Abstract
Detection of antibodies to an outer membrane protein 2 (OMP2) by enzyme-linked immunosorbent assay (ELISA) by using either the Chlamydia trachomatis- or the Chlamydia pneumoniae-specific protein was investigated. OMP2 is an immunodominant antigen giving rise to antibody responses in humans infected with different C. trachomatis serovars (A to C and D to K) or with C. pneumoniae, which could be detected by OMP2 ELISA. OMP2 ELISA is not species specific, but antibody titers were usually higher on the homologous protein. The sensitivity of this assay was high but varied according to the “gold standard” applied. Levels of antibody to C. pneumoniae OMP2 as detected by ELISA seem to return to background or near-background values within a shorter period of time compared to antibodies to C. pneumoniae detected by microimmunofluorescence (MIF), making it more likely that positive results in ELISA reflect recent infection. Thus, OMP2 ELISA has distinct advantages over MIF and commercially available ELISAs and might be a useful tool for the serodiagnosis of chlamydial infection.
Chlamydiae are major human pathogens. Their unique and complex reproductive cycle can enable the effective evasion of the host's defense mechanisms, leading to persisting infection. Immune responses stimulated by chlamydial infection can result in tissue damage and scar formation, particularly upon reinfection (reviewed in reference 8). Chlamydia trachomatis can cause eye or genitourethral infections and is the most common cause of preventable blindness in a “trachoma-belt” stretching from North Africa to Southeast Asia (serovars A to C) and is also a major reason for infertility in women due to chronic pelvic inflammatory disease (serovars D to K) (6, 20). More recently, Chlamydia pneumoniae, a common pathogen of respiratory infections, has been implicated in the pathogenesis of atherosclerosis due to its persistence in vascular tissue (18). Early detection of chlamydial infection and antibiotic therapy can prevent subsequent sequelae in a majority of patients.
Antibody responses have been utilized in the diagnosis of chlamydial infections in addition to detection of chlamydia by culture from patients' specimens, which is difficult and can only be performed in specialized laboratories, by detection of chlamydial genome by microbiological techniques (ligase chain reaction [LCR] or reverse transcription-PCR [RT-PCR]) or detection of chlamydial antigen by enzyme immunoassay (EIA). A microimmunofluorescence (MIF) test, which detects antibodies binding to Chlamydia elementary bodies (EBs), has long been considered to be the “gold standard” for the serodiagnosis of chlamydial infections (11). Alternative methods have been established, including various enzyme-linked immunsosorbent assays (ELISAs), which are much easier to perform than MIF and suitable for large-scale testing. This raises the question of what are the best antigens to use in ELISA-based serological diagnosis. An ideal antigen would be one recognized by all patients infected by a particular species, for example C. trachomatis. Antibodies to the major outer membrane protein (or outer membrane protein 1 [OMP1]) distinguish between different C. trachomatis serovars, which is not always helpful since several serovars would be a preferable target for serodiagnosis. However, it would also be desirable to use an antigen that is not recognized as part of the immune response to other Chlamydia species. This is necessary given the high frequency of infection with C. pneumoniae in the normal population so that many patients with C. trachomatis infection will already have encountered C. pneumoniae.
The major advantage of MIF over all other known serodiagnostic tools in chlamydial infection is its claim to species specificity, although recent reports have argued against it (26). Currently available ELISAs also show cross-reactivity between different chlamydial species despite various attempts at removing or blocking genus-reactive epitopes from whole EB preparations, by using the deacylated carbohydrate backbone of a recombinant chlamydial lipopolysaccharide epitopes or a recently identified, not-yet-characterized peptide epitope (1, 2, 12-15, 17). We have therefore selected an antigen, which is immunodominant and well conserved in all known C. trachomatis serovars but with the potential to distinguish between species. In immunoblots, antibody responses to proteins of 40 kDa (major outer membrane protein) and 60 kDa have been described for both C. trachomatis and C. pneumoniae (1, 3, 7, 11, 16). Likely candidates for the 60-kDa reactivity are the heat shock protein 60 (hsp60) and OMP2. Immune responses to chlamydial hsp60 have shown that hsp60 is not suitable for the serodiagnosis of chlamydial infection (17). OMP2 shows considerable variability between the different chlamydial species but is highly conserved within C. trachomatis serovars and C. pneumoniae isolates (23, 24). Therefore, we have developed ELISAs by using recombinant C. trachomatis and C. pneumoniae OMP2 and tested them for their utility in the diagnosis of chlamydial infection.
