Abstract
Most conventional vaccines consist of killed organisms or purified antigenic proteins. Such molecules are generally poorly immunogenic and need to be coupled to carrier proteins. We have identified a new carrier molecule, BB, derived from the G protein of Streptococcus strain G148. We show that BB is able to induce strong antibody responses when conjugated to peptides or polysaccharides. In order to localize T and B cell epitopes in BB and match them with the albumin-binding region of the molecule, we immunized mice with BB, performed B and T pepscan analyses, and compared the results with pepscan done with sera and cells from humans. Our results indicate that BB has two distinct T helper epitopes, seven linear B-cell epitopes, and one conformational B-cell epitope in BALB/c mice. Four linear B-cell epitopes were identified from human sera, three of which overlapped mouse B-cell epitopes. Finally, three human T-cell epitopes were detected on the BB protein. One of these T-cell epitopes is common to BALB/c mice and humans and was localized in the region that contains the albumin-binding site. These data are of interest for the optimization of new carrier molecules derived from BB.
Most conventional vaccines consist of killed organisms or purified antigenic proteins. However, there is considerable interest in the production of synthetic peptides (45) or polysaccharides (24) corresponding to immunogenic epitopes of pathogens. Such molecules are generally poorly immunogenic and need to be coupled to carrier proteins. Several carrier proteins have been used in humans for several years. Among them, tetanus toxoid (TT) (21), diphtheria toxoid (DT) (7), and cross-reacting material 197 of DT (CRM197) (14), the outer membrane protein complex of Neisseria meningitidis (19), are parts of marketed vaccines. Most of these proteins have been well characterized, especially TT and DT, where T helper epitopes have been localized (17, 34, 36). These peptides have been used in preclinical models or in clinical trials to initiate a CD4 T-cell response (43). Other carrier proteins have been fully characterized to identify T-cell epitopes that could be used as vaccines in a similar manner to TTp30, which is incorporated in antigens and used in cancer vaccines to break the tolerance (5, 6).
We have identified a new carrier molecule, BB, derived from the G protein of Streptococcus strain G148 (28). It binds serum albumin with high affinity (2, 10), and thus BB fusion proteins can be efficiently purified by affinity chromatography on albumin-Sepharose (27). The serum albumin-binding region has been located in the N-terminal portion (20, 23). It has been demonstrated that proteins fused to BB have a significantly increased in vivo half-life in rodent and nonhuman primate models (25, 29, 42, 44). Finally, we (22) and others (41) have demonstrated that BB exhibited carrier-related properties since antibody responses to peptides or proteins fused to BB were significantly enhanced. Clinical trials with BB fused to a protein derived from the respiratory syncytial virus are ongoing (33). In the present study, it is been shown that BB is able to induce strong antibody responses when conjugated to peptides or polysaccharides. In order to localize T- and B-cell epitopes in BB and match them with the albumin-binding region of the molecule to design new carrier molecules derived from BB, we immunized mice with BB and performed B- and T-cell Pepscan analyses. Our results indicate that BB has two distinct T helper epitopes and eight linear B-cell epitopes in BALB/c mice. Four linear B-cell epitopes have been identified from human sera, three of which overlapped mouse B-cell epitopes. Finally, three human T-cell epitopes were detected on the BB protein. One of these T-cell epitopes is common to BALB/c mice and humans and was localized in the region that contains the albumin-binding site.
MATERIALS AND METHODS
Protein, peptide, and polysaccharide.
Gene assembly, vector construction, and BB protein expression in Escherichia coli were undertaken as previously described (27). BB was purified by affinity chromatography on albumin-Sepharose, followed by cation-exchange chromatography and reversed-phase high-performance liquid chromatography (RP-HPLC). TT was purchased from SBL Vaccin AB (Stockholm, Sweden) and keyhole limpet hemocyanin (KLH) was from Pierce (Rockford, Ill.). The protected peptide chain corresponding to the G5 sequence [positions 144 to 159; (Cys)-Ser-Lys-Pro-Thr-Thr-Lys-Gln-Arg-Gln-Asn-Lys-Pro-Pro-Asn-Lys-Pro-(Cys)] of the attachment G protein of the RSV-A subgroup was synthesized with an additional cysteine at the N or C terminus, allowing coupling to the BB carrier protein. The chain was assembled by the solid-phase method on an Applied Biosystems 433A synthesizer by using Fmoc (9-fluorenylmethoxy carbonyl)/tbutyl (tBu) chemistry. The side chain protecting groups were trityl (Trt) for Asn, Gln, and Cys; tBu for Ser and Thr; pentamethylchromansulfonyl (Pmc) for Arg and Pro, and tert-butyloxycarbonyl (tBoc) for Lys. The crude peptide, cleaved from the resin with trifluoroacetic acid in the presence of scavengers, was lyophilized and purified by preparative RP-HPLC. The purity of the peptide was >95% according to RP-HPLC and free zone capillary electrophoresis. The measured mass (1,950.80 Da ± 0.31) of the purified peptide as determined by electrospray-mass spectrometry was in close agreement with that calculated from the theoretical sequence (1,951.29 Da). P40 (the outer membrane protein from Klebsiella pneumoniae with a molecular mass of 40 kDa) was prepared as previously described (13). Haemophilus influenzae type b (Hib) polysaccharide was kindly provided by Rino Rapuoli, Chiron Biocine Immunobiological Research Institute, Siena, Italy.
Peptide-carrier protein coupling.
Peptide G5, a major B-cell epitope of G2Na, the region from positions 130 to 230 of the G protein derived from the respiratory syncytial virus (32), was conjugated to BB by its C-terminal cysteine residue by using N-hydroxysuccinimidyl bromoacetate (Pierce) as a coupling reagent as previously described (15, 35). The peptide/protein molar ratio was determined by amino acid analysis by using the Waters Pico-Tag HPLC system. Amino acids released from the conjugates by hydrolysis with 6 N HCl at 160°C for 2 h were analyzed as their phenylthiohydantoin derivatives. The degree of reaction was determined by quantifying the amount of S-carboxymethylcysteine calculated from the comparison of its integrated value with a known amount introduced into an amino acid standard solution. Typically, the G5/BB ratio was between 5 and 8. The same method was applied for KLH-G5 conjugation.
