bacto Plate Count Agar; Difco Laboratories, Detroit, Mich.) and incubated at 20°C in the dark for about 5 days. The spores were suspended in Hanks' balanced salt solution (HBSS) (Gibco Life Technologies, Paisley, United Kingdom) containing 0.0001% Triton X-100 (BDH, Poole, United Kingdom) and sonicated in a water bath sonicator (Ultrasonic, Lahti, Finland) on ice for 40 min to obtain a single-spore suspension. Spore concentrations were counted under an epifluorescence microscope.
Animals.
Male NIH/S mice (age, 7 to 8 weeks) were obtained from the breeding colony of the Department of Environmental Health, National Public Health Institute, Kuopio, Finland. They were transferred from the barrier unit to a conventional animal room and housed singly in metal cages on aspen-wood chips (FinnTapvei, Kaavi, Finland) 1 week before the experiment. Animals received water and food (R36 maintenance diet for rats and mice [Lactamin, Stockholm, Sweden]) ad libitum. The mice were on a 12-h light (7 a.m. to 7 p.m.) and 12-h dark cycle in a room with an average temperature of 21°C and 26 to 47% relative humidity. The animals were treated humanely, and experimental protocols were approved by the Research Animal Committee of the University of Kuopio and the Provincial State Office.
Experimental design.
The dose-response induced by the spores was investigated by repeatedly exposing mice (16 animals/group) to S. californicus (2 × 103, 2 × 105, or 2 × 107 spores/animal) or HBSS (carrier control; Gibco) at 7-day intervals for 6 weeks by intratracheal instillation (six doses of 50 μl each per animal) while the animals were under anesthesia with 6% sevoflurane (Sevorane; Abbott, North Chicago, Ill.). Twenty-four hours after the last dosage, the mice were anesthetized with pentobarbital (100 mg/kg of body weight) and exsanguinated by cardiac puncture, and samples were collected.
Exposure to microbial spores.
The spores were instilled into the mouse lungs as described earlier (20). Shortly thereafter, an anesthetized mouse was placed at a 66° upward prone posture, and the incisors were placed on a wire. The dosing was performed under visual control by using a cold-light source (KL 1500 electronic; Schott, Mainz, Germany) placed against the throat. The nostrils were blocked, the tongue was pulled out, and the dose was delivered onto the vocal folds with a Finn pipette tip.
Sample collection.
Blood was collected, and serum was separated in a gel barrier capillary blood collection tube (Capiject T-MG; Terumo, Elkton, Md.) for cytokine analyses. The lungs (filled with 10% phosphate-buffered formalin), livers, spleens, and lymph nodes (mediastinal, axillary, brachial, and both superficial and deep cervical nodes) from three mice in each group were preserved in 10% phosphate-buffered formalin for histopathological evaluation. The lungs of seven animals per group were lavaged, cells and lavage fluid were collected and processed further, and both the total cell number and cell differentials in BALF were determined as described earlier (20).
Homogenization of lungs, lymph nodes, and spleens.
Inflammatory cells in the lungs, spleens, and lymph nodes (mediastinal, axillary, brachial, and both superficial and deep cervical nodes) were analyzed by flow cytometry. The tissues of six animals per group were dissected in ice-cold RPMI 1640 medium (Gibco). Lung lobes were moved onto a sterile metal mesh, rinsed with new medium, minced with a scalpel, homogenized by pushing them through the mesh with a sterile syringe plunger, and placed in 14 ml of ice-cold RPMI 1640 medium. The primary suspension was homogenized further with a plunger and filtered with a cell strainer (pore size, 70 μm; nylon; Falcon; Becton Dickinson, Paramus, N.J.) to obtain a single-cell suspension. The spleen and the pooled lymph nodes of each animal were rinsed with new medium and moved to the cell strainer. The spleens were minced with a scalpel, and both the spleens and the lymph nodes were homogenized with a plunger, filtered through the cell strainer, and placed in 14 ml of ice-cold RPMI 1640 medium. The cell suspensions were centrifuged (136 × g, 10 min, 4°C), and the media were discarded. Then, the red blood cells were hemolyzed from the cell pellets obtained from the lungs and spleen by hypotonic shock, as described earlier (20). After another centrifugation, the cells were resuspended in 1 ml of ice-cold RPMI 1640 medium, the hematopoietic cell concentration (erythrocytes excluded) of each sample (number of cells per milliliter of RPMI 1640 medium) was counted by the trypan blue exclusion method, and the total cell number was calculated for each organ.
Cell staining and flow cytometric analysis.
Approximately 1.5 × 105 lung, spleen, or lymph node cells were resuspended in triplicate in 1 ml of 2% fetal bovine serum (FBS) in HBSS. After centrifugation (240 × g, 5 min, 4°C), the supernatant was discarded and the cells were resuspended in 100 μl of 2% FBS. Nonspecific binding was blocked by adding 1 μg of CD16/CD32 monoclonal antibody (Fc Block; PharMingen, San Diego, Calif.) in 25 μl of 2% FBS to each sample, and the samples were then incubated on ice for 10 min. Then, all samples except nonspecific controls were stained by adding 0.6 μg of phycoerythrin (PE)-conjugated CD45 monoclonal antibody, 0.6 μg of CY-Chrome-conjugated CD3 monoclonal antibody, and either 0.6 μg of fluorescein isothiocyanate (FITC)-conjugated CD25 monoclonal antibody or 0.6 μg of FITC-conjugated CD4 monoclonal antibody (all monoclonal antibodies were obtained from PharMingen) in 25 μl of 2% FBS. Nonspecific controls were stained with anti-human monoclonal antibodies (PE-conjugated CD8 [Leu-2a; Becton Dickinson, San Jose, Calif.], peridinin chlorophyll-a protein-conjugated CD3 [Leu-4; Becton Dickinson, Calif.]), and FITC-conjugated anti-T-cell-receptor γδ-1 [Becton Dickinson]). The stained samples were incubated on ice for 30 to 60 min in the dark and then washed in 2 ml of 2% FBS. After centrifugation (240 × g, 5 min, 4°C), the supernatant was discarded, the cells were resuspended in 1% paraformaldehyde fixative, and the samples were stored at 4°C in the dark until analysis with a fluorescence-activated cell sorter.