MATERIALS AND METHODS
Patients.
The study population comprised eight different groups of patients (Table 1). The first group included 93 patients (age range, 13 to 60 years; median, 20 years) from The Gambia, a trachoma-endemic region. Of these, 27 (29%) had clinically active disease (11 with follicular trachoma, 5 with intense trachoma, and 11 with scarring trachoma) and 16 active disease plus positive results in an IDEIAssay for Chlamydia LPS in tear fluid. The second group comprised 25 patients with suspected C. trachomatis infection attending the local outpatient clinic for genitourinary diseases. The third group consisted of four patients with chlamydia-associated reactive arthritis. From one of these patients serum samples were available from the onset of disease and over a subsequent period of 2 years. The fourth group consisted of four patients with a history of acute respiratory disease and proven C. pneumoniae infection. These sera were kindly made available by M. Sillis, Public Health Laboratory, Norwich, United Kingdom. From these patients, serum samples were available from 4 to 12 weeks and from 0.5 to 3 years after the onset of symptoms, whereas an additional serum sample was obtained from two of these patients during the acute illness. The fifth group comprised 14 patients with atherosclerosis of the carotid artery (age range, 55 to 88 years; median, 76 years) who were undergoing thrombendarterectomy. The sixth group included sera from 100 patients with stable angina pectoris (age range, 39 to 85 years; median, 68 years), who were otherwise healthy and had no previous history of myocardial infarction. The seventh group consisted of 100 blood donors visiting the local blood donor center; serum samples were supplied anonymously. The ages of the blood donors ranged from 20 to 55 years, with median age of ca. 30 years. As negative controls, sera from 19 children aged 2 to 7 years were included. These sera were negative for chlamydial infection, as judged by C. trachomatis and C. pneumoniae MIF analyses. Plasma and serum samples were stored at −20°C prior to use.
TABLE 1.
Characteristics of the patient groups examined
| Patient group | No. of patients | Median age (yr) | Associated factor(s) | Method of chlamydial infection diagnosis |
|---|---|---|---|---|
| The Gambia (trachoma-endemic region) | 93 | 20 | C. trachomatis, serovars A and B | EIA |
| Suspected C. trachomatis-associated urethritis | 25 | 25 | C. trachomatis, serovars D to K | LCR |
| C. trachomatis-associated reactive arthritis | 4 | 28 | C. trachomatis, serovars D to K | Culture, PCR |
| C. pneumoniae-associated respiratory infection | 4 | 50 | C. pneumoniae | PCR |
| Carotid artery stenosis | 14 | 76 | (C. pneumoniae) | NA |
| Stable angina pectoris | 100 | 68 | (C. pneumoniae) | NA |
| Healthy blood donors | 100 | 30 | NAa | NA |
| Negative controls | 19 | 4 | NA | NA |
NA, not applicable.
Recombinant proteins.
The expression of recombinant protein was performed utilizing pQE60 plasmids (QIAexpressionist system [Qiagen, Hilden, Germany]). OMP2-encoding plasmids from C. trachomatis serovar B (strain B/jali20/OT) and genomic OMP2-DNA from C. pneumoniae (strain IOL 207) were a kind gift from I. N.Clarke, Southampton, United Kingdom. All genetic manipulation work was done with E. coli strain M15[pRep4].
