Abstract
Arabinomannan (AM) is a polysaccharide antigen of the mycobacterial capsule. However, it is uncertain whether AM constitutes an immunologically distinct fraction of Mycobacterium tuberculosis. In this study, we analyzed the repertoire and specificity of antibodies to AM by using AM-binding murine monoclonal antibodies (MAbs) and human serum samples. Murine MAbs were found to be diverse in their specificity to AM and cross-reactivity with other arabinose-containing mycobacterial polysaccharides, with MAb 9d8 binding exclusively to AM. Human antibodies to AM were detected in serum samples from patients with pulmonary tuberculosis (TB), as well as in those from healthy, purified protein derivative-negative controls, with significantly higher titers among patients. The binding of human antibodies to AM was inhibited by MAb 9d8 in three patients with TB but not in controls. MAb 5c11, which recognizes other mycobacterial arabinose-containing carbohydrates in addition to AM, inhibited the binding of serum samples from 75% of patients and 76% of controls. Analysis of human antibodies with murine MAbs to human VH determinants demonstrated diversity among antibodies to AM with qualitative and quantitative differences compared with antibodies to lipoarabinomannan. In summary, our study suggests that antibodies to AM are diverse and heterogeneous with respect to antigen recognition and VH determinant expression, with human serum samples containing different subsets of antibodies to AM with the specificities of AM-binding murine MAbs. One MAb and a subset of human antibodies bind AM specifically, suggesting that this polysaccharide is antigenically distinct and is expressed in human infection.
Tuberculosis (TB) is a leading cause of mortality worldwide, and it is estimated that 1.86 billion people are infected with Mycobacterium tuberculosis (13). Contributing factors to this severe health problem are the human immunodeficiency virus epidemic, the long and complicated course of treatment, drug resistance, and a lack of efficient diagnostic and preventive modalities. In addition, we lack a full understanding of the immunopathogenesis of TB. Our group is interested in the M. tuberculosis carbohydrate arabinomannan (AM), which is thought to be a component of the mycobacterial capsule. This interest arose from the generation of a monoclonal antibody (MAb), 9d8, that prolonged the survival of mice infected with M. tuberculosis (36) and binds AM. These findings suggested that AM may be an immunologically important fraction, prompting us to evaluate whether AM is an immunologically distinct component of the M. tuberculosis surface.
Although the concept of an M. tuberculosis polysaccharide capsule has not been widely accepted, there is considerable evidence that supports the existence of a capsule surrounding the mycobacterial surface (8). Chapman et al. used the term “capsular space” to refer to the space between the phagosomal membrane of the infected cell and the wall of the enclosed mycobacterium (5). In the 1980s, electron microscopic studies provided evidence of the existence of a capsule surrounding mycobacteria (8). The capsule is composed mainly of polysaccharides with a small protein component (8). One of these polysaccharides is AM. Little is known about its structure and the role it plays in the pathophysiology of TB. It was demonstrated to have an immunosuppressive effect on the activation of human lymphocytes in one study (14). Lipoarabinomannan (LAM), another polysaccharide of the mycobacterial surface (8), has been extensively studied with respect to TB pathogenesis and antibody response (6-8, 11, 15, 21, 24, 31, 35). The relationship between the antibody responses to AM and LAM remains unclear.
In this study, we analyzed the antibody response to AM and evaluated whether antibodies to AM represent an immunological response that is distinct from the immunological response to other arabinose-containing mycobacterial polysaccharides.
(This work was presented in part at the 39th Annual Meeting of the Infectious Diseases Society of America, San Francisco, Calif., October 2001 [abstract 337], and at the 4th NYC/Regional Tuberculosis Conference, Newark, New Jersey, April 2002.)
MATERIALS AND METHODS
Mycobacterial carbohydrates.
AM was isolated from a clinical strain of M. tuberculosis by precipitation with 70% ethanol and purified by column chromatography as previously described (33). AM purity was confirmed by gas-liquid chromatography and 13C nuclear magnetic resonance spectroscopy (33). LAM and arabinogalactan (AG) were kindly provided by J. T. Belisle (Department of Microbiology, Colorado State University, Fort Collins).
MAbs.
Murine MAbs 9d8 (immunoglobulin G3 [IgG3]) and 5c11 (IgM) have been described previously (20). MAbs CS-40 (IgG1) and CS-35 (IgG3) were kindly provided by J. T. Belisle. Murine MAbs that react with human VH determinants (kindly provided by R. Mageed, Kennedy Institute of Rheumatology, London, United Kingdom) were as follows. MAbs G6 (IgG2a) and G8 (IgG1) recognize determinants encoded by VH1 gene family elements, and MAbs D12 (IgG2a), B6 (IgG1), and 16:84 (IgG1) recognize determinants encoded by VH3 gene family elements.
Preparation of RNA and reverse transcription-PCR for murine MAb sequencing.
For RNA preparation, hybridoma cells producing MAbs 9d8 and 5c11 were grown in Dulbecco's modified Eagle's medium with fetal calf serum, NCTC 109, and nonessential amino acids. RNA was prepared with Trizol reagent (GIBCO, Grand Island, N.Y.) in accordance with the manufacturer's instructions. Briefly, 1 ml of Trizol reagent was used per 106 log-phase cells and 10 g of RNA was used immediately following preparation for cDNA synthesis from mRNA, with oligo(dT) primer and superscript II reverse transcriptase (GIBCO). The cDNA encoding the variable domains of 9d8 and 5c11 hybridoma immunoglobulin was then generated by PCR with universal 5′ (sense) variable-region and specific 3′ (antisense) constant-region primers (9) as follows: 5′VhUni, TGAGGTGCAGCTGGAGGAGTC; 5′VκUni, GACATTCTGATGACCCAGTCT; 3′msCγ, AGACCGATGGGGCTGTTGTTTTGGC; 3′msCμ, AGACATTTGGGAAGGACTGACTCTC; 3′msCκ, TGGATACAGTTGGTGCAGCATCAGC. A sample of 10 μg of template was used in the PCR with 2.5 mM each deoxynucleoside triphosphate and 125 nM each primer under the following conditions with Taq polymerase (Roche, Mannheim, Germany): 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min 30 s for 40 cycles, followed by a final 10-min extension at 72°C. Purified PCR products (QIAGEN) were then ligated into pCR2.1 (Invitrogen, Carlsbad, Calif.) in accordance with the manufacturer's instructions. Constructs containing inserts were detected by restriction digestion with EcoRI (Roche) and visualized by 2% agarose gel electrophoresis. The amplified variable-domain cDNAs of several selected clones were sequenced bidirectionally by automated gel sequencing (AECOM Cancer Center Sequencing Facility) with primers M13R and M13F.