The cells were analyzed by using a FACScan flow cytometer (Becton Dickinson) and CellQuest analysis program (Becton Dickinson). Forward light scatter, side light scatter, FL1 (FITC), FL2 (PE), and FL3 (CY-Chrome) were used. A total of 25,000 ungated events were recorded for lung samples, and 10,000 ungated events were recorded for spleen and lymph node samples. The proportions of both CD3+ and CD4+ cells within the CD45+ population in the lymphocyte gate were measured. The total numbers of activated and nonactivated T cells and the numbers of other cell populations were calculated by multiplying the calculated total cell number for each organ (lung, spleen, and lymph nodes) by the proportion of the cells of interest from the total CD45+ cells.
Analysis of IL-6 and TNF-α.
Cytokines were analyzed from BALF and serum by the enzyme-linked immunosorbent assay (ELISA) method. Antibody pairs were obtained from R&D Systems (Minneapolis, Minn.), and the analyses were performed according to the instructions of the manufacturer. The concentrations of monoclonal capture antibody were 1 μg/ml of phosphate-buffered saline for IL-6 analyses and 0.8 μg/ml of phosphate-buffered saline for TNF-α analyses, and the concentrations of biotinylated secondary antibody were 0.2 μg/ml for IL-6 analyses and 0.3 μg/ml for TNF-α.
Analysis of LDH, total protein, hemoglobin, and albumin concentrations.
The lactate dehydrogenase (LDH) concentration in BALF was analyzed with a Cytotoxicity Detection kit (Boehringer Mannheim, GmbH, Mannheim, Germany), the total protein concentration in BALF was determined by using the modified Lowry method (DC Protein Assay; Bio-Rad Laboratories, Hercules, Calif.), and the hemoglobin concentrations in the supernatants of hemolyzed cell pellets were analyzed by using the modified Stadie method (procedure no. 525; Sigma, St. Louis, Mo.), as described previously (20). The albumin concentration in BALF was analyzed by the ELISA method. Antibody pairs were obtained from Bethyl Laboratories (Montgomery, Tex.), and analyses were carried out according to the instructions of the manufacturer. The concentration of monoclonal capture antibody was 10 μg/ml of coating buffer (0.05 M sodium carbonate [pH 9.6]), and horseradish peroxidase-conjugated secondary antibody was diluted 1:80,000 in diluent (1% bovine serum albumin [BSA], 50 mM Tris [pH 8.0], 0.15 M NaCl, 0.05% Tween 20). Maxisorb (Nunc, Naperville, Ill.) 96-well microtiter plates were used. BALF samples (1:900) and the standards were diluted in diluent. Tetramethylbenzidine substrate solution (tetramethylbenzidine single solution; Zymed, South San Francisco, Calif.) was incubated with the samples and standards for 4 min. The absorbances were measured with an ELISA reader (iEMS Reader MF; Labsystems, Helsinki, Finland) at a wavelength of 450 nm.
Western blot analysis of iNOS.
Inducible NOS (iNOS) protein was analyzed as described previously, with some modifications (20). Briefly, the cells were lysed, and the supernatant was mixed with sample buffer and heated to 95°C for 7 min. Then, the samples (>38 μg of protein), positive controls, and markers (Bio-Rad) were subjected to electrophoresis (150 V, 50 min, 7.5% Tris-HCl gels; Criterion; Bio-Rad). Proteins were transferred electrophoretically to a polyvinylidene difluoride membrane (100 V, 1 h; Immun-Blot; Bio-Rad). After the membranes were blocked with 5% BSA (Sigma), they were incubated in primary antibody solution (0.1% rabbit anti-iNOS pAb [Transduction Laboratories, Lexington, Ky.] in 5% BSA) for 1 h. After six 5-min washings, the membranes were incubated in alkaline phosphatase-conjugated secondary antibody solution (0.1% alkaline phosphatase-goat anti-rabbit immunoglobulin G [Zymax; Zymed] in 5% BSA) for 1 h. The membranes were washed six more times and exposed to alkaline phosphatase developing buffer for 1 min. Finally, the membranes were developed by using 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium in developing buffer, and the reaction was stopped by rinsing the membranes in tap water.
Analysis of genotoxicity.
Genotoxicity was analyzed by using the alkaline single-cell gel (SCG) assay to measure the damage to the DNA in blood leukocytes from three mice from each group. Determination of DNA damage by the SCG assay was performed as described by Singh et al. (35), with some modifications. Briefly, 10 μl of whole blood obtained by cardiac puncture was suspended in 75 μl of 0.5% low-melting-point agarose (Bio-Rad) and spread on a microscope slide covered with 1% normal-melting-point agarose (Life Technologies, Paisley, United Kingdom). The slides were kept on ice for 5 min, after which the coverslips were removed. The cells were treated with a lysing solution (2.5 M NaCl, 100 mM disodium EDTA, 10 mM Tris [pH 10], 1% sodium lauryl sarcosinate, 1% Triton X-100) for 1 h at 4°C. The slides were then placed in a horizontal electrophoresis tank, and the DNA was allowed to unwind for 10 min in electrophoresis buffer (1 mM EDTA, 300 mM NaOH [pH > 13]) before the gels were run for 15 min at 25 V and 300 mA. After the electrophoresis run, the slides were neutralized with Tris buffer (0.4 M; pH 7.5). The SCG analysis was performed on ethidium bromide-stained, coded slides (with 200 cells per animal) by using an automated image analysis system (Komet 4.0.2; Kinetic Imaging Ltd., Bromborough, United Kingdom). The following comet response parameters were analyzed: tail DNA (tail percent DNA), tail extent moment [(tail length × tail percent DNA)/100], olive tail moment {[(tail mean − head mean) × tail percent DNA]/100}, and tail length.
Analysis of histopathological changes.
The tissue samples stored in 10% buffered formalin were trimmed, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin-eosin. Histopathological changes were evaluated under a light microscope.
Statistical analysis.
The normally distributed data, with equal variances between groups, were assessed by analysis of variance and Dunnett's test: exposed groups were compared to the carrier control group (flow cytometric data and total protein, albumin, and cytokine concentrations in BALF). In cases in which the variances were unequal, analysis of variance and Dunnett's C test were used (LDH data). Otherwise, the Kruskall-Wallis and Dunn tests were used (SPSS, version 9.0.1; SPSS Inc., 1999) (48). The difference was considered significant if the P value was <0.05.
RESULTS
Cells in BALF.