Oligonucleotides for use as sequencing or PCR primers were purchased from Perkin-Elmer (Perkin-Elmer Corp., Norwalk, Conn.). PCRs were performed in a Hybaid thermal cycler (Hybaid, Ashford, Middlesex, United Kingdom). OMP2 gene cassettes from C. pneumoniae were generated by PCR by using two synthetic, restriction site-containing oligonucleotides, each based on the sequences described by Watson et al. (24, 25): C. trachomatis OMP2 (5′-GTC CAT GGT GGC GAG TTT ATT TGC TAG C-3′ and 5′-ATG GAT CCG ATG TGT GTA TTC TCT GTA TC-3′) and C. pneumoniae OMP2 (5′-GTC CAT GGG CAC GAG TAT GGC GAG TTG CTT TG-3′ and 5′-GTG GAT CCA TAC ACG TGG GTA TTT TCT GTG TCT GA-3′) (the original chlamydial sequences are underlined). Cloning was done according to the manufacturer's recommendations. Transformants were screened for correct insertion of the coding fragment by restriction analysis of the pQE plasmid DNA, by PCR, and by directly screening the colonies for the expressed protein. In addition, the coding fragments were sequenced to exclude deviations from the published DNA sequences.
Protein purification.
The expression of recombinant protein was induced by the addition of isopropyl-β-d-thiogalactosidase to Escherichia coli cultures. The protein was purified by using NiSO4-charged HiTrap chelating Sepharose columns (Pharmacia Biotech, Uppsala, Sweden), which bind the His6 tail of the recombinant proteins, and applied to a modified continuous preparative elution electrophoresis system, a so-called prepcell (Bio-Rad, Hercules, Calif.). The purity of the obtained protein preparations was >98%, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with densitometry. C. trachomatis and C. pneumoniae OMP2 were refolded by stepwise dialysis into 10 mM Tris-HCl (pH 10).
ELISA.
Antigen was diluted in 0.1 M bicarbonate buffer (pH 9.5) to a concentration of 0.25 μg/ml, and 100 μl was dispensed into each well of a microtiter plate (Nunc Maxisorp). After incubation overnight at 4°C, plates were washed thrice with Tris buffer (0.01 M Tris.Cl, 0.15 M NaCl) and 150 μl of blocking buffer (5% [wt/vol] sucrose and 5% [vol/vol] Tween 20 in Tris buffer) was added for 1 h to inhibit nonspecific binding. Sera were diluted 1/200 in blocking buffer, and the peroxidase-conjugated anti-human immunoglobulin G (IgG) antibody (Jackson ImmunoResearch, West Grove, Pa.) was diluted 1/10,000 in washing buffer (0.5% Tween 20 in Tris buffer). Then, 100 μl of diluted serum or antibody was added per well, followed by incubation at room temperature for 2 and 1 h, respectively. Between each step, the plates were washed with washing buffer three times for 2 min. Next, 100 μl of K Blue substrate (Neogen, Lexington, Ky.) was placed in each well, and the mixtures were left at room temperature for 30 min. The enzyme reaction was stopped by the addition of 25 μl of 2 M H2SO4, and the A450 value was read in a Labsystems Multiskan ELISA reader (Labsystems OY, Helsinki, Finland). Each serum was tested in duplicate both in antigen-coated and in noncoated wells. A positive reference serum and a negative reference serum, as judged by MIF and ELISA, were included on each plate. The net absorbances of test samples were corrected to the positive reference serum by multiplying the measured net absorbance by the net absorbance of the positive reference serum obtained when the standard curve was constructed divided by the net absorbance of the positive reference serum. The reproducibility was >95%.
The mean net absorbance (x) of 19 serum samples from 2- to 7-year-old children nonreactive in MIF assay for IgG antibodies to either C. trachomatis or C. pneumoniae were used to determine the threshold of positivity. Net absorbances of test samples of less than x + 2 standard deviations (SD) were considered negative, i.e., nonreactive.