Human serum samples.
Serum samples were collected from 20 patients with pulmonary TB (patients) prior to treatment and 17 purified protein derivative-negative adults (controls). Written informed consent was obtained from all subjects, the experimental protocol was approved by the institutional ethical review board of the Albert Einstein College of Medicine and the New York University Medical School, and the guidelines for human experimentation were followed. Patients ranged in age from 16 to 65 years, with a mean of 32 years (17 males, two females, and one person of unknown gender). Controls ranged in age from 21 to 41 years, with a mean age of 30 years (nine males and eight females). For antigen detection, serum samples were obtained from another cohort of 11 patients with an age range of 20 to 65 years and an average age of 41 years and 10 controls with an age range of 21 to 56 years and an average age of 35 years. Serum samples used for antibody measurements were heated to 56°C for 30 min to inactivate complement prior to analysis by enzyme-linked immunosorbent assay (ELISA).
ELISA.
For the binding of murine MAbs to the mycobacterial carbohydrate fractions, microtiter plate wells (Corning Inc., Corning, N.Y.) were coated with 50 μl of AM, LAM, or AG at a concentration of 10 μg/ml in carbonate buffer (pH 9.6) and incubated at 37°C for 1 h. Carbonate buffer alone was added to control wells. The wells were blocked with 200 μl of 3% bovine serum albumin (BSA) in Tris-buffered saline (TBS) and incubated at 37°C for 1.5 h. The wells were washed three times, and 75 μl of MAb 9d8, 5c11, CS-40, or CS-35 at a concentration of 10 μg/ml in 1% BSA in TBS was added to the plates, serially diluted, and incubated. After washing, the plates were incubated with 1 μg of alkaline phosphatase-conjugated goat anti-mouse (GAM-AP) IgG1, IgG3, or IgM (Southern Biotechnology Associates, Inc., Birmingham, Ala.) per ml. The plates were washed five times, and 50 μl of 1 μg of p-nitrophenyl phosphate (PNPP; Sigma) substrate buffer (0.001 M MgCl2, 0.05 M Na2CO3 [pH 9.8]) per ml was added. All washes were done with TBS containing 0.05% Tween 20 (Sigma) with an ELISA plate washer (Skatron, Sterling, Va.). A405 was measured with a Multiscan MS microtiter plate reader (Labsystems Vantaa, Finland). Absorbance 1.5 times the background absorbance was considered positive for binding to the polysaccharide.
For the measurement of human serum IgG and IgM to AM, plates were coated with 50 μl of 3 μg of AM per ml in carbonate buffer and control plates were coated with carbonate buffer alone. After incubation for 1 h at 37°C, the wells were blocked as described above. Serum samples diluted 1:50 with 1% BSA in TBS were then added to the plates and serially diluted. After incubation and washing, 50 μl of alkaline phosphatase-conjugated goat anti-human (GAH-AP) IgG or IgM (Southern Biotechnology Associates, Inc.) at a concentration of 1 μg/ml was added and the plates were developed as described above. All samples were processed simultaneously. Titers were defined as the highest dilution giving an absorbance of 1.5 times the background absorbance.
The ELISA described here was used to determine IgG isotypes. This time, alkaline phosphatase-conjugated mouse anti-human (MAH-AP) IgG1, IgG2, IgG3, or IgG4 (Zymed Laboratories, San Francisco, Calif.) was added prior to the addition of PNPP. Absorbances of ≥0.1 were considered positive.
A competition ELISA with 5c11 and CS35 was done by coating microtiter plates with 3 μg of AM per ml and blocking with 3% BSA as described above. MAb 5c11 was added at a starting concentration of 25 μg/ml and serially diluted. Immediately, MAb CS35 or 3E5 (an irrelevant control), at a concentration of 5 μg/ml in 1% BSA in TBS, was added to designated wells. A solution of 1% BSA was added to control wells. After incubation at 37°C for 1 h, GAM-AP IgM at 1 μg/ml was added prior to the addition of PNPP.
For a competition ELISA with human serum samples and AM-binding murine MAbs, microtiter plates were coated with AM at a concentration of 3 μg/ml and blocked as described above. Serum samples were added at a starting dilution of 1:100 in 1% BSA and serially diluted. Immediately, MAb 9d8 or 5c11, at a concentration of 25 μg/ml, was added and the plates were incubated at 37°C for 1 h. GAH-AP IgG, at a concentration of 1 μg/ml, was added prior to the addition of PNPP. MAbs 3E5 (IgG3) and 2D10 (IgM) to Cryptococcus neoformans glucuronoxylomannan (25) were used as negative controls.