Polysaccharide-carrier protein coupling.
Conjugation of the Hib polysaccharides to BB, TT, and bovine serum albumin (BSA) was carried out as described by Chu et al. (4). The polysaccharide was activated with cyanogen bromide (Sigma) at pH 10.5 for 6 min and then reacted with 0.1 M adipic acid dihydrazide (Sigma) at pH 8.5 overnight at room temperature. After dialysis and purification by gel filtration on a Sephadex G-100 column (2.6 by 61 cm; Amersham Pharmacia Biotech, Saclay, France), the polysaccharide was freeze-dried. The percentage of hydrazide on the polysaccharide was measured by a 1,3,5-trinitrobenzenesulfonic assay with adipic acid dihydrazide as a standard. The resultant polysaccharide contained ca. 0.9 to 1.5% (wt/wt) adipic hydrazide. For conjugation, this adipic hydrazide polysaccharide derivative and proteins were used at 10 mg/ml each, and coupling was achieved with 0.1 M 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (Sigma) at pH 5 for 3 h at 4°C. The conjugates were purified from the reaction by a Sepharose CL-4B column equilibrated with phosphate-buffered saline (PBS). Protein fractions eluted in the void volume were combined, concentrated by ultrafiltration, and stored at 4°C. Conjugates were analyzed for carbohydrate with the orcinol-ferric chloride-HCl assay by using ribose as a standard (38) and for protein with the bicinchoninic acid assay (Pierce) with BSA as a standard. Polysaccharide/protein (wt/wt) ratios were determined to be 0.7, 1.1, and 1.0 for the BB-Hib, TT-Hib, and BSA-Hib conjugates, respectively. All conjugates were also characterized by gel filtration and sodium dodecyl sulfate-polyacrylamide gel electrophoresis analyses to demonstrate the covalency of the polysaccharide-protein linkage and the absence of uncoupled protein.
Immunization of mice.
Groups of five BALB/c mice were immunized subcutaneously (s.c.) on days 0, 14, and 28 with 0.5 μg of G5 peptide equivalent or 0.5 μg of Hib polysaccharide equivalent, conjugated with BB or TT, and suspended in PBS, with aluminum hydroxide (20% [vol/vol]; Superfos Biosector a/s, Vedbaek, Denmark). Mice were bled from the retro-orbital venous plexus at regular intervals to determine, respectively, anti-G5 or anti-Hib serum antibody titers. Control groups included mice immunized with PBS or immunogens plus adjuvant alone. For specific-epitopic suppression, experiments mice were primed with 100 μg of BB or TT before being immunized three times with 0.1 μg of G5 conjugated to BB or TT, respectively.
ELISA.
Briefly, for anti-G5 antibody titration, Immulon 2 microtiter plates (Dynatech, Chantilly, Va.) were coated overnight at 4°C with KLH-G5 (1 μg of peptide/ml) in carbonate buffer (pH 9.8). Nonspecific reactions were blocked with PBS-0.5% gelatin (Serva, Heidelberg, Germany). The antibody samples were serially diluted and stored for 2 h at room temperature. Plates were then extensively washed in PBS before the addition of 100 μl of peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG; Pierce) or goat anti-mouse IgG1 or IgG2a (Southern Biotechnology Associates, Birmingham, Ala.) per well for 1 h at 37°C. After a washing step, a solution of 3,3′,5,5′-tetramethylbenzidine (KPL) was added to each well. The reaction was proceeded for 10 min and stopped by the addition of 1 M H2SO4. The absorbance at 450 nm was determined by using a Labsystems IEMS reader (Labsystems, Helsinki, Finland). Titers were calculated as the reciprocal of the serum dilution that gave an A450 of >2 standard deviations above that of a negative control serum. The results are expressed in log10, and for each group of mice the means are calculated as geometric means. Vertical bars represent standard deviations. For anti-Hib antibody titration, Immulon-2 microtiter plates were coated with 25 μg of Hib conjugated to BSA. Thereafter, the method was performed as previously described. For these assays, it has been previously determined that the anti-BB and anti-TT antibody responses do not interfere with the coating molecules.
MAb production.
To generate monoclonal antibodies (MAbs), BALB/c mice were immunized twice s.c. with 3 μg of BB in complete Freund adjuvant for the first immunization and in incomplete Freund adjuvant for the boost. Four days later, a further intravenous inoculation of 3 μg of BB was performed. The mice were killed, and spleen cells were fused with SP2/0-Ag14 myeloma cells (American Type Culture Collection, Rockville, Md.) by using polyethylene glycol (Serva). Resultant hybridomas were initially screened for the secretion of BB-specific antibodies by ELISA. Positive reactors were subsequently cloned three times by serial limiting dilution and selected for their reactivity against different B-cell epitopes by Pepscan analysis.
Pepscan analysis for B-cell epitope.
Ninety-six overlapping 12-mers, with adjacent peptides overlapping by 10 amino acids, were synthesized on noncleavable derivatized rods (Mimotopes, Pty., Ltd., Clayton, Victoria, Australia) according to established procedures (12, 26). These peptides, spanning residues 1 to 116 and 176 to 257 of the BB protein, were tested for their reactivity with MAbs by ELISA. Briefly, nonspecific binding was blocked by incubation for 1 h at 37°C with PBS containing 0.1% Tween (Sigma) and 1% gelatin. The rods were subsequently incubated at 4°C overnight with MAbs, washed three times, and incubated for 1 h at room temperature with a horseradish peroxidase-conjugated goat anti-mouse antibody (1/5,000; Southern Biotechnology Associates). After being washed three times, the rods were transferred to a microtiter plate (Nunc, Roskilde, Denmark) containing 100 μl of tetramethylbenzidine (Dynatech). The reaction was terminated with 100 μl of 1 M H2SO4 per well. The optical densities were measured at 450 nm. A reactivity was considered a positive signal when the optical density was higher than twice the background level, which was defined as the mean of the tenth minor optical densities of the plate. It has been established previously that the mean of the tenth minor optical densities of the plate are representative of what is observed with a nonrelevant serum.