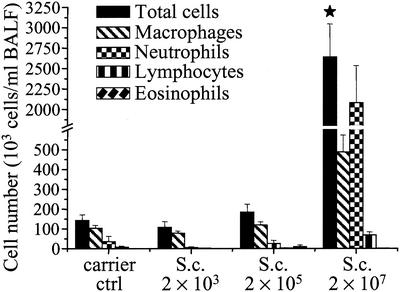
The cell profile and the numbers of cells in BALF (as well as all other parameters) were analyzed 24 h after the last dosage. Repeated dosing of S. californicus spores induced inflammation in the lungs. The cell response was dramatic at the spore dose of 2 × 107 (Fig. 1). At that dose the total number of cells, consisting mainly of neutrophils and macrophages, increased more than 18-fold. The number of lymphocytes increased ninefold at the same dose level. The increase in the total cell number at the dose of 2 × 105 spores was 29%, mainly due to macrophages. Eosinophils were occasionally observed in the BALF samples of the groups exposed to the two highest spore doses. The lowest spore dose did not increase the cell numbers in BALF.
FIG. 1.
Inflammatory cells in BALF 24 h after the last (sixth) instillation of graded doses of S. californicus spores (2 × 103, 2 × 105, and 2 × 107) at 7-day intervals (n = 5 to 7). Carrier ctrl, carrier control; S.c., S. californicus. Each column represents the mean + standard error. The asterisk indicates a statistically significant difference from the result for the carrier control (P < 0.05).
Hemoglobin concentrations in BALF were similar in the exposed and the control groups (data not shown), indicating that at the time point studied the spore exposure did not induce severe vascular leakage or vascular damage in the airways.
Recruitment, expansion, and activation of lymphocyte populations in lungs, lymph nodes, and spleen.
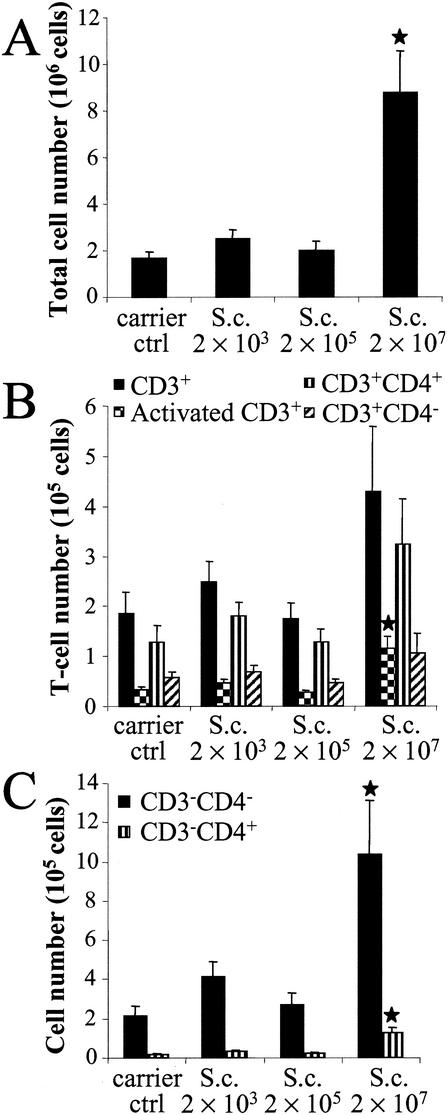
To further assess the spore exposure-induced alterations in cell populations in the lungs, lymph nodes, and spleen, the organs were homogenized, the cells were isolated, the total cell number was calculated, and the cells were subjected to flow cytometric analysis. The highest spore dose (2 × 107) increased the total cell number in lung tissue by more than fivefold compared to that in the control group (Fig. 2A). The total cell number also increased in the groups exposed to 2 × 103 and 2 × 105 spores (51 and 22%, respectively), although these increases were not statistically significant. The T-lymphocyte number (CD3+ cells) more than doubled at the highest dose level (Fig. 2B). Concomitantly, the number of activated T lymphocytes, i.e., the cells expressing both CD3+ and CD25+ surface molecules, in the lungs increased by 3.5-fold compared to that in the control group (P = 0.003). The proportion of activated T cells (CD3+ CD25+ cells/CD3+ cells) was clearly higher in the spore-exposed group than in the control group (27 versus 18%, respectively). There was a 2.5-fold increase in the number of helper T lymphocytes (CD3+ CD4+ cells); and the number of CD3+ CD4− cells, among which are included killer T lymphocytes (CD3+ CD8+) and γδ+ T cells, almost doubled at the highest spore dose compared to the numbers in the control group. Moreover, the highest spore dose recruited abundant CD3− CD4− cells and, to a lesser extent, CD3− CD4+ cells into the lungs (Fig. 2C). The count of CD3− CD4− cells was almost five times higher in the spore-exposed group than in the control group (P = 0.007), and the number of unconventional CD3− CD4+ lymphocytes was more than seven times higher in the spore-exposed group than in the control group (P = 0.001). The CD3− CD4− cells within the lymphocyte gate include B lymphocytes and natural killer cells. The lower spore doses did not markedly affect the cell populations in the lungs. The cell numbers tended to be lower at the spore dose of 2 × 105 than at the spore dose of 2 × 103 (Fig. 2A to C).
FIG. 2.
Total cell (A), T-lymphocyte (B), and CD3− cell (C) numbers in the lungs 24 h after the last (sixth) instillation of graded doses of S. californicus spores (2 × 103, 2 × 105, and 2 × 107) at 7-day intervals (n = 6). Carrier ctrl, carrier control; S.c., S. californicus. Each column represents the mean + standard error. The asterisk indicates a statistically significant difference from the result for the carrier control (P < 0.05).