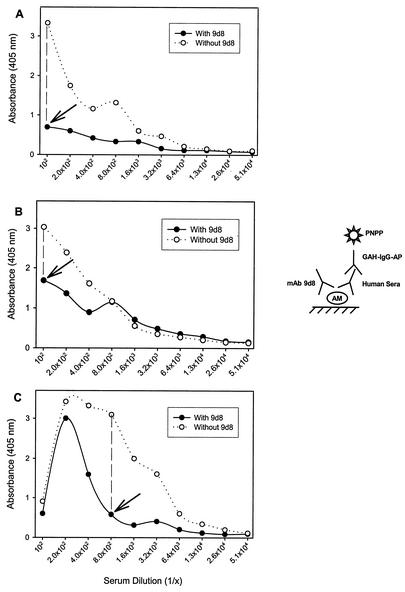
Results were read, and the presence or absence of inhibition was determined. In those assays demonstrating inhibition, the percentage of maximal inhibition was determined by identifying the point of maximal inhibition on the graph (see Fig. 1) and calculating the percent reduction of A405 in the presence of MAb for that point.
FIG. 1.
Competition ELISA between human antibodies from three individuals with TB and MAb 9d8. The ELISA configuration demonstrates the binding of TB patients' serum IgG to AM in the presence or absence of MAb 9d8 (A, patient B; B, patient C; C, patient E). The graph shows decreased binding of human antibodies to AM in the presence of MAb 9d8, and arrows indicate points of maximal inhibition.
VH determinant expression by antibodies to AM and LAM.
The ELISA used was adapted from an assay previously described (1, 17, 18). Briefly, microtiter plates were coated with 3 μg of AM or LAM per ml in carbonate buffer. Plates were incubated at 37°C for 1 h and blocked with 200 μl of 3% BSA in TBS. Serum samples were added at a starting dilution of 1:50, serially diluted, and incubated for 1.5 h. MAb directed to human VH determinants at a concentration of 5 μg/ml in 1% BSA in TBS was then added to designated wells. After incubation and washing, 50 μl of GAM-AP IgG1 or IgG2a at a concentration of 1 μg/ml was added. Plates were developed as described above. Absorbance of 1.5 times the background absorbance was considered positive.
AM detection in serum.
Detection of AM in serum was done with a capture ELISA similar to that which was previously described (33). Briefly, microtiter plate wells were coated with 50 μl of 1 μg of unlabeled goat anti-mouse IgG3 per ml in TBS; this was followed by blocking with 200 μl of 1% BSA in TBS. MAb 9d8, at a concentration of 1 μg/ml, was added to each well, and the plates were incubated for 1 h at 37°C. Undiluted serum samples (predigested with proteinase K as described below) were then added and serially diluted, and the plates were incubated for 1 h at 37°C. The plates were then incubated with MAb 5c11 at a concentration of 10 μg/ml, and after further washing, 1 μg of GAM-AP IgM per ml was added to all of the wells. Washes between the various steps and development of plates were done as described above. Purified AM was used as a positive control.
Preparation of samples for capture ELISA.
The serum samples used for detection of AM were digested with proteinase K (Boehringer Mannheim, Indianapolis, Ind.) to eliminate antigen-antibody complexes and other interfering proteins prior to testing by capture ELISA. Proteinase K diluted in PBS was added to undiluted serum samples to a final concentration of 0.1 μg/ml. After overnight incubation at 37°C, samples were boiled for 15 min to inactivate proteinase K and centrifuged; the supernatant solution was diluted in 1% BSA and assayed by capture ELISA.
Analysis of data.
Geometric mean titers of IgG and IgM of human antibodies to AM were calculated, and differences between patients and controls were analyzed by the Kruskal-Wallis test. The results of the competition ELISA were analyzed by the chi square test. Statistical significance was defined as P < 0.05.
Nucleotide sequence accession numbers.
The consensus sequences obtained in this study were deposited in the GenBank database under the following accession numbers: 9d8 Vh, AJ416413; 9d8 Vκ, AJ416412; 5c11 Vh, AJ416415; 5c11 Vκ, AJ416414.
RESULTS
Binding of murine MAbs to AM, LAM, and AG.
The binding of murine MAbs 5c11, 9d8, CS-40, and CS-35 to the mycobacterial fractions AM, LAM, and AG was assessed, and the results are shown in Table 1. All of the MAbs recognized AM. However, while MAb 9d8 recognized AM exclusively, the other three MAbs recognized other surface polysaccharides as well. MAbs 5c11 and CS-35 recognized LAM and AG in addition to AM, and MAb CS-40 recognized LAM but not AG.
TABLE 1.
Analysis of murine MAbs to AM in terms of isotype, binding patterns, and gene sequencing
| MAb | Isotype | Antigen(s) | VH family | JH family | CDR3 H3 (aa)a | VK family | JK family | CDR3 L3 (aa) |
|---|---|---|---|---|---|---|---|---|
| 5c11 | IgM | AM, LAM, AG | 12 (subgroup 3C) | 3 | 9 | 10 (subgroup 3) | 2 | 8 |
| 9d8 | IgG3 | AM | 7 (subgroup 2A) | 3 | 8 | 2 (subgroup 1) | 4 | 9 |
| CS-40 | IgG1 | AM, LAM | NDb | ND | ND | ND | ND | ND |
| CS-35 | IgG3 | AM, LAM, AG | ND | ND | ND | ND | ND | ND |
aa, amino acids.
ND, not done.
Sequencing of MAb variable domains.
To learn more about the differences of antibodies to AM, the Vh and Vκ domains of murine MAbs 5c11 and 9d8 were sequenced and the sequences were deposited in the GenBank database under the accession numbers listed in Materials and Methods. Analysis of the sequences revealed that the MAbs used different germ line family sequences for VH, Vκ, and Jκ but the same germ line family for JH (Table 1). Comparison of these heavy- and light-chain rearranged V region sequences to similar sequences found in the GenBank database has shown that the V region rearrangement events encode various immunoglobulin binding specificities ranging from double-stranded DNA to protein epitopes (3, 4, 22, 26-28, 30).
Measurement of IgG and IgM to AM in serum samples.
