Abstract
Recombinase activation gene 1 (RAG-1) function is essential for V(D)J recombination in T-cell-receptor and immunoglobulin rearrangements whereby the immune system may encode memories of a vast array of antigens. The RAG-1 gene is also localized to neurons in the hippocampal formation and related limbic regions that are involved in spatial learning and memory as well as other parameters of neurobehavioral performance. Since the unique ability to encode memory is shared by the immune system and the brain, we tested the hypothesis that loss of the RAG-1 gene in the brain would influence learning and memory performance and examined several different domains of behavior in RAG-1-knockout and control mice. Compared to control mice, RAG-1-knockout mice exhibited increased locomotor activity in an open field under both dim and bright lighting conditions and decreased habituation (reduction in the expected decline in locomotor activity with increasing familiarity with the novel environment in a 1-h test session) in bright lighting. RAG-1-knockout mice also showed reduced levels of fearfulness for some measures of fear-motivated behavior in both the open-field behavior test and elevated-plus maze test. Contrary to our hypothesis, no differences in spatial learning and memory were found between the groups, although modest differences were observed visible-platform testing in the Morris water maze. Neither prepulse inhibition, a measure of sensorimotor gating, nor reflexive acoustic startle responses differed between the RAG-1-knockout and control mice. It remains to be determined if these changes are due to the loss of RAG-1 gene expression in the brain, are due to the absence of the gene in the immune system (e.g., the loss of cytokines with neuromodulatory activities), or are due to some combination of both effects. Study of the neurobiological actions of RAG-1 in the brain may provide new insights into important processes involved in normal brain function and disease.
It is well established that the central nervous and immune systems share a number of genes such as common signaling molecules, receptors, and enzymes (1). Both systems possess the unique ability to encode memory, although it is unclear whether such complex events in the brain and the immune system also share a common molecular basis. The ability of the immune system to form memories of vast numbers of ever changing antigens is associated with the process of V(D)J recombination that occurs in T-cell-receptor and immunoglobulin rearrangements (3, 11, 24). Recombinase activation gene 1 (RAG-1) plays a pivotal role in V(D)J recombination, and deletion of this gene results in a lack of mature, functional B and T lymphocytes (15). Chun et al. (4) showed that in the forebrain of normal mice, RAG-1 mRNA expression is localized primarily to neurons in the hippocampal formation and related limbic regions, areas known to mediate cognitive function and other measures of neurobehavioral performance (e.g., emotional and motor behaviors and sensorimotor gating). The regional distribution of RAG-1 mRNA was of particular interest to our laboratory, as it is remarkably similar to the distribution of interleukin-2/15 receptor-β (IL-2/15Rβ) gene expression in situ (17). Like RAG-1 mRNA, IL-2/15Rβ is also expressed by developing and mature brain neurons (4, 7, 17), and IL-2/15Rβ-knockout mice exhibit alterations in neurobehavioral performance (17). Although the brains of RAG-1 mice were not found to have gross structural abnormalities or visible neurological deficits on gross inspection (15), systematic examination of neurobehavioral performance to assess brain function has not been conducted with these mice.
The present study therefore compared systematically the neurobehavioral performances of B6.129S7-Rag1-knockout and B6129SF2/J control mice. In light of the aforementioned role of the RAG-1 gene in processes associated with immunological memory and its expression in the hippocampal formation, an area of the limbic forebrain that mediates spatial learning and memory, we sought to test the hypothesis that deletion of the RAG-1 gene from the brain would result in impaired spatial learning and memory. We therefore compared the performances of RAG-1 and control mice in the Morris water maze, an aversively motivated spatial learning paradigm that has been used extensively to study the neurobiology of spatial learning and memory in rodents (14, 16). Although the primary aim of the study was to test the effects of loss of the RAG-1 gene on spatial learning and memory, we also sought to perform additional behavioral analyses to determine if other domains of behavior might be altered by the loss of this gene. Therefore, we also compared RAG-1-knockout and control mice on measures of fearfulness and locomotor activity in response to the novel conditions of an open field (5, 27) and elevated-plus maze (12, 25), as well as reflexive startle responses to acoustic stimuli and sensorimotor gating using prepulse inhibition (PPI; the suppression of the startle response observed when the startling stimulus is immediately preceded by a weak prepulse stimulus) (18-20, 23). These additional tests were conducted because of the well-established role of the hippocampal formation and related limbic circuitry (and those of the efferent neurons projecting from these areas) in mediating these behaviors and because alterations in these behaviors have been associated with deletion of genes of immunological origin (e.g., cytokine and cytokine receptors) in mice (18, 20).