Serum samples from blood donors positive in MIF and ELISA on C. trachomatis and C. pneumoniae were used to create standard curves at the beginning of the work, and all subsequent assays interpreted as follows. Seven serum samples were tested in serial twofold dilutions beginning at 1/25. The endpoint titer was defined as the reciprocal of the highest serum dilution nonreactive in ELISA. For each antigen and serum dilution, a linear regression line was fitted to the net absorbance as a function of the endpoint titer. The coefficients of linear correlation for the 1/100 dilution were 0.94 for C. trachomatis OMP2 and 0.96 for C. pneumoniae OMP2. In subsequent experiments, antibody titers were read off the respective standard curve, when the results resided in the linear range. In all other cases, the antibody titer was determined by twofold dilutions beginning at 1/100.
MIF.
C. trachomatis and C. pneumoniae MIF was performed with a commercially available test kit (Labsystems OY) according to the manufacturer's instructions.
Statistical methods.
Data were analyzed by use of the Fisher exact t test wherever appropriate.
RESULTS
Antibodies to OMP2 in acute Chlamydia infection. (i) C. trachomatis infections.
To determine the antibody responses to OMP2 in C. trachomatis infection, sera from two different populations were tested: one group consisted of patients with infections generally caused by serovars A to C and the other group consisted of patients with infections generally caused by serovars D to K.
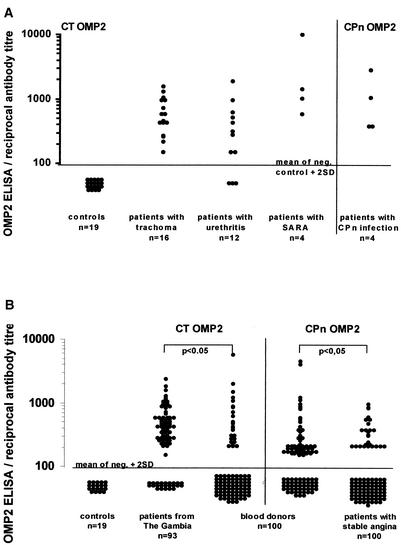
Sixteen patients sera from the Gambia were examined. All had follicular conjunctivitis and positive results in an IDEIAssay for Chlamydia LPS in tear fluid. All sixteen patients were positive in the C. trachomatis OMP2 ELISA. Twelve sera were from patients attending the local genitourinary outpatient clinic, whose urethral or vaginal swabs were positive for chlamydiae by EIA and LCR. Nine sera (75%) gave positive results in the C. trachomatis OMP2 ELISA. In addition, four sera from patients with sexually acquired reactive arthritis, two of which had culture-proven C. trachomatis infection, were tested. All sera were drawn shortly after the onset of symptoms. All four (100%) were positive in the C. trachomatis OMP2 ELISA (Fig. 1A).
FIG. 1.
(A) Distribution of antibody titers to OMP2 in 16 patients with trachoma (C. trachomatis serovars A to C), 12 patients with urethritis, 4 patients with sexually acquired reactive arthritis (C. trachomatis serovars D to K), and 4 patients with acute C. pneumoniae infection as measured by ELISA on the homologous protein. In all patients, acute infection with C. trachomatis or C. pneumoniae was proven by IDEIA, LCR, culture, or PCR, respectively. The cutoff was defined as the mean of negative control sera + 2 SD. (B) Distribution of antibody titers to OMP2 (C. trachomatis and C. pneumoniae OMP2), as measured by ELISA on the protein indicated, in 100 healthy blood donors (median age, ca. 30 years), 93 patients from The Gambia (median age, 20 years), and 100 patients with stable angina (median age, 68 years), who were otherwise healthy and had no recent history of myocardial infarction. The cutoff was defined as the mean of negative control sera + 2 SD.
(ii) C. pneumoniae infections.
Four patients with acute respiratory infection (serologically and antigen positive for C. pneumoniae) were tested, all of whom gave positive results in the C. pneumoniae OMP2 ELISA (Fig. 1A).
Antibodies to OMP2 in healthy individuals, patients with ischemic heart disease, and inhabitants of a trachoma-endemic region.