IgG and IgM to AM were detected in serum samples of patients and controls (Tables 2 and 3). The geometric mean titer showed a 10-fold higher level of IgG (16,858 versus 1,579) and a 4-fold higher level of IgM (171 versus 38) in serum samples from patients compared to those from controls (Table 2). The differences were statistically significant by the Kruskal-Wallis test at P < 0.01 and P < 0.05, respectively. The predominant IgG isotype among antibodies to AM was IgG2 (Table 2), which was detected in 16 (80%) of 20 patients and 11 (65%) of 17 controls, with mean absorbances of 1.15 ± 1 and 0.29 ± 0.28, respectively. IgG1 was detected in the serum samples of seven (35%) patients, with absorbance values significantly lower than those measured for IgG2 (absorbance data not shown).
TABLE 2.
Titers and isotype determination of human antibodies to AM
| Group (no. of persons) | GMTa
|
% of persons expressing antibodies of subclass:
|
||||
|---|---|---|---|---|---|---|
| IgM | IgG | IgG1 | IgG2 | IgG3 | IgG4 | |
| Pulmonary TB patients (20) | 171b | 16,858c | 35 | 80 | 0 | 0 |
| Controls (17) | 38 | 1,579 | 0 | 65 | 6 | 6 |
GMT, geometric mean titer.
P < 0.05.
P < 0.01.
TABLE 3.
Antibody response among Individuals with Pulmonary Tb and Controls
| Subject | Titer
|
Predominant IgG subclass | % of maximal inhibition by:
|
||
|---|---|---|---|---|---|
| IgM | IgG | MAb 5c11 | MAb 9d8 | ||
| Pulmonary TB patients | |||||
| A | 1:150 | 1:109,350 | IgG2 | NIa | NI |
| B | 1:150 | 1:36,150 | IgG1 | 70 | 79 |
| C | 1:50 | 1:109,350 | IgG2 | 65 | 44 |
| D | 1:150 | 1:36,150 | IgG1 | 46 | NI |
| E | 1:450 | 1:109,350 | IgG2 | 77 | 81 |
| F | 1:1,350 | 1:1,350 | IgG2 | 56 | NI |
| G | 1:450 | 1:109,350 | IgG2 | 78 | NI |
| H | 1:50 | 1:12,150 | IgG2 | 56 | NI |
| I | 1:1,350 | 1:12,150 | NRb | 57 | NI |
| J | 1:50 | 1:450 | NR | 40 | NI |
| K | NR | 1:109,350 | IgG2 | NI | NI |
| L | 1:4,050 | 1:12,150 | IgG2 | 58 | NI |
| M | 1:150 | 1:4,050 | IgG2 | 52 | NI |
| N | 1:50 | 1:450 | NR | NI | NI |
| O | 1:150 | 1:12,150 | IgG2 | 57 | NI |
| P | 1:150 | 1:36,150 | IgG2 | 63 | NI |
| Q | 1:150 | 1:36,150 | IgG2 | 76 | NI |
| R | 1:1,350 | 1:36,150 | IgG2 | NI | NI |
| S | 1:150 | 1:12,150 | IgG2 | NI | NI |
| T | 1:150 | 1:12,150 | IgG2 | NI | NI |
| Controls | |||||
| 1 | 1:50 | 1:1,350 | IgG2 | 68 | NI |
| 2 | 1:1,350 | 1:4,050 | NR | 67 | NI |
| 3 | NR | 1:12,150 | IgG2 | 33 | NI |
| 4 | NR | 1:12,150 | IgG2 | 59 | NI |
| 5 | 1:50 | 1:1,350 | NR | 44 | NI |
| 6 | 1:1,350 | 1:450 | IgG2 | 51 | NI |
| 7 | 1:50 | 1:4,050 | NR | 58 | NI |
| 8 | 1:50 | 1:1,350 | NR | 59 | NI |
| 9 | 1:50 | NR | NR | NI | NI |
| 10 | 1:50 | 1:1,350 | IgG2 | 67 | NI |
| 11 | 1:150 | 1:4,050 | NR | NI | NI |
| 12 | 1:450 | 1:36,150 | IgG2 | 75 | NI |
| 13 | NR | 1:1,350 | IgG2 | NI | NI |
| 14 | NR | 1:450 | IgG4 | 77 | NI |
| 15 | 1:50 | 1:4,050 | IgG2 | NI | NI |
| 16 | 1:150 | 1:450 | IgG2 | 71 | NI |
| 17 | 1:50 | 1:4,050 | IgG2 | 52 | NI |
NI, no inhibition.
NR, nonreactive.
Competition ELISA.
A competition ELISA was performed to determine whether IgG to AM included a subset of antibodies with MAb 9d8 specificity. Among the patients, inhibition of binding by MAb 9d8 was observed in three serum samples (those of patients B, C, and E; Table 3), representing 15% of the patient population tested. Inhibition by MAb 9d8 was not found in control serum samples. The differences between the two groups were not statistically significant (P = 0.234). Inhibition by MAb 9d8 was detected in individuals with a high absorbance value detected upon measurement of total IgG to AM (data not shown), and the percent maximal inhibition ranged from 44 to 81% (Fig. 1). No inhibition was observed when MAb 3E5 was used instead of MAb 9d8. Inhibition by MAb 5c11 was found in serum samples of 15 patients and 13 controls (75 and 76%, respectively). Percent maximal inhibition by MAb 5c11 ranged from 40 to 78% among patients and from 33 to 77% among the controls who demonstrated inhibition of binding (Table 3).
A competition ELISA was also performed with MAbs 5c11 and CS-35 to determine whether these MAbs, which demonstrated binding patterns similar to those of the mycobacterial carbohydrates, recognize the same epitope on these carbohydrates. The results showed no inhibition of MAb 5c11 binding to AM by MAb CS-35, suggesting that these MAbs recognize different epitopes.