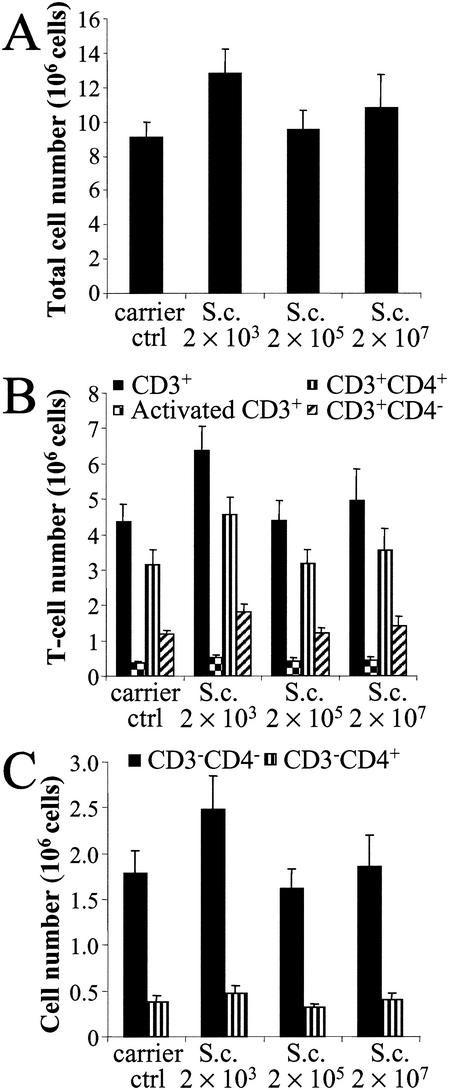
The highest increase in the total cell number (41%) in lymph nodes was detected at the lowest spore dose (2 × 103) (Fig. 3A). The middle dose (2 × 105) did not change the total cell number, and at the highest dose (2 × 107), the change was only 19%. The same dose-response pattern was seen for all cell populations in the lymph nodes (Fig. 3B and C). Even though the number of T lymphocytes increased during the spore exposure, the proportion of activated T cells remained as low as 8 to 10% (Fig. 3B). The most obvious change in the cell populations at the lowest spore dose (2 × 103) was the 53% increase in the number of CD3+ CD4− cells. The middle dose (2 × 105) had only a slight effect on the cell populations in the lymph nodes. The highest spore dose (2 × 107) was most effective at increasing the total cell number (19%) and the number of activated lymphocytes (18%).
FIG. 3.
Total cell (A), T-lymphocyte (B), and CD3− cell (C) numbers in lymph nodes 24 h after the last (sixth) instillation of graded doses of S. californicus spores (2 × 103, 2 × 105, and 2 × 107) at 7-day intervals (n = 5 to 6). Carrier ctrl, carrier control; S.c., S. californicus. Each column represents the mean + standard error.
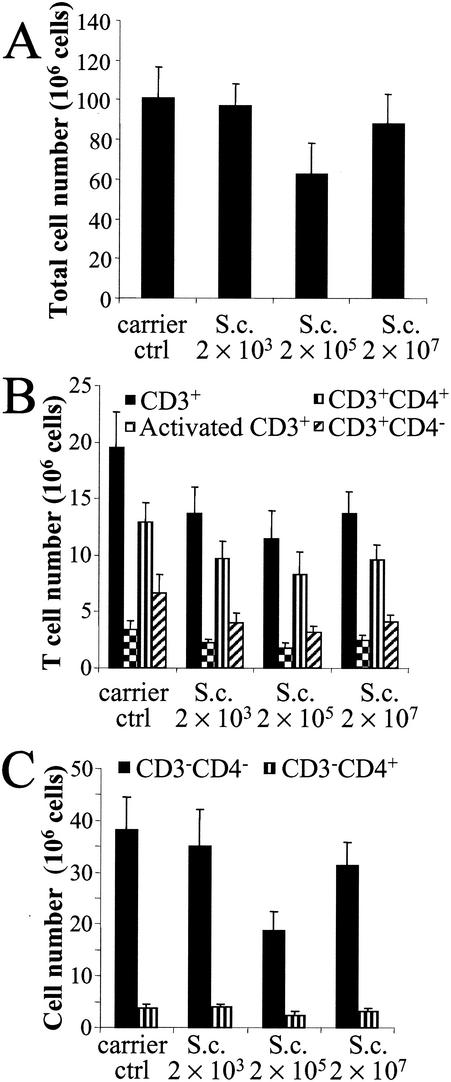
The total cell number decreased in the spleen during spore exposure (Fig. 4A). This effect was observed especially at the dose of 2 × 105 spores, the decrease being 38%. Interestingly, the effect was partly reversed at the highest spore dose (2 × 107), when the total cell number was only 13% lower than that in the controls. The numbers of T lymphocytes (CD3+ cells), activated T cells (CD3+ CD25+ cells), and helper T cells (CD3+ CD4+ cells) and especially the amounts of other T cells (CD3+ CD4− cells) decreased at all spore doses, and the strongest effect was again detected at the dose of 2 × 105 spores, when the decreases were 41, 47, 36, and 52%, respectively (Fig. 4B). The lowest spore dose (2 × 103) affected the amount of CD3− cells to a lesser extent than it did the amount of CD3+ cells. The higher doses, particularly the dose of 2 × 105 spores, decreased both CD3− CD4− cell (P = 0.057) and CD3− CD4+ cell numbers (Fig. 4C). The changes in cell populations in the lymph nodes and spleen were not statistically significant.
FIG. 4.
Total cell (A), T-lymphocyte (B), and CD3− cell (C) numbers in the spleen 24 h after the last (sixth) instillation of graded doses of S. californicus spores (2 × 103, 2 × 105, and 2 × 107) at 7-day intervals (n = 5 to 6). Carrier ctrl, carrier control; S.c., S. californicus. Each column represents the mean + standard error.
Production of proinflammatory cytokines.
To evaluate whether repeated dosing of the spores of S. californicus maintained proinflammatory cytokine production, the concentrations of TNF-α and IL-6 in BALF and serum were measured. At 24 h after the last dosage, both cytokines were at the control level or below the detection limit in both BALF and serum (data not shown).
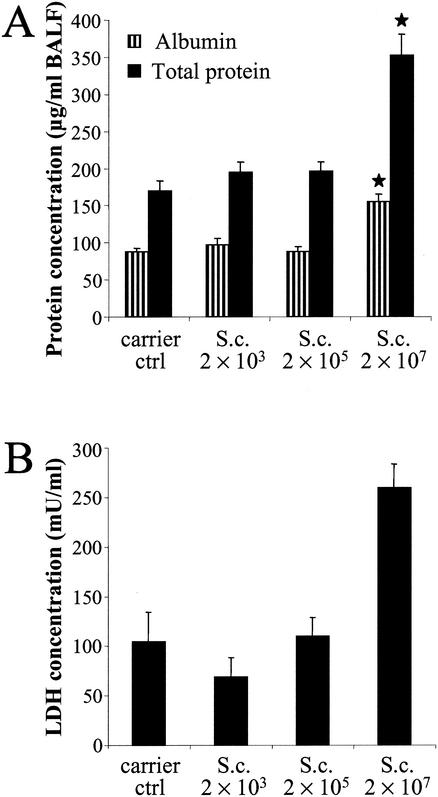
Albumin, total protein, and LDH concentrations.