MATERIALS AND METHODS
Animals.
The mice used in these experiments were obtained from Jackson Laboratories and were cared for in accordance with the guidelines of the National Institutes of Health (16a). Mice were housed in groups under specific-pathogen-free conditions in a temperature-controlled (23 ± 1°C) animal facility with free access to food and water and were acclimated to our colony for 10 days prior to testing. The subject groups consisted of 12 male B6.129S7-Rag1−/−tm1Mom mice and 12 age-matched male B6129SF2/J mice that were used as the appropriate genetic background controls. The sequence in which the behavioral tests were performed was as follows: (i) Morris water maze test, (ii) elevated-plus maze test, (iii) open-field behavior test in dim and bright lighting (counterbalanced for test order; see below), and (iv) acoustic startle and PPI. Sequential behavioral testing is likely to modify the behaviors of animals in succeeding tests, and although such effects are random across subject groups, group-test sequence interactions cannot be ruled out. Since spatial learning and memory were the primary behavioral domains of interest, as noted earlier, the Morris water maze test was conducted first. There was a 2-day interval between each of the behavioral tests. All of the mice (n = 12/group) were tested in the elevated-plus maze and open-field behavior test (except that the data for one control mouse and two RAG-1-knockout mice generated from tests under the dim light testing condition could not be used because they were incompletely collected due to computer failure during testing), and 10 mice per group were tested in the Morris water maze. Animals were tested during the light phase of the light-dark cycle.
Morris water maze.
As described previously (18, 20), the water maze consists of a circular pool (diameter, 120 cm; height, 45 cm) filled with opaque water containing an escape platform, either visible or submerged, which the mouse must locate beginning from one of four starting points (the starting point is changed randomly for each trial). During each trial, the latency (in seconds) to reach the platform and the swim-path length (in centimeters) required to reach the platform were measured by using an overhead video tracking system (Chromotrack; San Diego Instruments). To assess the mice for gross physical, sensory, motor, or motivational impairments, the mice were first trained in a task with a visible escape platform for eight trials per day on 2 successive days. This was followed by a task with a submerged escape platform (eight trials per day on 4 successive days) and a 60-s postacquisition probe trial with no escape platform (ninth trial on day 6 of testing), used to measure spatial learning and memory performance (14, 16, 18). During the probe trial, the percentage of the swim time in each of the four pool quadrants was assessed.
Elevated-plus maze.
As described previously (18, 20), the elevated-plus maze used is made of black Plexiglas and consists of an elevated (38.5-cm) central platform (5 by 5 cm) surrounded by four perpendicular arms (30 by 5 cm). Two arms are fully open and 180° apart, whereas the distal halves of the two other (closed) arms have sidewalls (height, 14.5 cm). The mouse was placed in the center of the central platform at an angle of 45° from the open and closed arms. Entry into an arm was counted when all four legs of the mouse were on the arm. The variables measured include the percentage of time spent in the open and closed areas of the maze, the latency to leave the central platform, the total number of crossings between the various open and closed areas of the maze, and the number of crossings into the open areas of the maze divided by the total number of crossings. Testing was conducted in dim lighting, and the behavior of the animals was monitored for 5 min.
Open-field behavior.
Open-field activity was measured with a Tru Scan activity monitor (Coulbourn, Allentown, Pa.). The monitors consist of four clear Plexiglas walls (26.5 by 37.5 cm) that form an enclosure with two rows of 16 photocell beams to measure horizontal activity, with one row being located front to back and the other being located side to side. These beams are 2.5 cm above the floor and are spaced 0.76 cm apart. The Tru Scan software allows the recording of several parameters; this experiment focused on two measures: total movements and the number of entries into the center of the arena (outside of the region within a 2.5-beam margin of the walls). The latter measure indicates when an animal is not engaged in thigmotaxis (movement in areas adjacent to the walls). Locomotor activity was operationally defined as total movements in the open-field arena. In the Tru Scan activity monitor, total movements are derived from the sum of the movement episodes, in which a movement episode is defined as a series of successive coordinate changes that occur without rest and that last at least 1 s. Recordings were made in 5-min increments over 60 min for each lighting condition. The mice were subjected to the open-field behavior test under both bright lighting conditions and dim lighting conditions. The order was counterbalanced so that half of each group of mice was subjected to testing in bright light first and the other half was subjected to testing in dim light first.
Acoustic startle reactivity and PPI.