Peptide synthesis for T-cell analysis.
Ninety-two synthetic peptides representing the fragments from positions 1 to 120 and positions 116 to 257 of BB were produced by using the multipin DKP system (Mimotopes). Peptides were synthesized as 16-mers, with adjacent peptides overlapping by 14 amino acids. This synthesis was performed by using classical Fmoc/tBu chemistry. After side chain deprotection with a mixture of trifluoroacetic acid, ethanedithiol, and anisole (38:1:1), the peptides were cleaved in ammonium bicarbonate buffer (0.1 M, pH 8.4) with 40% (vol/vol) acetonitrile via a cyclization mechanism, leaving at the C terminus a cyclo(lysylpropyl) moiety. Finally, the peptides were freeze-dried and stored as a dry powder before use.
Murine T-cell proliferation assays.
Ten days after s.c. immunization of mice with BB, the lymph nodes were removed and a cell suspension was obtained by teasing them. Cultures were performed in triplicate in RPMI 1640 (Gibco) containing 50 U of penicillin/ml, 50 μg of streptomycin/ml, 0.25 M glutamine, and 10% fetal calf serum. The cells were stimulated in vitro by incubating 4 × 105 cells/well in 96 round-bottom plates (Costar) with various concentrations of recombinant BB. Background proliferation was measured with cells derived from PBS-immunized mice. At 54 h after plating, methyl [3H]thymidine (1 μCi; Amersham) was added to each well, and the plates were incubated for an additional 18 h. Culture were harvested onto a filter by using a semiautomatic harvester (Skatron), and the incorporated [3H]thymidine was determined by using a 1900CA-Tricarb beta counter (Packard Instruments). The stimulation index was calculated as the experimental amount of radioactivity/the basal amount of radioactivity.
Preparation of PBMC and T-cell proliferation assays.
Peripheral blood mononuclear cells (PBMC) were isolated from the blood of healthy volunteers by centrifugation on Ficoll-Paque (Amersham Pharmacia Biotech). The PBMC were adjusted to 0.25 × 106 cells/ml on RPMI 1640, 2 mmol of l-glutamine, 100 U of penicillin per ml, and 100 mg of strepotmycin per ml (Sigma) with 10% fetal calf serum. A total of 2.5 × 105 PBMC (in 100 μl) were added to each well of 96-well flat-bottom plates still containing 25 μg of each peptide to be tested. These peptides consisted of ninety-two 16-mer peptides spanning residues 1 to 120 and residues 116 to 257 of the BB and overlapping by 14 residues. Each peptide was tested in triplicate, and 2.5 μg of phytohemagglutinin per ml was then added in quadruplicate wells as a positive control. The cells were incubated at 37°C in 5% CO2 for 4 days. The cultures were pulsed with 1 μCi of [3H]thymidine (Amersham) per well for the final 18 h. Cultures were harvested onto a filter by using a semiautomatic harvester (Skatron), and the incorporated [3H]thymidine was determined by using a Packard 1900CA-Tricarb beta counter. The stimulation index was calculated as the experimental amount of radioactivity/the basal amount of radioactivity.
Human serum collection.
Sera from healthy donors were provided by the blood center of the Hôpital Cantonal, Geneva, Switzerland. These sera were screened for anti-BB antibody responses. The sera from the highest responders were diluted for Pepscan analysis.
RESULTS
Antibody responses to peptides and polysaccharides conjugated to BB.
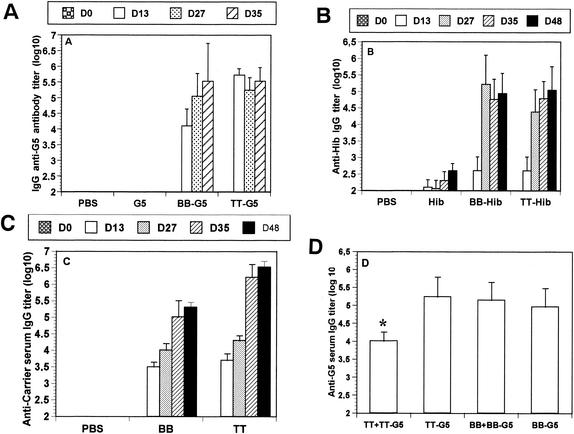
It has been shown that BB can induce an immune response against sequences from malaria antigen when genetically fused to them (41). To confirm the efficacy of BB as a carrier protein when conjugated to antigens, two models have been used. The first one involved the G5 peptide derived from the RSV G protein. For the second one, Hib polysaccharide from H. influenzae was used. For these experiments, antigens were injected alone or conjugated to BB or TT in the presence of the adjuvant aluminum hydroxide. The results presented in Fig. 1A showed that 10 μg of the G5 peptide injected s.c. into BALB/c mice failed to induce an antibody response, even in the presence of aluminum hydroxide. When injected coupled to BB, a significant anti-G5 antibody response was observed even after the first immunization. This antibody response increased after a boost and reached a peak of 5.6 log10 after three injections. After two or three immunizations, the antibody responses compared well with those observed in mice immunized with G5 coupled to the “gold standard” carrier protein TT (Fig. 1A). In the experiments with Hib polysaccharide conjugates, a significant and maximal response was observed after two injections of BB-Hib. In this case, the kinetics of antibody production were close to those observed in mice injected with TT-Hib (Fig. 1B). Figure 1C shows the anti-carrier titers observed for BB and TT. After three immunizations, the anti-TT antibody response was significantly higher than the anti-BB response at a comparable amount of carrier protein injected (0.42 μg for BB and 0.45 μg for TT). BB is a part of the streptococcal G protein, and so it is likely that people could be primed for this protein. In this context, it was particularly interesting to have some data related to specific epitopic suppression. For that purpose, mice were primed with 100 μg of BB and then immunized three times with 0.1 μg of G5 coupled to BB or TT. A significant specific epitopic suppression was observed when TT-primed mice were immunized with TT-G5, with a significant reduction of the IgG titer (Fig. 1D). In the same conditions no specific epitopic suppression was observed in BB-primed mice.