Vascular leakage and cytotoxicity in lungs were assessed further by measuring the albumin, total protein, and LDH concentrations in BALF. The highest spore dose increased the concentrations of all three. The albumin concentration was almost 80% higher in the exposed group than in the control group (Fig. 5A). The total protein concentration doubled, and the LDH concentration increased by 2.5-fold in the exposed group (2 × 107 spores) compared to that in the control group (Fig. 5A and B).
FIG. 5.
Albumin, total protein (A), and LDH (B) concentrations in BALF 24 h after the last (sixth) instillation of graded doses of S. californicus spores (2 × 103, 2 × 105, and 2 × 107) at 7-day intervals (n = 7). Carrier ctrl, carrier control; S.c., S. californicus. Each column represents the mean + standard error. The asterisk indicates a statistically significant difference from the result for the carrier control (P < 0.05).
Expression of iNOS.
To assess NO production in lungs, the expression of iNOS protein in BALF cells was determined by Western blotting. The iNOS protein was not detected at any dose level 24 h after the last dosage.
Genotoxicity.
The genotoxic potential of the spores was evaluated by analyzing damage to the DNA in circulating leukocytes by the alkaline SCG assay at 24 h after the last dosage. No increased DNA damage was detected after the spore exposure (data not shown).
Histopathological changes in lungs.
No notable macroscopic changes were observed at necropsy. By histopathological analysis, the highest spore dose (2 × 107) induced moderate to abundant increases in the numbers of mononuclear cells and neutrophils in the alveoli and bronchiolar lumen. The amounts of peribronchial and vascular mononuclear cells also increased. Moreover, in one of the three mice, these mononuclear cells also created granuloma-like formations. In one of the three animals exposed to the lowest spore dose (2 × 103), there was a slight to moderate increase in the numbers of neutrophils in the alveoli and bronchiolar lumen. These changes either were focal or existed in larger aggregates. The dose of 2 × 105 spores did not cause any histopathological changes. The highest spore dose induced reactive changes, i.e., mild follicular hyperplasia, in the lymph nodes. Lower doses did not cause detectable changes in the lymph nodes, nor did any of the doses evoke any observable changes in the spleen or liver.
DISCUSSION
Our results indicate that repeated pulmonary exposure to the spores of S. californicus induces complex dose-dependent immunological responses in the mouse. Severe inflammation was evident in the lungs, reflected by the intense inflammatory cell response as well as by histopathological analysis. The decrease in the cellularity of the spleen was seen. In lymph nodes, immunostimulation was observed at the lowest spore dose. The dose-response patterns in the spleen, lymph nodes, and even the lungs resembled each other, indicating that the exposure-induced effects were not limited to the lungs.
The spores of S. californicus caused a dose-dependent inflammation in the lungs. The highest spore dose (2 × 107) caused a dramatic inflammatory cell flow into the airways. This flow mainly consisted of neutrophils, but the numbers of macrophages and lymphocytes also increased. Neutrophils and macrophages are the cells typically found in nonadaptive immunological responses. Macrophages, as antigen-presenting cells, and lymphocytes are important in adaptive immunological responses. These cellular responses suggest that both adaptive and nonadaptive immunological responses are involved in the inflammation caused by the spores. The observed neutrophil and macrophage responses were markedly stronger after repeated intratracheal dosing of 2 × 107 spores than those detected after a single dosage at the present or an even much higher dose level (20). This kind of potentiation in lymphocyte numbers was not observed. In line with the present results, it has been shown that during repeated airway exposure to 90 μg (for 3 successive days a week for up to 9 weeks) of killed Thermoactinomyces vulgaris, which is also a gram-positive bacterium, macrophages were increasingly recruited into the airways for up to 9 weeks and neutrophils were increasingly recruited into the airways for up to 6 weeks (41). Lymphocyte numbers were also increased by the T. vulgaris exposure, but the response did not change after 3 weeks. Regulation of the lymphocyte response during long-term airway exposure is a complex issue. It has been shown that repeated intratracheal dosing with particulate T-cell antigen (sheep red blood cells) can even diminish lymphocyte numbers via an apoptotic pathway in the mouse lungs compared to the response caused by administration of a single dose (26, 42). One explanation for the relatively subdued lymphocyte response in the present study may be the active microbial secondary metabolites which are released during the exposure. S. californicus has been reported to produce Ca2+- and calmodulin-dependent phosphodiesterase (PDE1) inhibitor (25), which increases intracellular cyclic AMP and cyclic GMP levels via PDE1 inhibition. This inhibition can affect the function of activated lymphocytes (21) and cause apoptosis in proliferating lymphocytes (14, 18, 22, 24). Moreover, the streptomycetes are capable of producing very powerful immunosuppressive substances, such as rapamycin, which inhibits the clonal expansion and activation of T cells by inhibiting signaling through the IL-2 receptor (CD25) and FK506 (tacrolimus), which has the same effect but which acts by inhibiting IL-2 synthesis (5, 29, 45).
The results of flow cytometric analysis corroborated the recruitment of lymphocytes into the lungs during the spore exposure. Both the influx of T cells into the lungs and increases in their activities were detected. The majority of the cells consisted of helper T cells, but the amounts of other T-cell populations also increased. The observed CD3+ CD4− population includes CD8+ killer T cells and γδ+ T cells. However, the adaptive immune response is not totally T-cell mediated, since an intense increase in the CD3− CD4− population was observed. The CD3− CD4− population within the lymphocyte gate includes not only B lymphocytes but also natural killer cells. Interestingly, a marked increase in the CD3− CD4+ cell population was evident during the spore exposure. It has previously been shown that unconventional lymphocytes can be found in the lungs and other tissues; for example, extrathymic T cells appeared in murine lungs during mycobacterial cord factor-induced inflammation (39). In addition, infection with the intracellular pathogen Chlamydia pneumoniae has been reported to increase dramatically the atypical CD3− CD4+ CD8+ cell population in NIH/S mouse lungs (31). The same population was also observed during influenza A virus infection, indicating that the population was not chlamydia specific. Even though it is not known whether the spore exposure-induced cell population coexpresses the CD8 receptor, it is possible that these two cell populations are of a similar type. These results suggest that an extracellular microorganism may also provoke an increase in this atypical cell population. In the few clinical studies whose results have been published, exposure to microbes in moisture-damaged buildings has been associated with changes in lymphocyte populations in blood (6, 19). Even though these changes were relatively minor and many factors (e.g., the age of the subjects, exposure levels, and the microbial flora in the buildings) may affect the responses, making the interpretation even more difficult, the lymphocyte populations may play a role in causing adverse health effects.