VH determinant expression.
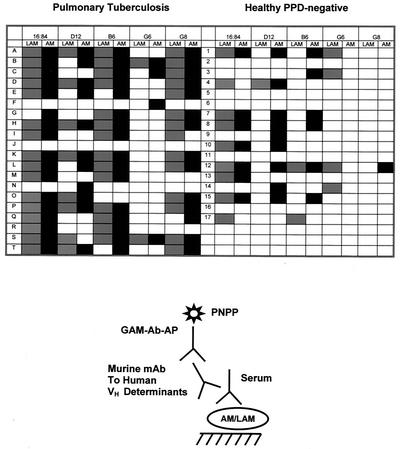
Extrapolating from the murine MAb analysis, indicating that different VH gene families could encode VH regions of antibodies to AM, we utilized VH determinant expression as a tool with which to explore the diversity of human antibodies to AM. We also used this method to detect subtle similarities and differences between antibodies to AM and LAM. Antibodies to AM in serum samples of patients expressed predominantly VH determinants recognized by MAbs 16:84, D12, B6, and G8 (Fig. 2). Antibodies to AM in serum samples of controls were recognized by MAbs 16:84, D12, and B6, but little or no reactivity with MAbs G6 and G8 was detected (Fig. 2). Diversity was observed with respect to the number and composition of the VH determinants expressed by antibodies to AM in different individuals. Our results also show different patterns of VH determinant expression by antibodies to AM and LAM among different individuals. We use the term “correspondence” to describe these findings. Correspondence is defined as the presence of antibodies to both AM and LAM that are recognized by a specific anti-idiotypic MAb(s). Among the patients, 40% (patients A, D, H, K, M, O, P, and R) showed complete correspondence in antibody response to AM and LAM (Fig. 2). In other words, in these individuals, the results suggest that the pattern of VH determinant expression of antibodies to AM matched that of antibodies to LAM. Partial correspondence in antibody response to AM and LAM was demonstrated in 55% of the patients (patients B, C, E, F, G, I, L, N, Q, S, and T); some of their antibodies that are recognized by certain anti-idiotypic MAbs bind to either AM or LAM but not to both. In these patients, the pattern of VH determinant expression of antibodies to AM did not have an exact match with the antibodies to LAM. One individual (5%) showed no expression of the VH determinants tested. Differences and commonalities in VH determinant expression by antibodies to AM and LAM were also found in controls. However, overall, a lesser degree of VH determinant expression was detected in this group.
FIG. 2.
Detection of VH determinant expression among antibodies to AM (black areas) and LAM (grey areas) recognized by specific mouse anti-human MAbs to VH determinants in human serum samples of patients with pulmonary TB and healthy, purified protein derivative (PPD)-negative individuals.
AM detection in serum samples.
To determine whether antigen-antibody complexes interfered with the measurement of antibodies to AM, we tested serum samples for the presence of AM with a sensitive capture ELISA. The limit of detection of the assay is 0.45 μg/ml. AM was not detected in any of the serum samples tested.
DISCUSSION
AM is a major component of the M. tuberculosis capsule (8). Little is known about its exact structure or biological effects or how it differs from the AM component of LAM. Previous studies by our group demonstrated that the survival of mice infected with M. tuberculosis precoated with MAb 9d8, directed to AM, was enhanced (36), suggesting that AM may be an immunologically important fraction. Our earlier studies also suggested that the AM antigen has unique characteristics (33). For instance, it is highly sensitive to the effect of the detergent Tween 80, which is used to grow M. tuberculosis in suspension (33). In fact, MAb 9d8 was generated by immunizing mice with M. tuberculosis grown in the absence of the detergent Tween 80 (20). A similar Tween-associated effect was not observed with LAM. In addition, its expression during growth in vitro is different from that of LAM (33). These qualities suggest that AM may possess distinct antigenic characteristics.
To evaluate antibody binding to AM, we used several MAbs generated to mycobacterial carbohydrates and compared their binding to various mycobacterial fractions. Studying the binding of murine MAbs 9d8, 5c11, CS-40, and CS-35 (Table 1) to AM and other arabinose-containing fractions of M. tuberculosis revealed that all four MAbs recognized AM but each differed in its characteristics of binding to the other arabinose-containing fractions. While MAb 9d8 recognizes AM exclusively (20, 36), MAbs 5c11, CS-40, and CS-35 recognized both AM and LAM. Moreover, MAbs 5c11 and CS-35 recognized the cell wall carbohydrate AG, a part of the mycolyl-AG-peptidoglycan complex. These results suggest that AM and LAM share antigenic determinants, as both were recognized by MAbs 5c11, CS-40, and CS-35. The ability of MAbs 5c11 and CS-35 to recognize AG, in addition to AM and LAM, can be explained by the cross-reactivity between AM and AG previously described (8). The exclusive binding of MAb 9d8 to AM supports the idea that, despite some antigenic similarities, AM may be structurally different from LAM. The concept that antigenic differences between AM and LAM exist is also supported by our previous studies demonstrating that the method of tissue processing required to perform immunostaining with MAb 9d8 (33) differs from that used for immunostaining with MAb 5c11 (21). The immunostaining patterns of lung tissue from mice infected with M. tuberculosis in the presence of these MAbs were different as well. Furthermore, while the AM epitope recognized by MAb 9d8 is affected by the presence of Tween 80 in the M. tuberculosis culture medium, the epitopes recognized by MAbs CS-40 and 5c11 remain unaffected (33). These findings emphasize the uniqueness of AM and certain antibodies that are directed to it.
DNA sequencing of MAbs 5c11 and 9d8 demonstrated the usage of different VH and VK region genes by these antibodies (Table 1). The heavy- and light-chain V regions, and especially the CDR3 region, are important for antigen recognition. The differences in antigen specificity among the AM-binding MAbs are echoed by differences in variable-gene usage. The finding that MAbs that bind AM are heterogeneous in V region usage suggests that antibodies to AM are structurally diverse.