FIG. 1.
Anti-peptide (A), anti-polysaccharide (B), or anti-carrier (C) serum antibody responses. Mice were immunized three times with 10 μg of G5 or 0.5 μg of Hib alone or conjugated to BB or TT in presence of aluminum hydroxide. After each immunization, sera were collected and anti-G5, anti-Hib, or anti-carrier IgG content was measured by ELISA. (D) Specific epitopic suppression studies. Naive mice or mice primed, respectively, with TT or BB were then immunized three times with 0.1 μg of G5 conjugated, respectively, to TT or BB. Anti-G5 IgG titers were measured by ELISA. The results are expressed as the geometric means ± the standard deviation (n = 5). ∗, P < 0.05.
Isolation and characterization of B-cell epitopes on the BB protein.
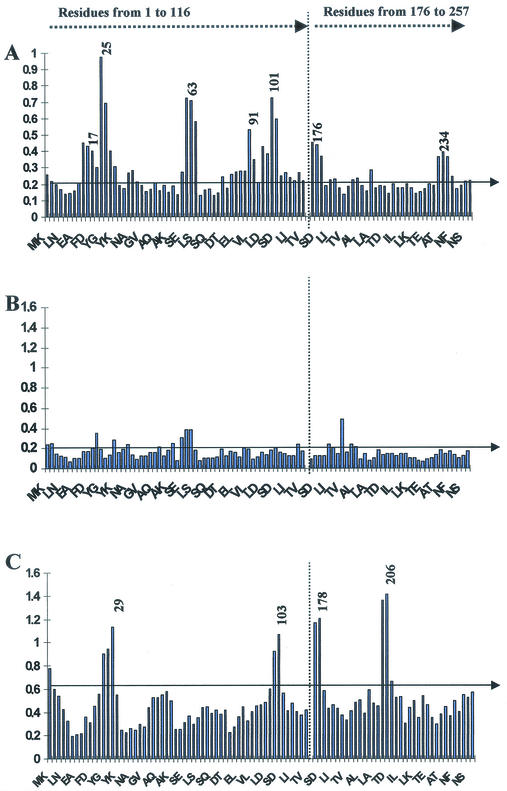
To localize B-cell determinants on the BB protein, MAbs were generated by immunizing mice with BB. Supernatants from positive clones were tested by Pepscan analysis. For this analysis, a series of peptides spanning the entire BB molecule were synthesized as 12-mers, with the exception of peptides corresponding to the region from positions 126 to 176, which were the same as those covering the fragment from positions 48 to 100 (Fig. 2). Each peptide overlapped the adjacent one by 10 amino acids. As illustrated in Table 1, seven different linear B-cell epitopes were described on the BB protein. Four of the seven MAbs generated recognized more than one peptide on the plate due to the repetitive structure of the A/B region on the BB protein. 13F10 and 5C4 MAbs recognized very close sequences, 13F10 recognized both SDYYKNLINN and SDYHKNLINN sequences, and the 5C4 MAb only recognized the GVSDYYKNLIN peptide. The reactivities observed with MAbs were in agreement with those obtained with a pool of polyclonal sera from BALB/c mice immunized with BB, with the exception of B epitopes recognized by 8B5 and 3B5 MAbs, which recognized ELAEAKVLANRE and TVEGVKDLQAQ, respectively (Fig. 3A). Some MAbs, like 5G7, seemed to be conformational MAbs recognizing BB with high titers in ELISA (5.7 log10) but did not significantly bind any peptides on the Pepscan plate (Fig. 3B). A comparison between the mouse polyclonal B-cell epitope profile and Pepscan analysis of sera from normal volunteers immunized with BBG2Na (Fig. 3C) indicated that the epitope usage was quite different, with only three overlapping reactivities located within the regions from positions 29 to 38, 103 to 112, and 177 to 186 and corresponding, respectively, to DYYKNLINNAKT, SDYHKNLINNAK, and VSDYYKNLINNA, respectively. In this Pepscan, a particular reactivity was noticed in the region from positions 206 to 215.
FIG. 2.
Structure of the BB protein. The green region correspond to the HSA-binding region. The large letters in this region are residues critical for HSA binding. The red lines indicate the BALB/c T-cell epitopes, and the broken lines indicate the human epitopes.
TABLE 1.
Characterization of the B-cell determinants of the BB protein
| MAb/isotype | Major reactivities observed during Pepscan analysis |
|
|---|---|---|
| Amino acid range | Sequencea | |
| 13F10/IgG1k | 27-38 | VSDYYKNLINNA |
| 101-112 | GVSDYHKNLINN | |
| 178-189 | SDYYKNLINNAK | |
| 5C4/IgG1k | 25-36 | YGVSDYYKNLIN |
| 176-187 | GVSDYYKNLINN | |
| 7B5/IgG1k | 17-28 | ANFDQFNKYGVS |
| 97-108 | LDKYGVSDYHKN | |
| 6B1/IgG1k | 234-245 | ARSFNFPILENS |
| 8B5/IgG1k | 85-96 | ELAEAKVLANRE |
| 11A10/IgG1k | 65-76 | DGLSDFLKSQTP |
| 3B5/IgG1k | 39-50 | KTVEGVKDLQAQ |
| 115-126 | TVEGVKDLQAQV | |
Italic type indicates consensus sequences. Underscoring indicates the amino acid differences within consensus sequences.
FIG. 3.
BB reactivities of polyclonal sera from BALB/c mice immunized three times s.c. with 3 μg of BB adjuvanted in complete Freund adjuvant for the first immunization and in incomplete Freund adjuvant for the second and third injections (A) or supernatants from the 5G7, an anti-BB monoclonal MAb generated by mouse immunization with 10 μg of BB adjuvanted as previously described (B) or polyclonal sera from a normal volunteer (C) against overlapping 12-mer peptides spanning the entire BB protein, with the exception of peptides from the region from positions 128 to 175. The mouse serum was a pool of sera from five mice and was diluted 1/5,000. Human serum was diluted 1/5,000. The supernatant from the 5G7 MAb was undiluted. The first two residues of every third peptide are indicated in the x axis. Reactivity peaks are numbered to facilitate direct comparison of the various sera; the numbers correspond to the first amino acid residues of the implicated peptide. The horizontal line indicates the background as defined in Materials and Methods.