The spores instilled in the lungs also affected the cell populations in the lymph nodes and spleen. There was a trend toward increased numbers of cells in the lymph nodes but decreased numbers of cells in the spleen after the spore exposure. The dose-responses were not linear, suggesting an interplay of opposing effects. The decrease in the amount of the splenocytes is of interest, since it is well known that streptomycetes are producers of anthracyclines, such as daunorubicin and doxorubicin, drugs widely used in cancer chemotherapy (2). Their deleterious effects on splenocytes have been demonstrated in several studies (8, 9, 46). These agents have been shown to cause a rapid and massive depletion of T and B lymphocytes, especially in the spleen, moderate levels of depletion in the lymph nodes, and lower levels of depletion in the thymus after injection of a single low intraperitoneal dose in the mouse (8). Moreover, this could also partly explain the relatively low lymphocyte numbers observed in the airways after repeated spore exposure, since these substances are cytotoxic to both nonactivated and activated human peripheral lymphocytes via an apoptotic pathway in vitro (8).
Even the lowest spore dose (2 × 103) induced a slight but detectable cellular response in the lungs and lymph nodes. The middle dose (2 × 105) seemed to abolish the immunological stimulation, presumably due to immunosuppressive or other immunotoxic effects. The highest spore dose (2 × 107) caused severe inflammation by markedly stimulating both nonadaptive and adaptive immunological host defenses in the lungs. At this dose level, the immunostimulation was also observed by histopathological analysis as reactive changes in the lymph nodes. This powerful immunological response might partly overcome the immunotoxic effects of the spores in the spleen and lymph nodes.
The highest spore dose also had other effects in the lungs. It increased both albumin and total protein concentrations in BALF. The increase in albumin levels was likely due to the increased vascular permeability during the inflammation. The total protein concentration increased more than the albumin concentration did, indicating that the concentrations of other proteins in the airways were also increased. LDH, which is an indicator of cytotoxicity, increased in BALF concomitantly with the albumin and total protein concentrations. The spores of S. californicus are also not highly toxic to mouse RAW264.7 macrophages in vitro (10-12), and clear cytotoxicity was evident only after a single high dose of 3 × 108 spores in mice (20).
We have previously shown (20) that the same batch of spores increased the production of proinflammatory cytokines (TNF-α and IL-6) after a single intratracheal spore dose. The cytokine responses in BALF had already peaked at 6 h, but a moderate IL-6 response induced by 2 × 107 spores was also detectable at 24 h. In the present study, no increased production of these cytokines was observed 24 h after the last dosage. This may be due to waning of the proinflammatory response, and subsequently, other mediators may become operative during the longer-term exposure. This kind of phenomenon has been observed in another murine model by particulate antigen exposure (42). Likewise, the iNOS protein was not detected in BALF cells at 24 h after the last dosage, although the spores were able to induce the expression of iNOS in mouse cells both in vitro and in vivo (10-12, 20). After a single intratracheal spore dose and during the consequent acute inflammation, the iNOS protein was transiently detectable (20). Although streptomycetes are capable of producing genotoxic substances (4, 13, 40), no genotoxicity was detected in blood leukocytes after repeated intratracheal dosing of the spores. However, local genotoxicity, especially in the lungs, cannot be excluded, even though no genotoxicity was observed in the blood.
In the present study, even the lowest dose of S. californicus spores (2 × 103) affected the cell populations in the lungs and lymph nodes, although the differences were not statistically significant. A clear immunotoxic effect was observed at the dose level of 2 × 105 spores. In a single-dose study, 108 spores caused a transient inflammation in mouse lungs which almost disappeared within 7 days (20). Also, the lowest single dose studied (2 × 107) evoked some inflammation. Hence, considerably smaller spore amounts are sufficient to cause responses upon repeated exposure, and the effects are not limited to the lungs. The spores of S. californicus provoked some effects in the lungs at the same level of exposure, based on spore numbers, as toxic spores of Stachybotrys chartarum after repeated intranasal instillations in mice (28). This implies that the spores of S. californicus should also be considered a health hazard in water-damaged buildings.
In summary, repeated exposure of the lungs to S. californicus spores originally isolated from a moisture-damaged building provoked immunological responses that were dependent on the dose. The responses were presumably determined by the immunotoxic and immunostimulating properties of the spores. Severe inflammation mediated by both nonadaptive and adaptive immunological mechanisms was observed at a higher dose level than systemic immunotoxicity was.
Acknowledgments
This study was supported by The Finnish Research Programme on Environmental Health, The Academy of Finland.
We thank Leena Heikkinen, Kati Huttunen, Marjut Roponen, Heli Martikainen, Arja Rönkkö, Anne Seppä, Irma Väänänen, and Arja Kinnunen for excellent technical assistance. We are also grateful to Anne Hyvärinen for microbiological expertise, Jenni Vuola for consultation about tissue homogenization and cell isolation, and Ewen MacDonald for reading and commenting on the manuscript.