Analysis of the human antibody response to AM demonstrated that IgG and IgM to AM were detected in the serum samples of both patients and controls, with antibody titers being significantly higher in serum samples from patients. The presence of higher titers of antibodies to AM among the patients is probably due to active replication of bacilli during disease. In this regard, we have previously demonstrated that the titers of IgG and IgM to AM in mice infected with M. tuberculosis increased in direct proportion to the CFU count (33). The presence of antibodies to AM among the controls may be explained by exposure to environmental mycobacteria. Various mycobacteria can be isolated from the environment (12, 16, 23), and structural similarities between AMs of different mycobacterial species were previously described (13, 24). AM-binding MAb 9d8, for example, was previously found to recognize three mycobacterial species other than M. tuberculosis (M. kansasii, M. gordonae, and M. gastri) (20). Several studies have provided supportive evidence for the role played by environmental exposure in eliciting immune responses (including antibodies) to mycobacterial antigens (15, 23, 29, 37). Cross-reactivity of human antibodies induced by environmental mycobacteria could thus explain the presence of antibodies to M. tuberculosis AM in healthy individuals in this study. The presence of IgM to AM in controls is intriguing and may suggest recent or continuous exposure to environmental mycobacteria.
IgG2 was the predominant isotype among antibodies to AM (Tables 2 and 3). This finding is consistent with the predominance of this isotype among antibodies to other bacterial polysaccharide antigens, such as those of Streptococcus pneumoniae, Haemophilus influenzae type b (19, 32, 34), and C. neoformans (10), in adults. Our results are also consistent with previous studies demonstrating IgG2 predominance in the antibody response to LAM in TB and leprosy (7, 11). The stronger IgG2 response, as manifested by the higher absorbance values detected in the patients than in the controls (individual absorbance values are not shown), is most probably a reflection of the higher total IgG titers detected in the former (34). The finding of IgG1 in the serum samples of few patients is consistent with studies demonstrating that this isotype is the second most prevalent isotype elicited by bacterial polysaccharides (32, 34).
To exclude interference by AM in our assay, serum samples were tested for the presence of this antigen by capture ELISA. None of the undiluted serum samples tested demonstrated the presence of AM. The absence of AM in patients' serum samples may be due to either small quantities of the antigen produced or rapid clearance, the later possibly as a result of their antibody responses. Previous experiments, for instance, showed that LAM is cleared from serum within a few hours and that its clearance is enhanced by the presence of antibody (21). We hypothesize that the same effective clearance occurs with AM.
Since MAb 9d8, which binds AM exclusively, identified an immunologically important epitope of AM, we attempted to determine if antibodies with the same specificity as MAb 9d8 were present in human serum samples by using a competition ELISA. Inhibition of binding to AM by MAb 9d8 was found in three patients (Fig. 1) but not in controls. It is possible that other serum samples contained antibodies to AM with the specificity of MAb 9d8 as well; however, their titers may be below the limit of detection of our assay. Alternately, the affinity of human antibody to AM may be stronger than that of MAb 9d8, thus preventing inhibition by the latter. Structural differences between AMs from different strains of M. tuberculosis may also account for the lack of inhibition in certain individuals. Inhibition of serum antibody binding by MAb 5c11 was more common than inhibition by MAb 9d8. This result is most probably due to the higher prevalence of the MAb 5c11 epitope in AM. Alternatively, MAb 5c11 may bind more strongly to AM than does MAb 9d8, thus preventing easy displacement by human antibodies to AM.
The analysis of murine MAbs (Table 1) and the competition ELISA suggest that antibodies to AM may vary in specificity and cross-reactivity. To further explore the diversity among human antibodies to AM, we used murine MAbs to human VH determinants to test our samples (Fig. 2). VH determinants are specific areas in the variable regions of antibodies that are coded for by specific gene families. Our results demonstrated that antibodies to AM in patients' serum samples expressed mostly VH determinants that are recognized by MAbs 16:84, D12, B6, and G8. The control antibodies expressed VH determinants recognized by MAbs 16:84, D12, and B6 but little or none recognized by MAb G6 or G8. The expression of VH3 gene family determinants (recognized by MAbs 16:84, D12, and B6) by antibodies to AM is similar to that found in the antibodies to pneumococcal polysaccharide (1), H. influenzae (2) and C. neoformans glucuronoxylomannan (17, 18). However, unlike antibodies to these polysaccharides, antibodies to AM also expressed determinants encoded by the VH1 gene family (recognized by MAbs G6 and G8). These findings suggest that the antibody response to AM is not restricted to the expression of one or few idiotypic determinants.
We also used VH determinant expression to differentiate between the repertoire of antibodies generated to AM and the repertoire of antibodies generated to LAM, as both contain arabinose and mannose. Qualitative differences and commonalities in VH determinant expression were found among antibodies, from different individuals, generated to the two polysaccharides (Fig. 2) in both patients and controls. The differences noted between the antibody responses to AM and LAM support the notion that these polysaccharides have antigenic differences. It also suggests that certain individuals may differ in the ability to elicit antibody responses to these antigens. The similarities in VH determinant expression of antibodies to AM and LAM seen among both groups, particularly in the TB patients, may be explained by a possible cross-reactivity of antibodies to AM and LAM or by epitopes shared by both fractions. MAbs 5C11, CS40, and CS35 (Table 1), for instance, were shown to react with both AM and LAM. Some individuals in both groups appear to have antibodies to either AM or LAM which did not express most or any of the VH determinants recognized by the VH MAbs used in this study. These individuals may have antibodies to AM and LAM that express VH determinants recognized by other MAbs not used in this study. Although there appears to be more VH determinant expression among patients, this probably reflects the higher antibody titers among individuals in this group.