T-cell proliferative responses to synthetic peptides.
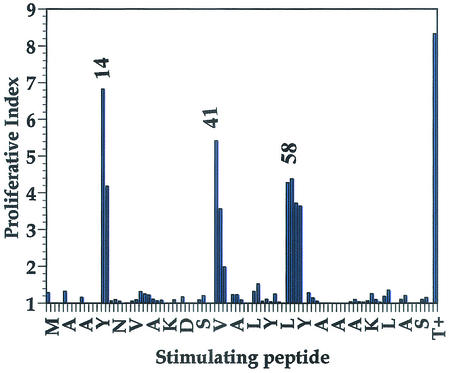
T-cell epitopes were identified by measuring the in vitro stimulatory properties of overlapping peptides on T cells from BALB/c mice immunized with BB. The results in Fig. 4 showed that three sequences located in positions 25, 81, and 174 and corresponding, respectively, to peptides 14 (VSDYYKNLINNAKTVE), 41 (VKSIELAKVLANRE), and 58 (KYGVSDYYKNLINNAK), were able to induce a significant proliferative response when added to T cells from lymph nodes of BB-immunized BALB/c mice. Peptides 14 and 58 both included the sequence VSDYYKNLINNAKT. Peptide 51, located at the region from positions 101 to 117 of BB, also contained this sequence, with the exception of a histidine residue at position 106. However, this peptide showed no T-cell epitope activity, indicating that in the VSDYYKLINNAK sequence, the second tyrosine residue was particularly involved in the interaction with the T-cell receptor. To confirm that peptides 14 and 41 were T-cell epitopes, groups of BALB/c mice were immunized with BB, peptide 14, or peptide 41. T cells from lymph nodes were collected and then stimulated in vitro with BB, peptide 14, or peptide 41 (Fig. 5). When T cells from mice immunized with BB were stimulated in vitro with BB, peptide 14, or peptide 41, a significant proliferation was observed. This result was in agreement with those obtained by Pepscan analysis. However, when mice were immunized with peptide 14, a proliferative response was observed when T cells were stimulated in vitro with BB or peptide 14 but not when they were stimulated with peptide 41. Similarly, T cells from mice immunized with peptide 41 proliferated when stimulated with peptide 41 or BB but, as expected, did not proliferate when cultivated with peptide 14. Taken together, these results confirm that the VSDYYKNLINNAKTVE and VKSIELAEAKVLANRE sequences are BALB/c T-cell epitopes on the BB protein.
FIG. 4.
Proliferative response of T cells derived from BB-immunized BALB/c mice to stimulation with BB or a panel of overlapping BB peptides. The first residues of every nine peptides are indicated in the x axis. Reactivity peaks are numbered to facilitate correlations with the sequence shown in Fig. 1; the numbers correspond to the first amino acid residues of the implicated peptide. The results are reported as the average of the proliferative index of triplicate sample experiments. This experiment is representative of three separate experiments.
FIG. 5.
Proliferative response of T cells derived from PBS-, BB-, peptide 14-, or peptide 41-immunized mice to stimulation with BB, peptide 14, or peptide 41. The results are reported as the average of triplicate proliferative index values.
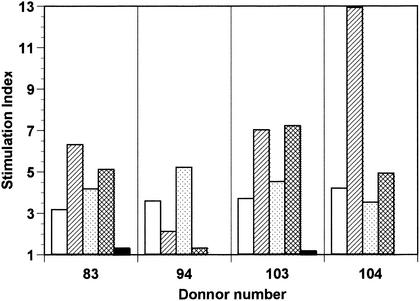
To determine whether these two peptides were also T-cell epitopes for humans, PBMC from a panel of donors were tested for their reactivity to BB. PBMC from 12 of 24 donors showed reactivity to BB, with a proliferative index from 3.1 to 16.5. This high frequency of response could be due to the fact that the majority of people could be primed for BB when infected by Streptococcus. Subsequently, the cells from five reactive donors were cultured in the presence of the synthetic peptide 14 (Fig. 6). A proliferative response to peptide 14 was observed for four of five donors. In addition to peptide 14, a variety of specific proliferative responses were also seen with peptides 33 (DGLSDFLKSQTPAEDT) and 39 (AEDTVKSIELAEAKVL) located, respectively, in positions 63 to 78 and positions 74 to 89 of the BB sequence. In contrast to the results obtained in BALB/c mice, no objective responses (index from 0.74 to 1.6) were observed with peptide 41 (VKSIELAKVLANRE) in PBMC. These results indicated that three human T-cell epitopes were found on the BB protein. No other peptides induced reproducible significant PBMC proliferation.
FIG. 6.
Proliferative response of human PBMC to peptides of the BB protein. PBMC from four of twelve donors with reactivity to the BB protein were cultured in the presence of peptides 33 (▨), 39 (▩), 14 (░⃞), or 41 (▪) at 50 μg/ml; BB (□) at 20 μg/ml; or medium alone. The data are presented as the mean values obtained from duplicate experiments.
DISCUSSION
In earlier studies, it has been shown that BB was able to induce an antibody response when fused to weak immunogens (22, 33). To demonstrate the advantage of using this carrier protein in a future conjugate vaccine, we compared the anti-peptide antibody titers elicited in mice immunized with BB-G5 or BB-Hib conjugates to those observed in mice immunized with TT-G5 or TT-Hib. Our results showed strong anti-peptide responses independently of the carrier used. Similarly, anti-Hib antibody titers were not significantly different after immunization with TT-Hib or BB-Hib. Altogether, these results indicated that BB is as effective as TT in inducing antibodies and could be considered as an alternative carrier for human vaccination.