REFERENCES
- 1.Alderson, G., D. A. Ritchie, C. Cappellano, R. H. Cool, N. M. Ivanova, A. S. Huddleston, C. S. Flaxman, V. Kristufek, and A. Lounes. 1993. Physiology and genetics of antibiotic production and resistance. Res. Microbiol. 144:665-672. [DOI] [PubMed] [Google Scholar]
- 2.Arcamone, F. M. 1998. From the pigments of the actinomycetes to third generation antitumor anthracyclines. Biochimie 80:201-206. [DOI] [PubMed] [Google Scholar]
- 3.Arndt, C., M. C. Cruz, M. E. Cardenas, and J. Heitman. 1999. Secretion of FK506/FK520 and rapamycin by Streptomyces inhibits the growth of competing Saccharomyces cerevisiae and Cryptococcus neoformans. Microbiology 145:1989-2000. [DOI] [PubMed] [Google Scholar]
- 4.Bolzan, A. D., and M. S. Bianchi. 2001. Genotoxicity of streptonigrin: a review. Mutat. Res. 488:25-37. [DOI] [PubMed] [Google Scholar]
- 5.Brazelton, T. R., and R. E. Morris. 1996. Molecular mechanisms of action of new xenobiotic immunosuppressive drugs: tacrolimus (FK506), sirolimus (rapamycin), mycophenolate mofetil and leflunomide. Curr. Opin. Immunol. 8:710-720. [DOI] [PubMed] [Google Scholar]
- 6.Dales, R., D. Miller, J. White, C. Dulberg, and A. I. Lazarovits. 1998. Influence of residential fungal contamination on peripheral blood lymphocyte populations in children. Arch. Environ. Health 53:190-195. [DOI] [PubMed] [Google Scholar]
- 7.Dales, R. E., H. Zwanenburg, R. Burnett, and C. A. Franklin. 1991. Respiratory health effects of home dampness and molds among Canadian children. Am. J. Epidemiol. 134:196-203. [DOI] [PubMed] [Google Scholar]
- 8.Ferraro, C., L. Quemeneur, A. F. Prigent, C. Taverne, J. P. Revillard, and N. Bonnefoy-Berard. 2000. Anthracyclines trigger apoptosis of both G0-G1 and cycling peripheral blood lymphocytes and induce massive deletion of mature T and B cells. Cancer Res. 60:1901-1907. [PubMed] [Google Scholar]
- 9.Fornasiero, M. C., M. Ferrari, P. Gnocchi, D. Trizio, and A. M. Isetta. 1992. Immunodepressive activity of FCE 23762 on humoral and cell-mediated immune responses in normal mice: comparison with doxorubicin. Agents Actions 37:311-318. [DOI] [PubMed] [Google Scholar]
- 10.Hirvonen, M.-R., A. Nevalainen, N. Makkonen, J. Mönkkönen, and K. Savolainen. 1997. Streptomyces spores from mouldy houses induce nitric oxide, TNFα and IL-6 secretion from RAW264.7 macrophage cell line without causing subsequent cell death. Environ. Toxicol. Pharmacol. 3:57-63. [DOI] [PubMed] [Google Scholar]
- 11.Hirvonen, M.-R., A. Nevalainen, N. Makkonen, J. Mönkkönen, and K. Savolainen. 1997. Induced production of nitric oxide, tumor necrosis factor, and interleukin-6 in RAW264.7 macrophages by streptomycetes from indoor air of moldy houses. Arch. Environ. Health 52:426-432. [DOI] [PubMed] [Google Scholar]
- 12.Hirvonen, M.-R., M. Ruotsalainen, K. Savolainen, and A. Nevalainen. 1997. Effect of viability of actinomycete spores on their ability to stimulate production of nitric oxide and reactive oxygen species in RAW264.7 macrophages. Toxicology 124:105-114. [DOI] [PubMed] [Google Scholar]
- 13.Holmalahti, J., J. Mäki-Paakkanen, L. Kangas, and A. von Wright. 1996. Genotoxicity of dihydroabikoviromycin, a secondary metabolite of Streptomyces anulatus. Mutat. Res. 368:157-163. [DOI] [PubMed] [Google Scholar]
- 14.Hurwitz, R. L., K. M. Hirsch, D. J. Clark, V. N. Holcombe, and M. Y. Hurwitz. 1990. Induction of a calcium/calmodulin-dependent phosphodiesterase during phytohemagglutinin-stimulated lymphocyte mitogenesis. J. Biol. Chem. 265:8901-8907. [PubMed] [Google Scholar]
- 15.Hyvärinen, A., T. Reponen, T. Husman, and A. Nevalainen. 2001. Comparison of the indoor air quality in mould damaged and reference buildings in a subarctic climate. Cent. Eur. J. Public Health 9:133-139. [PubMed] [Google Scholar]
- 16.Hyvärinen, A., T. Reponen, T. Husman, J. Ruuskanen, and A. Nevalainen. 1993. Characterizing mold problem buildings—concentrations and flora of viable fungi. Indoor Air 3:337-343. [Google Scholar]
- 17.Ichimura, M., R. Eiki, K. Osawa, S. Nakanishi, and H. Kase. 1996. KS-505a, an isoform-selective inhibitor of calmodulin-dependent cyclic nucleotide phosphodiesterase. Biochem. J. 15:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, X., J. Li, M. Paskind, and P. M. Epstein. 1996. Inhibition of calmodulin-dependent phosphodiesterase induces apoptosis in human leukemic cells. Proc. Natl. Acad. Sci. USA 93:11236-11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johanning, E., R. Biagini, D. Hull, P. Morey, B. Jarvis, and P. Landsbergis. 1996. Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in a water-damaged office environment. Int. Arch. Occup. Environ. Health 68:207-218. [DOI] [PubMed] [Google Scholar]
- 20.Jussila, J., H. Komulainen, K. Huttunen, M. Roponen, A. Hälinen, A. Hyvärinen, V.-M. Kosma, J. Pelkonen, and M.-R. Hirvonen. 2001. Inflammatory responses in mice after intratracheal instillation of spores of Streptomyces californicus isolated from indoor air of a moldy building. Toxicol. Appl. Pharmacol. 171:61-69. [DOI] [PubMed] [Google Scholar]
- 21.Kanda, N., and S. Watanabe. 2001. Regulatory roles of adenylate cyclase and cyclic nucleotide phosphodiesterases 1 and 4 in interleukin-13 production by activated human T cells. Biochem. Pharmacol. 62:495-507. [DOI] [PubMed] [Google Scholar]
- 22.Kizaki, H., K. Suzuki, T. Tadakuma, and Y. Ishimura. 1990. Adenosine receptor-mediated accumulation of cyclic AMP-induced T-lymphocyte death through internucleosomal DNA cleavage. J. Biol. Chem. 265:5280-5284. [PubMed] [Google Scholar]