To determine the antibody response in a control population, sera from 100 healthy United Kingdom blood donors were tested by C. trachomatis and C. pneumoniae OMP2 ELISA. A total of 27 (27%) were positive on C. trachomatis OMP2, and 54 (54%) were positive on C. pneumoniae OMP2. In contrast, sera from 93 individuals from The Gambia were examined by using C. trachomatis OMP2 ELISA. A total of 70 (75%) gave positive results (Fig. 1B).
In addition, sera from 100 patients with stable angina pectoris, who were otherwise healthy and had no previous history of myocardial infarction, were examined by C. pneumoniae OMP2 ELISA. Twenty-nine (29%) of these were positive in the C. pneumoniae OMP2 ELISA (Fig. 1B).
Species specificity of OMP2 ELISA.
Sera from the patients shown in Fig. 1A were tested on both the homologous and the heterologous protein. In 25 of 33 (76%) cases, the antibody titers were higher on the homologous protein (data not shown).
Longitudinal studies.
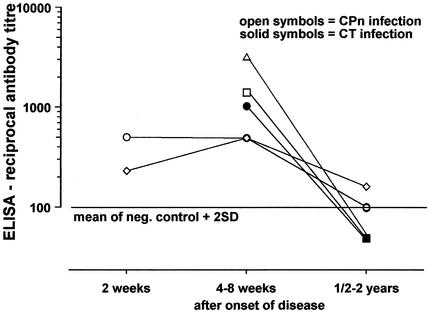
Serial serum samples were available from five patients, four patients with C. pneumoniae infection, and one patient with C. trachomatis. The level of antibodies against chlamydial OMP2 declined to background levels over a period of 1 to 2 years (Fig. 2).
FIG. 2.
Longitudinal study showing the distribution of antibody titers of serial serum samples from four patients with acute C. pneumoniae and one patient with acute C. trachomatis infection. The cutoff was defined as the mean of negative control sera + 2 SD.
Clinical applications of OMP2 ELISA.
Chronic chlamydial infection has been implicated in the pathogenesis of atherosclerosis, and most of the serological data supporting this theory were obtained by using MIF. We therefore tested sera from 14 patients with end-stage atherosclerosis who had undergone thrombendarterectomy of the carotid artery (age range, 55 to 88 years; median, 76 years) by ELISA and MIF. Only two (14%) of these patients were found to be positive by C. pneumoniae OMP2 ELISA, whereas nine (64%) of these patients were positive by MIF on C. pneumoniae EBs: three with titers of >1/512 and six with titers that were between 1/128 and 1/32 (data not shown).
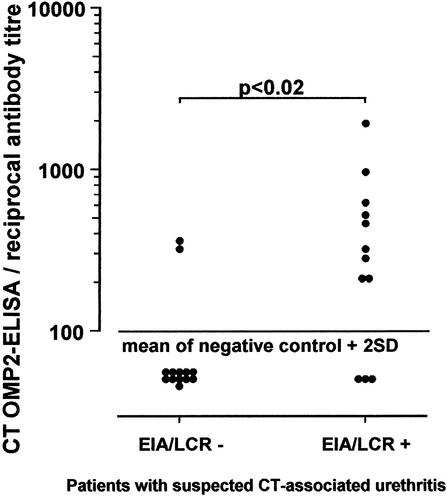
A reliable serologic test for the diagnosis of chlamydial infection is especially desirable in pelvic inflammatory disease, since the available tests are invasive. We therefore compared sera from patients with suspected C. trachomatis infection attending the local genitourinary outpatient clinic whose vaginal or urethral swab tests were negative (13) or positive (12) by LCR and EIA. In the first subgroup two (15%) sera and in the second subgroup nine (75%) sera were positive in the C. trachomatis OMP2 ELISA (Fig. 3).
FIG. 3.
Distribution of antibody titers to C. trachomatis OMP2 in sera from patients with suspected C. trachomatis-associated urethritis attending the local genitourinary clinic, whose vaginal or urethral swab tests were positive or negative for chlamydiae by LCR and EIA. The cutoff was defined as the mean of negative control sera + 2 SD.