Many studies (9, 16, 39, 40) have demonstrated that preimmunization with TT can affect responses to haptens linked to TT. The phenomenon of epitope-specific suppression through preimmunization with a carrier protein could be a drawback to their repeated use in human programs, given the fact that the majority of subjects have been vaccinated against tetanus in early childhood. The mechanism responsible for suppression of the anti-hapten antibody response to hapten-carrier conjugates upon high-dose carrier priming is still unclear. Involvement of both T suppressor cells and carrier-specific B memory cells (11, 16, 40) has been reported. Another possible explanation might be that circulating antibodies against the carrier protein are responsible for scavenging the antigens, or that they induce the formation of immune complexes which inhibit the immune response (31). This last mechanism could occur with BB because of the potential streptococcus infections in humans. To mimic this situation, mice were primed with BB before immunization with BB-G5. No specific epitopic suppression was observed, in contrast to that observed with TT, thus confirming that BB could be a good alternative carrier.
To characterize our carrier, MAbs were generated to map B-cell epitopes. Seven different linear and one conformational B-cell epitope have been identified in mice. This high number of B-cell epitopes could explain the high immunogenicity observed against BB in mice (data not shown). Two of these epitopes are shared by mice and humans. Although no specific epitope suppression has been observed in mice with BB used as the carrier in our G5 model, it is not possible to predict what could occur in humans. Thus, further work could include the reduction of immunogenicity by removal of identified B-cell epitopes of the molecule. For this purpose an extensive knowledge of the T-cell epitope would be necessary in order to delete B-cell epitopes without impairing T helper functions. Another option could be to use only the T-cell epitopes of the molecule, as has been the case for other carriers. T-cell epitopes from DT (32) and TT have been identified (8, 17, 30, 36, 37) and are currently used as defined T-cell carriers in characterized synthetic vaccines (18, 45). Efforts to create a subunit vaccine that would include the minimal components of the outer surface protein A from Borrelia burgdorferi, which causes Lyme disease, have been investigated (3). The use of synthetic T-cell epitope has also been reported with the PADRE molecule (1). Recently, TTp30 has been incorporated into different antigens in order to break immune tolerance (5, 6). In order to determine T-cell epitopes on the BB molecule, Pepscan analysis was performed. Our experiments showed that BB contained two murine T-cell epitopes located, respectively, at positions 25 to 40 (VSDYYKNLINNAKTVE) and positions 81 to 96 (VKSIELAKVLANRE) of the BB sequence. Each of these two epitopes are repeated twice in the BB protein, which could explain the efficacy of BB as a carrier in mouse models. Our results suggested that, in the T-cell epitope from positions 25 to 40 (25-40), the tyrosine residue in position 31 of the sequence was of particular interest for the T helper activity. Indeed, the peptide 102-117, which displays the same sequence with the exception of this tyrosine (VSDYHKNLINNAKTVE), showed no T helper activity. Experiments done with PBMC from healthy donors showed that 50% of them (12 of 24) proliferated when incubated with BB. Our results indicated that the peptide 25-40 seemed to be a T-cell epitope for both BALB/c mice and humans. It is interesting that this T helper epitope also contained a B-cell epitope located at the 46-amino-acid region spanning from positions 161 to 206 and was responsible for the human serum albumin (HSA) binding. Experiments done by Linhult et al. (23) showed that mutations at positions S178, Y181, K182, N183, L184, K189, and E192 decreased significantly the albumin binding. Of particular importance were the mutations at Y180 and Y181, which were shown to have the most influence on the affinity toward HSA. Taken together with the fact that our results indicated that Y181 was also critical for the T helper function, it seemed that binding to HSA and T helper activity could not be dissociated. It has been previously shown that the efficient binding of BB to serum albumin could result in an extended half-life in vivo. Indeed, fusion to BB prolonged the in vivo half-life of CD4 (22, 29, 44) and human soluble complement receptor type I (25). This property could in part explain the strong carrier activity of BB. To test this hypothesis, it would be interesting to evaluate in mice the carrier properties of BB when the T-cell epitope 25-40 is deleted or mutated.
No proliferation was observed when PBMC were cultured in the presence of the second mouse T-cell epitope. However, it appeared that two other peptides, 33 and 39, could also induce the proliferation of PBMC. These peptides are located in the same region of the mouse T-cell epitope 81-96. These results must therefore be considered and analyzed in the context of our clinical trials where PBMC from BB-immunized people will be available.
Further work will involve immunological studies in mice with various constructs containing one or many copies of each T-cell epitope conjugated to haptens to determine the optimal combination to be used in a synthetic vaccine. The use of second-generation synthetic-based vaccines, with specific T-cell epitopes from BB, will enable further directions for the design of new vaccines.
Acknowledgments
We thank A. M. Lepecquet, F. Derouet, S. Jarosz, and L. Zanna for their excellent technical assistance and A. Alder for review of the English.
REFERENCES
- 1.Alexander, J., M. F. del Guercio, A. Maewal, L. Qiao, J. Fikes, R. W. Chesnut, J. Paulson, D. R. Bundle, S. DeFrees, and A. Sette. 2000. Linear PADRE T helper epitope and carbohydrate B-cell epitope conjugates induce specific high titer IgG antibody responses. J. Immunol. 164:1625-1633. [DOI] [PubMed] [Google Scholar]
- 2.Bjorck, L., W. Kastern, G. Lindahl, and K. Wideback. 1987. Streptococcal protein G, expressed by streptococci or by Escherichia coli, has separate binding sites for human albumin and IgG. Mol. Immunol. 24:1113-1122. [DOI] [PubMed] [Google Scholar]
- 3.Bockenstedt, L. K., E. Fikrig, S. W. Barthold, R. A. Flavell, and F. S. Kantor. 1996. Identification of a Borrelia burgdorferi OspA T-cell epitope that promotes anti-OspA IgG in mice. J. Immunol. 157:5496-5502. [PubMed] [Google Scholar]
- 4.Chu, C., R. Schneerson, J. B. Robbins, and S. C. Rastogi. 1983. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect. Immun. 40:245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalum, I., M. R. Jensen, P. Hindersson, H. I. Elsner, and S. Mouritsen. 1996. Breaking of B cell tolerance toward a highly conserved self protein. J. Immunol. 157:4796-4804. [PubMed] [Google Scholar]
- 6.Dalum, I., M. R. Jensen, K. Gregorius, C. M. Thomasen, H. I. Elsner, and S. Mouritsen. 1997. Induction of cross-reactive antibodies against a self protein by immunization with a modified self protein containing a foreign T helper epitope. Mol. Immunol. 34:1113-1120. [DOI] [PubMed] [Google Scholar]
- 7.Davey, S. 1996. The vaccine challenge, p. 1-25. In State of the world's vaccines and immunization. World Health Organization/United Nations Children's Fund, Geneva, Switzerland.