- 23.Lacey, J., and B. Crook. 1988. Fungal and actinomycete spores as pollutants of the workplace and occupational allergens. Ann. Occup. Hyg. 32:515-533. [DOI] [PubMed] [Google Scholar]
- 24.Lerner, A., D. H. Kim, and R. Lee. 2000. The cAMP signaling pathway as a therapeutic target in lymphoid malignancies. Leukoc. Lymphoma 37:39-51. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda, Y., and H. Kase. 1987. KS-619-1, a new inhibitor of Ca2+ and calmodulin-dependent cyclic nucleotide phosphodiesterase from Streptomyces californicus. J. Antibiot. (Tokyo) 40:1104-1110. [DOI] [PubMed] [Google Scholar]
- 26.Milik, A. M., V. A. Buechner-Maxwell, J. Sonstein, S. Kim, G. D. Seitzman, T. F. Beals, and J. L. Curtis. 1997. Lung lymphocyte elimination by apoptosis in the murine response to intratracheal particulate antigen. J. Clin. Investig. 99:1082-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nevalainen, A., A.-L. Pasanen, M. Niininen, T. Reponen, P. Kalliokoski, and M. J. Jantunen. 1991. The indoor air quality in Finnish homes with mold problems. Environ. Int. 17:299-302. [Google Scholar]
- 28.Nikulin, M., K. Reijula, B. B. Jarvis, P. Veijalainen, and E. L. Hintikka. 1997. Effects of intranasal exposure to spores of Stachybotrys atra in mice. Fundam. Appl. Toxicol. 35:182-188. [PubMed] [Google Scholar]
- 29.Ochiai, T., Y. Gunji, M. Nagata, A. Komori, T. Asano, and K. Isono. 1993. Effects of rapamycin in experimental organ allografting. Transplantation 56:15-19. [DOI] [PubMed] [Google Scholar]
- 30.Panzone, G., A. Trani, P. Ferrari, L. Gastaldo, and L. Colombo. 1997. Isolation and structure elucidation of 7,8-dideoxy-6-oxo-griseorhodin C produced by Actinoplanes ianthinogenes. J. Antibiot. (Tokyo) 50:665-670. [DOI] [PubMed] [Google Scholar]
- 31.Penttilä, J. M., R. Pyhälä, M. Sarvas, and N. Rautonen. 1998. Expansion of a novel pulmonary CD3− CD4+ CD8+ cell population in mice during Chlamydia pneumoniae infection. Infect. Immun. 66:3290-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pirhonen, I., A. Nevalainen, T. Husman, and J. Pekkanen. 1996. Home dampness, moulds and their influence on respiratory infections and symptoms in adults in Finland. Eur. Respir. J. 9:2618-2622. [DOI] [PubMed] [Google Scholar]
- 33.Platt, S. D., C. J. Martin, S. M. Hunt, and C. W. Lewis. 1989. Damp housing, mould growth, and symptomatic health state. Br. Med. J. 24:1673-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samson, R. A., B. Flannigan, M. E. Flannigan, A. P. Verhoeff, O. C. C. Adan, and E. S. Hoekstra. 1994. Health implications of fungi in indoor environments. In R. A. Samson, B. Flannigan, M. E. Flannigan, A. P. Verhoeff, O. C. C. Adan, and E. S. Hoekstra (ed.), Air quality monographs, vol. 2. Elsevier Science B.V., Amsterdam, The Netherlands.
- 35.Singh, N. P., M. T. McCoy, R. R. Tice, and E. L. Schneider. 1988. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 175:184-191. [DOI] [PubMed] [Google Scholar]
- 36.Sorenson, W. G. 1999. Fungal spores: hazardous to health? Environ. Health. Perspect. 107:469-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spengler, J., L. Neas, S. Nakai, D. Dockery, F. Speizer, J. Ware, and M. Raizenne. 1994. Respiratory symptoms and housing characteristics. Indoor Air 4:72-82. [Google Scholar]
- 38.Strachan, D. P. 1988. Damp housing and childhood asthma: validation of reporting of symptoms. Br. Med. J. 12:1223-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabata, A., K. Kaneda, H. Watanabe, T. Abo, and I. Yano. 1996. Kinetics of organ-associated natural killer cells and intermediate CD3 cells during pulmonary and hepatic granulomatous inflammation induced by mycobacterial cord factor. Microbiol. Immunol. 40:651-658. [DOI] [PubMed] [Google Scholar]
- 40.Tai, K. W., Y. C. Chang, L. S. Chou, and M. Y. Chou. 1998. Cytotoxic effect of pingyangmycin on cultured KB cells. Oral Oncol. 34:219-223. [DOI] [PubMed] [Google Scholar]
- 41.Takizawa, H., M. Suko, N. Kobayashi, K. Ohta, S. Shoji, T. Horiuchi, H. Okudaira, T. Miyamoto, and J. Shiga. 1989. Spontaneous regression in murine hypersensitivity pneumonitis: lack of immunological tolerance. Int. Arch. Allergy Appl. Immunol. 89:173-180. [DOI] [PubMed] [Google Scholar]
- 42.Todt, J., J. Sonstein, T. Polak, G. D. Seitzman, B. Hu, and J. L. Curtis. 2000. Repeated intratracheal challenge with particulate antigen modulates murine lung cytokines. J. Immunol. 164:4037-4047. [DOI] [PubMed] [Google Scholar]
- 43.Tsuge, N., K. Furihata, K. Shin-Ya, Y. Hayakawa, and H. Seto. 1999. Novel antibiotics pyrisulfoxin A and B produced by Streptomyces californicus. J. Antibiot. (Tokyo) 52:505-507. [DOI] [PubMed] [Google Scholar]
- 44.Waegemaekers, M., N. Wageningen, B. Brunekreef, and J. S. M. Boleij. 1989. Respiratory symptoms in damp homes. Allergy 44:192-198. [DOI] [PubMed] [Google Scholar]
- 45.Wallemacq, P. E., and R. Reding. 1993. FK506 (tacrolimus), a novel immunosuppressant in organ transplantation: clinical, biomedical, and analytical aspects. Clin. Chem. 39:2219-2228. [PMC free article] [PubMed] [Google Scholar]
- 46.Zaleskis, G., S. Verstovsek, T. S. Tzai, E. Mihich, and M. J. Ehrke. 1995. Doxorubicin and cyclosporin A affect murine lymphoid cells expressing different antigenic determinants. Oncol. Res. 7:307-315. [PubMed] [Google Scholar]
- 47.Zar, J. H. 1996. Multiple comparisons, p. 227. In J. H. Zar (ed.), Biostatistical analysis. Prentice-Hall Inc., Englewood Cliffs, N.J.