DISCUSSION
A reliable test for the serodiagnosis of chlamydial infection is not currently available. We have therefore established an ELISA with recombinant C. trachomatis and C. pneumoniae OMP2, since OMP2 is very well conserved within a chlamydial species but shows variable regions between different species (9, 22, 23, 24). Additionally, recombinant proteins have the advantage of being well-defined (in contrast to chlamydial EBs) and are easily applied to quantitative production.
OMP2 has been shown to be an immunodominant antigen leading to antibody responses both in humans and rabbits as detected by immunoblot (23, 25). A recent report has confirmed these findings by ELISA utilizing peptides from the variable N-terminal and constant C-terminal regions of C. trachomatis OMP2 (15). However, data concerning species specificity derived from these studies are controversial since in the ELISA study, denatured, truncated polypeptides were used, whereas in the immunoblotting study 60- or 62-kDa polypeptide bands reacting in immunoblot with serum antibodies were assumed to represent OMP2 but were not actually positively identified.
For the validation of this assay, sera were chosen from patients with both clinical evidence of chlamydial infection and unequivocal evidence of chlamydial infection as shown by culture, LCR, or EIA. In these patients we were able to demonstrate a high sensitivity for OMP2 ELISA with sera from patients with acute chlamydial infection, although only a relatively small group of patients with acute C. pneumoniae infection was available for analysis. These findings are in contrast with sensitivities reported for MIF and commercially available ELISAs, although a direct comparison is not possible since different gold standards were defined for each study (4, 21). The lowest sensitivity was calculated for C. trachomatis OMP2 ELISA with sera from patients attending the local genitourinary outpatient clinic, which might be due to a delayed seroconversion (5). Thus, although it is evident that the diagnosis of C. trachomatis infection in genitourinary clinics must rest primarily on the demonstration of the organism (by culture, staining, or molecular techniques), OMP2 serology may be a useful adjunct in this clinical setting by giving an indication of the likelihood that the patient has been previously infected by C. trachomatis.
Reports on the prevalence of IgG antibodies to C. pneumoniae as judged by MIF gave similar results (∼50%) in young, healthy adults (19) compared to the results obtained by C. pneumoniae OMP2 ELISA in our blood donor group (54%). Whereas the prevalence of IgG antibodies to whole chlamydia reported for MIF increases with age (19), the prevalence of IgG antibodies to C. pneumoniae OMP2 as detected by ELISA decreases to 29%, which might be due to a lower sensitivity of ELISA compared to MIF, as proposed by Hermann et al. (10). Alternatively, this finding may be explained by the fact that the antibody response against C. pneumoniae recognized by MIF persist for a longer period of time (3), thus indicating chronic infection, than than those recognized by OMP2 ELISA, as suggested by our results in the longitudinal study (Fig. 2).
Also, there was a marked difference in the results obtained by MIF and C. pneumoniae OMP2 ELISA in older patients with atherosclerosis (median age, 76 years). C. pneumoniae OMP2 ELISA may therefore be more useful in assessing how recently C. pneumoniae infection has occurred, in itself an important issue in the current discussion concerning the role of chlamydiae in the pathogenesis of atherosclerosis.
Clearly, OMP2 ELISA is not species specific. Nevertheless, in 75% of cases the antibody titer was higher on the homologous protein. This suggests that there may be antibodies directed against the N-terminal region which shows sequence variation between species. Such antibodies were not detected by the study of Mygind et al. (15), but epitopes deriving from this region might still be conformational in nature and not present on the truncated and denatured proteins used in their study. In contrast, our study used full-length refolded proteins. The same comments apply to the study of Watson et al. (25), who also argued that the variable N-terminal region of OMP2 has no or limited immunogenicity.