- 8.Diethelm-Okita, B. M., D. K. Okita, L. Banaszak, and B. M. Conti-Fine. 2000. Universal epitopes for human CD4+ cells on tetanus and diphtheria toxins. J. Infect. Dis. 181:1001-1009. [DOI] [PubMed] [Google Scholar]
- 9.Etlinger, H. M., D. Gillessen, H. W. Lahm, H. Matile, H. J. Schonfeld, and A. Trzeciak. 1990. Use of prior vaccinations for the development of new vaccines. Science 249:423-425. [DOI] [PubMed] [Google Scholar]
- 10.Falkenberg, C., L. Bjorck, and B. Akerstrom. 1992. Localization of the binding site for streptococcal protein G on human serum albumin: identification of a 5.5-kilodalton protein G binding albumin fragment. Biochemistry 31:1451-1457. [DOI] [PubMed] [Google Scholar]
- 11.Galelli, A., and B. Charlot. 1990. Clonal anergy of memory B cells in epitope-specific regulation. J. Immunol. 145:2397-2405. [PubMed] [Google Scholar]
- 12.Geysen, H. M., S. J. Rodda, T. J. Mason, G. Tribick, and P. G. Schoofs. 1987. Strategies for epitope analysis using peptide synthesis. J. Immunol. Methods 102:259-274. [DOI] [PubMed] [Google Scholar]
- 13.Goetsch, L., A. Gonzalez, H. Plotnicky-Gilquin, J. F. Haeuw, J. P. Aubry, A. Beck, J. Y. Bonnefoy, and N. Corvaia. 2001. Targeting of nasal mucosa-associated antigen-presenting cells in vivo with an outer membrane protein A derived from Klebsiella pneumoniae. Infect. Immun. 69:6434-6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta, R. K., R. J. Collier, R. Rappuoli, and G. R. Siber. 1997. Differences in the immunogenicity of native and formalinized cross reacting material (CRM197) of diphtheria toxin in mice and guinea pigs and their implications on the development and control of diphtheria vaccine based on CRMs. Vaccine 15:1341-1343. [DOI] [PubMed] [Google Scholar]
- 15.Haeuw, J. F., I. Rauly, L. Zanna, C. Libon, C. Andreoni, T. Nguyen, T. Baussant, J. Y. Bonnefoy, and A. Beck. 1998. The recombinant Klebsiella pneumoniae outer membrane protein OmpA has carrier properties for conjugated antigenic peptides. Eur. J. Chem. 255:446-454. [DOI] [PubMed] [Google Scholar]
- 16.Herzenberg, L. A., and T. Tokuhisa. 1982. Epitope-specific regulation. I. Carrier-specific induction of suppression for IgG anti-hapten antibody responses. J. Exp. Med. 155:1730-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho, P. C., D. A. Mutch, K. D. Winkel, A. J. Saul, G. L. Jones, T. J. Doran, and C. M. Rzepczyk. 1990. Identification of two promiscuous T-cell epitopes from tetanus toxin. Eur. J. Immunol. 20:477-483. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann, P., M. Loleit, K. Mittenbuhler, W. Beck, K. H. Wiesmuller, G. Jung, and W. G. Bessler. 1997. Induction of an epitope-specific humoral immune response by lipopeptide-hapten conjugates: enhancement of the anti-melittin response by a synthetic T helper (Th)-cell epitope. FEMS Immunol. Med. Microbiol. 17:225-234. [DOI] [PubMed] [Google Scholar]
- 19.Käyhty, H., H. Ahman, P. R. Rönnberg, R. Tillikainen, and J. Eskola. 1995. Pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine is immunogenic in infants and children. J. Infect. Dis. 172:1273-1278. [DOI] [PubMed] [Google Scholar]
- 20.Kraulis, P. J., P. Jonasson, P. A. Nygren, M. Uhlen, L. Jendeberg, B. Nilsson, and J. Kordel. 1996. The serum albumin-binding domain of streptococcal protein G is a three-helical bundle: a heteronuclear NMR study. FEBS Letter 378:190-194. [DOI] [PubMed] [Google Scholar]
- 21.Kurikka, S., H. Käyhty, H. Peltola, L. Saarinen, J. Eskola, and H. Mäkelä. 1995. Neonatal immunization: response to Haemophilus influenzae type b-tetanus toxoid conjugate vaccine. Pediatrics 95:815-822. [PubMed] [Google Scholar]
- 22.Libon, C., N. Corvaïa, J. F. Haeuw, T. N. Nguyen, S. Stahl, J. J. Bonnefoy, and C. Andreoni. 1999. The serum albumin-binding region of streptococcal protein G (BB) potentiates the immunogenicity of the G130-230 RSV-A protein. Vaccine 17:406-414. [DOI] [PubMed] [Google Scholar]
- 23.Linhult, M., H. K. Binz, M. Uhlen, and S. Hober. 2002. Mutational analysis of the interaction between albumin-binding domain from streptococcal protein G and human serum albumin. Protein Sci. 11:206-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo-Man, R., S. Vichier-Guerre, S. Bay, E. Deriaud, D. Cantacuzene, and C. Leclerc. 2001. Anti-tumor immunity provided by a synthetic multiple antigenic glycopeptide displaying a tri-Tn glycotope. J. Immunol. 166:2849-2854. [DOI] [PubMed] [Google Scholar]
- 25.Makrides, S. C., P. A. Nygren, B. Andrews, P. J. Ford, K. S. Evans, E. G. Hayman, H. Adari, M. Uhlen, and C. A. Toth. 1996. Extended in vivo half-life of human soluble complement receptor type 1 fused to a serum albumin-binding receptor. J. Pharmacol. Exp. Ther. 277:534-5442. [PubMed] [Google Scholar]