In summary, our results demonstrate the heterogeneity of the repertoire of antibodies to AM in terms of specificity, cross-reactivity, and VH determinant expression. Our data also indicate that humans mount antibody responses to AM, particularly as a result of disease, and that antibodies with the specificity of MAb 9d8 to AM, which is protective against murine TB, can be found in human serum samples. The results also suggest that AM is antigenically different from LAM despite the fact that both contain arabinose and mannose. Further studies are required to define the presence of particular subsets of antibodies to AM in serum and correlate them with disease progression and outcome.
Acknowledgments
A.G.-F. was supported by National Institutes of Health (NIH) grants AI001691 and AI053192 and a grant from the Sequella Global Tuberculosis Foundation. A.C. is supported by NIH grants AI033142, AI033774, and HL059842. L.P. is supported by NIH grants AI045459, AI044374, and AI035370.
Many thanks to John T. Belisle for providing LAM and AG, as well as MAbs CS-40 and CS-35, as part of NIH contract N01-AI-75320 (Tuberculosis Research Materials and Vaccine Testing). We also thank R. Mageed of the Kennedy Institute of Rheumatology, Chesterfield, Derbyshire, United Kingdom, for providing murine MAbs to human VH determinants.
REFERENCES
- 1.Abadi, J., J. Friedman, R. A. Mageed, R. Jefferis, M. C. Rodriguez-Barradas, and L. Pirofski. 1998. Human antibodies elicited by a pneumococcal vaccine express idiotypic determinants indicative of VH3 gene segment usage. J. Infect. Dis. 178:707-716. [DOI] [PubMed]
- 2.Adderson, E. E., P. G. Shackelford, A. Quinn, P. M. Wilson, M. W. Cunningham, R. A. Insel, and W. L. Carroll. 1993. Restricted immunoglobulin VH usage and VDJ combinations in the human response to Haemophilus influenzae type b capsular polysaccharide. Nucleotide sequences of monospecific anti-Haemophilus antibodies and polyspecific antibodies cross-reacting with self antigens. J. Clin. Investig. 91:2734-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridges, A., A. Birch, G. Williams, M. Aguet, D. Schlatter, W. Huber, G. Garotta, and J. A. Robinson. 1995. Variable region cDNA sequences and characterization of murine anti-human interferon gamma receptor monoclonal antibodies that inhibit receptor binding by interferon gamma. Mol. Immunol. 32:1329-1338. [DOI] [PubMed] [Google Scholar]
- 4.Brown, J. C., B. A. Brown II, Y. Li, and C. C. Hardin. 1998. Construction and characterization of a quadruplex DNA selective single-chain autoantibody from a viable moth-eaten mouse hybridoma with homology to telomeric DNA binding proteins. Biochemistry 37:16338-16348. [DOI] [PubMed] [Google Scholar]
- 5.Chapman, G. B., J. H. Hanks, and J. H. Wallace. 1959. An electron microscope study of the disposition and fine structure of Mycobacterium lepraemurium in mouse spleen. J. Bacteriol. 77:205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costello, A. M., A. Kumar, V. Narayan, M. S. Akbar, S. Ahmed, C. Abou-Zeid, G. A. Rook, J. Stanford, and C. Moreno. 1992. Does antibody to mycobacterial antigens, including lipoarabinomannan, limit dissemination in childhood tuberculosis? Trans. R. Soc. Trop. Med. Hyg. 86:686-692. [DOI] [PubMed] [Google Scholar]
- 7.Da Costa, C. T., S. Khanolkar-Young, A. M. Elliott, K. M. Wasunna, and K. P. McAdam. 1993. Immunoglobulin G subclass responses to mycobacterial lipoarabinomannan in HIV-infected and non-infected patients with tuberculosis. Clin. Exp. Immunol. 91:25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daffe, M., and P. Draper. 1998. The envelope layers of mycobacteria with reference to their pathogenicity. Adv. Microb. Physiol. 39:131-203. [DOI] [PubMed] [Google Scholar]
- 9.Dattamajumdar, A. K., D. P. Jacobson, L. E. Hood, and G. E. Osman. 1996. Rapid cloning of any rearranged mouse immunoglobulin variable genes. Immunogenetics 43:141-151. [DOI] [PubMed] [Google Scholar]
- 10.Deshaw, M., and L. A. Pirofski. 1995. Antibodies to the Cryptococcus neoformans capsular glucuronoxylomannan are ubiquitous in serum from HIV+ and HIV− individuals. Clin. Exp. Immunol. 99:425-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhandayuthapani, S., S. Izumi, D. Anandan, and V. N. Bhatia. 1992. Specificity of IgG subclass antibodies in different clinical manifestations of leprosy. Clin. Exp. Immunol. 88:253-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.du Moulin, G. C., K. D. Stottmeier, P. A. Pelletier, A. Y. Tsang, and J. Hedley-Whyte. 1988. Concentration of Mycobacterium avium by hospital hot water systems. JAMA 260:1599-1601. [DOI] [PubMed] [Google Scholar]
- 13.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 14.Ellner, J. J., and T. M. Daniel. 1979. Immunosuppression by mycobacterial arabinomannan. Clin. Exp. Immunol. 35:250-257. [PMC free article] [PubMed] [Google Scholar]
- 15.Fairchok, M. P., J. H. Rouse, and S. L. Morris. 1995. Age-dependent humoral responses of children to mycobacterial antigens. Clin. Diagn. Lab. Immunol. 2:443-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falkinham, J. O., III, B. C. Parker, and H. Gruft. 1980. Epidemiology of infection by nontuberculous mycobacteria. I. Geographic distribution in the eastern United States. Am. Rev. Respir. Dis. 121:931-937. [DOI] [PubMed] [Google Scholar]