In conclusion, OMP2 is an immunodominant antigen giving rise to antibody responses by humans infected with C. trachomatis (serovars A to C or serovars D to K) and with C. pneumoniae; these antibodies can be reliably detected by OMP2 ELISA. The sensitivity of this assay is high. The fact that antibodies to C. pneumoniae OMP2 in ELISA decline much faster than those detected by C. pneumoniae MIF may make C. pneumoniae OMP2 ELISA a useful tool, since positive results are more likely to reflect recent infection. OMP2 ELISA might therefore have a role in the serodiagnosis of chlamydial infection.
REFERENCES
- 1.Black, C. M., J. E. Johnson, C. E. Farshy, T. M. Brown, and B. P. Berdal. 1991. Antigenic variation among strains of Chlamydia pneumoniae. J. Clin. Microbiol. 29:1312-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brade, L., H. Brunnemann, M. Ernst, Y. Fu, O. Holst, P. Kosma, H. Naher, K. Persson, and H. Brade. 1994. Occurrence of antibodies against chlamydial lipopolysaccharide in human sera as measured by ELISA using an artificial glycoconjugate antigen. FEMS Immunol. Med. Microbiol. 8:27-41. [DOI] [PubMed] [Google Scholar]
- 3.Campbell, L. A., C.-C. Kuo, S.-P. Wang, and J. T. Grayston. 1990. Serological response to Chlamydia pneumoniae infection. J. Clin. Microbiol. 28:1261-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chernesky, M., K. Luinstra, J. Sellors, J. Schachter, J. Moncada, O. Caul, I. Paul, L. Mikaelian, B. Toye, J. Paavonen, and J. Mahony. 1997. Can serology diagnose upper genital tract Chlamydia trachomatis infection? Sex. Transm. Dis. 25:14-19. [DOI] [PubMed] [Google Scholar]
- 5.Clad, A., H. M. Freidank, M. Kunze, U. Schnoeckel, S. Hofmeier, U. Flecken, and E. E. Petersen. 2000. Detection of seroconversion and persistence of Chlamydia trachomatis antibodies in five different serological tests. Eur. J. Clin. Microbiol. Infect. Dis. 19:932-937. [DOI] [PubMed] [Google Scholar]
- 6.Dawson, C. R., B. R. Jones, and M. L. Tarrizzo (ed.). 1985. Guide to trachoma control in programs for the prevention of blindness, p. 8-47. World Health Organization, Geneva, Switzerland.
- 7.Deane, K. H., R. M. Jecock, J. H. Pearce, and J. S. Gaston. 1997. Identification and characterization of a DR4-restricted T-cell epitope within chlamydia heat shock protein 60. Clin. Exp. Immunol. 109:439-445. [DOI] [PMC free article] [PubMed]
- 8.Grayston, J. T., S. P. Wang, L. J. Yeh, and C. C. Kuo. 1985. Importance of reinfection in the pathogenesis of trachoma. Rev. Infect. Dis. 7:717-725. [DOI] [PubMed] [Google Scholar]
- 9.Hanuka, N. M. Glaser, and I. Sarov. 1988. Detection of IgG and IgA antibodies to Chlamydia trachomatis in sera of patients with chlamydial infection: use of immunoblotting and peroxidase assay. Sex. Transm. Dis. 15:93-99. [DOI] [PubMed] [Google Scholar]
- 10.Hermann, C., K. Graf, A. Groh, E. Straube, and T. Hartung. 2002. Comparison of eleven commercial tests for Chlamydia pneumoniae-specific immunoglobulin G in asymptomatic healthy individuals. J. Clin. Microbiol. 40:1603-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ijima, Y., N. Miyashita, T. Kishimoto, Y. Kanamoto, R. Soejima, and A. Matsumoto. 1994. Characterization of Chlamydia pneumoniae species-specific proteins immunodominant in humans. J. Clin. Microbiol. 32:583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladany, S., C. M. Black, C. E. Farshy, J. M. Ossewaarde, and R. B. Barnes. 1989. Enzyme immunoassay to determine exposure to Chlamydia pneumoniae (strain TWAR). J. Clin. Microbiol. 27:2778-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laferriere, C., R. W. Peeling, E. S. Tackaberry, J. Hamel, J.-A. Dillon, and B. R. Brodeur. 1993. A novel approach to the laboratory diagnosis of Chlamydia trachomatis infections using monoclonal anti-idiotype antibodies. J. Immunol. Methods 163:123-131. [DOI] [PubMed] [Google Scholar]