- 26.Meaji, N. J., A. M. Bray, and H. M. Geysen. 1990. Multi-pin peptide synthesis strategy for T-cell determinant analysis. J. Immunol. Methods 134:23-33. [DOI] [PubMed] [Google Scholar]
- 27.Nygren, P. A., M. Eliasson, E. Palmcrantz, L. Abrahmsen, and M. Uhlen. 1988. Analysis and use of the serum albumin binding domains of streptococcal protein G. J. Mol. Recogn. 1:60-74. [DOI] [PubMed] [Google Scholar]
- 28.Nygren, P. A., C. Ljungquist, H. Tromborg, K. Nustad, and M. Uhlen. 1990. Species-dependent binding of serum albumins to the streptococcal receptor protein G. Eur. J. Biochem. 193:143-148. [DOI] [PubMed] [Google Scholar]
- 29.Nygren, P. A., P. Flodby, R. Andersson, H. Wigzell, and M. Uhlén. 1991. In vivo stabilization of a human recombinant CD4 derivative by fusion to a serum-albumin-binding receptor. Vaccines 91:363-368. [Google Scholar]
- 30.Panina-Bordignon, P., A. Tan, A. Termijtelen, S. Demotz, G. Corradin, and A. Lanzavecchia. 1989. Universally immunogenic T-cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur. J. Immunol. 19:2237-2242. [DOI] [PubMed] [Google Scholar]
- 31.Peeters, C. C., A. M. Tenbergen-Meekes, J. T. Poolman, M. Beurret, B. J. Zegers, and G. T. Rijkers. 1991. Effect of carrier priming on immunogenicity of saccharide-protein conjugate vaccines. Infect. Immun. 59:3504-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plotnicky-Gilquin, H., L. Goetsch, T. Huss, T. Champion, A. Beck, J. F. Haeuw, T. N. Nguyen, J. Y. Bonnefoy, N. Corvaia, and U. F. Power. 1999. Identification of multiple protective epitopes (protectopes) in the central conserved domain of a prototype human respiratory syncytial virus G protein. J. Virol. 73:5637-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Power, U. F., T. N. Nguyen, E. Rietveld, R. L. de Swart, J. Groen, A. D. Osterhaus, R. de Groot, N. Corvaia, A. Beck, N. Bouveret-Le-Cam, and J. Y. Bonnefoy. 2001. Safety and immunogenicity of a novel recombinant subunit respiratory syncytial virus vaccine (BBG2Na) in healthy young adults. J. Infect. Dis. 184:1456-1460. [DOI] [PubMed] [Google Scholar]
- 34.Raju, R., D. Navaneetham, D. Okita, B. Diethelm-Okita, D. McCormick, and B. M. Conti-Fine. 1995. Epitopes for human CD4+ cells on diphtheria toxin: structural features of sequence segments forming epitopes recognized by most subjects. Eur. J. Immunol. 25:3207-3214. [DOI] [PubMed] [Google Scholar]
- 35.Rauly, I., L. Goetsch, J. F. Haeuw, C. Tardieux, T. Baussant, J. Y. Bonnefoy, and N. Corvaia. 1999. Carrier properties of a protein derived from outer membrane protein A of Klebsiella pneumoniae. Infect. Immun. 67:5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reece, J. C., H. M. Geysen, and S. J. Rodda. 1993. Mapping the major human T helper epitopes of tetanus toxin. J. Immunol. 151:6175-6184. [PubMed] [Google Scholar]
- 37.Reece, J. C., D. L. McGregor, H. M. Geysen, and S. J. Rodda. 1994. Scanning for T helper epitopes with human PBMC using pools of short synthetic peptides. J. Immunol. Methods 172:241-254. [DOI] [PubMed] [Google Scholar]
- 38.Reuter, G., and R. Schauer. 1994. Determination of sialic acids. Methods Enzymol. 230:168-199. [DOI] [PubMed] [Google Scholar]
- 39.Schutze, M. P., C. Leclerc, M. Jolivet, F. Audibert, and L. Chedid. 1985. Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J. Immunol. 135:2319-2322. [PubMed] [Google Scholar]
- 40.Schutze, M. P., E. Deriaud, G. Przewlocki, and C. Leclerc. 1989. Carrier-induced epitopic suppression is initiated through clonal dominance. J. Immunol. 142:2635-2640. [PubMed] [Google Scholar]
- 41.Sjolander, A., P. A. Nygren, S. Stahl, K. Berzins, M. Uhlen, P. Perlmann, and R. Andersson. 1997. The serum albumin-binding region of streptococcal protein G: a bacterial fusion partner with carrier-related properties. J. Immunol. Methods 201:115-123. [DOI] [PubMed] [Google Scholar]
- 42.Stahl, S., A. Sjolander, P. A. Nygren, K. Berzins, P. Perlmann, and M. Uhlen, M. 1989. A dual expression system for the generation, analysis and purification of antibodies to a repeated sequence of the Plasmodium falciparum antigen Pf155/RESA. J. Immunol. Methods 124:43-52. [DOI] [PubMed] [Google Scholar]
- 43.Valmori, D., A. Pessi, E. Bianchi, and G. Corradin. 1992. Use of human universally antigenic tetanus toxin T-cell epitopes as carriers for human vaccination. J. Immunol. 149:717-721. [PubMed] [Google Scholar]
- 44.Watanabe, M., K. A. Reimann, P. A. DeLong, T. Liu, R. A. Fisher, and N. L. Letvin. 1984. Effect of recombinant soluble CD4 in rhesus monkeys infected with simian immunodeficiency virus of macaques. Nature 337:267-270. [DOI] [PubMed] [Google Scholar]
- 45.Zauner, W., K. Lingnau, F. Mattner, A. von Gabain, and M. Buschle. 2001. Defined synthetic vaccines. Biol. Chem. 382:581-595. [DOI] [PubMed] [Google Scholar]