- 17.Fleuridor, R., R. H. Lyles, and L. Pirofski. 1999. Quantitative and qualitative differences in the serum antibody profiles of human immunodeficiency virus-infected persons with and without Cryptococcus neoformans meningitis. J. Infect. Dis. 180:1526-1535. [DOI] [PubMed] [Google Scholar]
- 18.Fleuridor, R., Z. Zhong, and L. Pirofski. 1998. A human IgM monoclonal antibody prolongs survival of mice with lethal cryptococcosis. J. Infect. Dis. 178:1213-1216. [DOI] [PubMed] [Google Scholar]
- 19.Freijd, A., L. Hammarstrom, M. A. Persson, and C. I. Smith. 1984. Plasma anti-pneumococcal antibody activity of the IgG class and subclasses in otitis prone children. Clin. Exp. Immunol. 56:233-238. [PMC free article] [PubMed] [Google Scholar]
- 20.Glatman-Freedman, A., J. M. Martin, P. F. Riska, B. R. Bloom, and A. Casadevall. 1996. Monoclonal antibodies to surface antigens of Mycobacterium tuberculosis and their use in a modified enzyme-linked immunosorbent spot assay for detection of mycobacteria. J. Clin. Microbiol. 34:2795-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glatman-Freedman, A., A. J. Mednick, N. Lendvai, and A. Casadevall. 2000. Clearance and organ distribution of Mycobacterium tuberculosis lipoarabinomannan (LAM) in the presence and absence of LAM-binding immunoglobulin M. Infect. Immun. 68:335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalinke, U., A. Oxenius, C. Lopez-Macias, R. M. Zinkernagel, and H. Hengartner. 2000. Virus neutralization by germ-line vs. hypermutated antibodies. Proc. Natl. Acad. Sci. USA 97:10126-10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsson, L. O., B. E. Skoogh, M. W. Bentzon, M. Magnusson, J. Olofson, J. Taranger, and A. Lind. 1991. Sensitivity to sensitins and tuberculin in Swedish children. II. A study of preschool children. Tubercle 72:37-42. [DOI] [PubMed] [Google Scholar]
- 24.Lemassu, A., A. Ortalo-Magne, F. Bardou, G. Silve, M. A. Laneelle, and M. Daffe. 1996. Extracellular and surface-exposed polysaccharides of non-tuberculous mycobacteria. Microbiology 142:1513-1520. [DOI] [PubMed] [Google Scholar]
- 25.Lendvai, N., A. Casadevall, Z. Liang, D. L. Goldman, J. Mukherjee, and L. Zuckier. 1998. Effect of immune mechanisms on the pharmacokinetics and organ distribution of cryptococcal polysaccharide. J. Infect. Dis. 177:1647-1659. [DOI] [PubMed] [Google Scholar]
- 26.Moncharmont, B., J. L. Su, and I. Parikh. 1982. Monoclonal antibodies against estrogen receptor: interaction with different molecular forms and functions of the receptor. Biochemistry 21:6916-6921. [DOI] [PubMed] [Google Scholar]
- 27.Mueller, C. M., and R. Jemmerson. 1996. Maturation of the antibody response to the major epitope on the self antigen mouse cytochrome c. Restricted V gene usage, selected mutations, and increased affinity. J. Immunol. 157:5329-5338. [PubMed] [Google Scholar]
- 28.Orlandini, M., A. Santucci, A. Tramontano, P. Neri, and S. Oliviero. 1994. Cloning, characterization, and modeling of a monoclonal anti-human transferrin antibody that competes with the transferrin receptor. Protein Sci. 3:1476-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilkington, C., A. M. Costello, G. A. Rook, and J. L. Stanford. 1993. Development of IgG responses to mycobacterial antigens. Arch. Dis. Child. 69:644-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Putterman, C., B. Deocharan, and B. Diamond. 2000. Molecular analysis of the autoantibody response in peptide-induced autoimmunity. J. Immunol. 164:2542-2549. [DOI] [PubMed] [Google Scholar]
- 31.Sada, E., P. J. Brennan, T. Herrera, and M. Torres. 1990. Evaluation of lipoarabinomannan for the serological diagnosis of tuberculosis. J. Clin. Microbiol. 28:2587-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarvas, H., N. Rautonen, S. Sipinen, and O. Makela. 1989. IgG subclasses of pneumococcal antibodies—effect of allotype G2mn. Scand J. Immunol. 29:229-237. [DOI] [PubMed] [Google Scholar]
- 33.Schwebach, J. R., A. Casadevall, R. Schneerson, Z. Dai, X. Wang, J. B. Robbins, and A. Glatman-Freedman. 2001. Expression of a Mycobacterium tuberculosis arabinomannan antigen in vitro and in vivo. Infect. Immun. 69:5671-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siber, G. R., P. H. Schur, A. C. Aisenberg, S. A. Weitzman, and G. Schiffman. 1980. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N. Engl. J. Med. 303:178-182. [DOI] [PubMed] [Google Scholar]
- 35.Sousa, A. O., S. Henry, F. M. Maroja, F. K. Lee, L. Brum, M. Singh, P. H. Lagrange, and P. Aucouturier. 1998. IgG subclass distribution of antibody responses to protein and polysaccharide mycobacterial antigens in leprosy and tuberculosis patients. Clin. Exp. Immunol. 111:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teitelbaum, R., A. Glatman-Freedman, B. Chen, J. B. Robbins, E. Unanue, A. Casadevall, and B. R. Bloom. 1998. A MAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc. Natl. Acad. Sci. USA 95:15688-15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thayer, W. R., Jr., C. M. Bozic, R. T. Camphausen, and M. McNeil. 1990. Implications of antibodies to pyruvylated glucose in healthy populations for mycobacterioses and other infectious diseases. J. Clin. Microbiol. 28:714-718. [DOI] [PMC free article] [PubMed] [Google Scholar]