- 14.Marston, E. L., A. V. James, J. T. Parker, J. C. Hart, T. M. Brown, T. O. Messmer, D. L. Jue, C. M. Black, G. M. Carlone, E. W. Ades, and J. Sampson. 2002. Newly characterized species-specific immunogenic Chlamydia pneumoniae peptide reactive with murine monoclonal and human serum antibodies. Clin. Diagn. Lab. Immunol. 9:446-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mygind, P., G. Christiansen, K. Persson, and S. Birkelund. 1998. Analysis of the humoral immune response to Chlamydia outer membrane protein 2. Clin. Diagn. Lab. Immunol. 5:313-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newhall, V., W. J., B. Batteiger, and R. B. Jones. 1982. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect. Immun. 38:1181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peeling, R. W., J. Kimani, F. Plummer, I. Maclean, M. Cheang, J. Bwayo, and R. C. Brunham. 1997. Antibody to chlamydial hsp60 predicts an increased risk for chlamydial pelvic inflammatory disease. J. Infect. Dis. 175:1153-1158. [DOI] [PubMed] [Google Scholar]
- 18.Saikku, P., M. Leinonen, K. Mattila, M. R. Ekman, M. S. Nieminen, P. H. Makela, J. K. Huttunen, and V. Valtonen. 1988. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary artery disease and acute myocardial infarction. Lancet ii:983-986. [DOI] [PubMed]
- 19.Saikku, P. 1999. Epidemiology of Chlamydia pneumoniae in atherosclerosis. Am. Heart J. 138:S500-503. [DOI] [PubMed] [Google Scholar]
- 20.Schachter, J., and C. R. Dawson (ed.). 1978. Human chlamydial infections, p. 63-69. PSG Publishing Co., Littleton, Colo.
- 21.Verkooyen, R. P., D. Willemse, S. C. A. M. Hiep-van Casteren, S. A. Mousavi-Joulandan, R. J. Snijder, J. M. M. van den Bosch, H. P. T. van Helden, M. F. Peeters, and H. A. Verbruch. 1998. Evaluation of PCR, culture, and serology for diagnosis of Chlamydia pneumoniae respiratory infections. J. Clin. Microbiol. 36:2301-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagar, E. A., J. Schachter, P. Bavoil, and R. S. Stephens. 1990. Differential human serologic response to 60,000 molecular weight Chlamydia trachomatis antigens. J. Infect. Dis. 162:922-927. [DOI] [PubMed] [Google Scholar]
- 23.Wagels, G., S. Rasmussen, and P. Timms. 1994. Comparison of Chlamydia pneumoniae isolates by Western blot (immunoblot) analysis and DNA sequencing of the OMP2 gene. J. Clin. Microbiol. 32:2820-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson, M. W., P. R. Lambden, and I. N. Clarke. 1991. Genetic diversity and identification of human infection by amplification of the chlamydial 60-kD cysteine-rich outer membrane protein gene. J. Clin. Microbiol. 29:1188-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson, M. W., P. R. Lambden, J. S. Everson, and I. N. Clarke. 1994. Immunoreactivity of the 60-kDa cysteine-rich proteins of Chlamydia trachomatis, Chlamydia psittaci, and Chlamydia pneumoniae expressed in Escherichia coli. Microbiology 140:2003-2011. [DOI] [PubMed] [Google Scholar]
- 26.Wong, Y. K., J. M. Sueur, C. H. D. Fall, J. Orfila, and M. E. Ward. 1999. The species specificity of the microimmunofluorescence antibody test and comparisons with a time resolved fluoroscopic immunoassay for measuring IgG antibodies against Chlamydia pneumoniae. J. Clin. Pathol. 52:99-102. [DOI] [PMC free article] [PubMed] [Google Scholar]