Two SR-LAB test chambers (San Diego Instruments) were used to measure acoustic startle response and PPI as described previously (18, 20). Mice were placed in a small cylindrical enclosure (3.8 by 9.5 cm) located in a dark, ventilated chamber. A speaker located 30 cm above the cylinder delivered the background noise (65 dB), startle stimuli, and prepulse stimuli, all of which consisted of broadband white noise. Mice were allowed a 5-min acclimation period during which the background noise was delivered. Five different startle stimulus intensities (80, 90, 100, 110, and 120 dB) and two prepulse stimulus intensities (80 and 90 dB), presented 70 ms before a 120-dB probe stimulus was delivered, were presented in a pseudo-random sequence. Each was presented eight times for a total 56 trials over a 10-min period. The startle stimuli and the prepulse stimuli were of 30 ms in duration and were separated by 70 ms. Startle responses were recorded during a 100-ms period following the onset of each startle stimulus.
Statistical analyses.
Analysis of variance (ANOVA) was used to analyze the data. For some dependent variables, a repeated-measures ANOVA was performed. Main effects (e.g., group) and interactions (e.g., group-time block) were examined for the various dependent variables of interest generated as part of each of the behavioral tests. Statistically significant differences are reported as P values less than 0.05. Analyses were carried out by using the SYSTAT (version 9) program.
RESULTS
Open-field behavior.
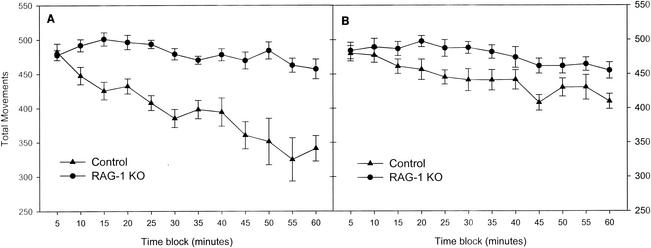
Figure 1 compares the number of movements of RAG-1-knockout and control mice in the open field in bright and dim lighting. Statistical analyses revealed that the testing order (whether subjects were exposed to bright or dim lighting first) had no effect on either the RAG-1-knockout or the control mice. As seen in Fig. 1, compared to the control mice, the RAG-1-knockout mice showed increased movements under both bright and dim lighting conditions in the open field. For the bright lighting condition, analysis of open-field movements showed significant main effects of group [F(1,22) = 46.0; P < 0.001] and time block [F(11,242) = 9.4; P < 0.001] and a significant group-time block interaction [F(11,242) = 4.6; P = 0.001]. For the dim lighting condition, analysis of open-field movements showed significant main effects of group [F(1,19) = 7.5; P = 0.013] and time block [F(11,209) = 6.9; P < 0.001] but no group-time block interaction. As seen in Fig. 1 and indicated by the group-time block interactions, there were notable differences in habituation between the two groups across the 1-h session in bright lighting compared to that in dim lighting. In the bright lighting condition, the mean ± standard error of the mean of the slope generated from 12 time blocks for control mice versus that generated from 12 time blocks for RAG-1-knockout mice (−2.77 ± 0.95 versus −12.46 ± 3.03) differed significantly [F(1,22) = 9.238; P < 0.01]. In dim lighting, however, the mean slopes were not significantly different between the groups. Comparisons within subject groups also revealed that whereas control mice showed the expected decrease in total movements under bright lighting conditions compared to the total movements under dim lighting conditions [F(1,22) = 10.535; P = 0.004], there was no such difference for the RAG-1-knockout mice.
FIG. 1.
Comparison of total movements in the open field between RAG-1-knockout (KO) and control mice under bright (left) and dim (right) lighting conditions (mean ± standard error of the mean).
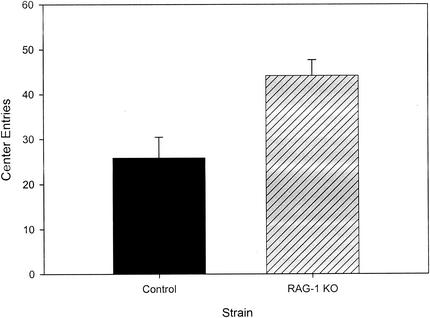
The number of center entries made by mice during their initial exposure to the open field in bright light is often used as a measure to assess fearfulness. We therefore compared this measure for control and RAG-1-knockout mice that were tested first in the open field under bright lighting conditions, a condition that is aversive or anxiogenic to mice. As depicted in Fig. 2, during the first 5-min time block, the knockout mice exhibited approximately 70% more center entries than the control mice [F(1,10) = 9.9; P = 0.006]. This effect was not restricted to the initial 5 min in the open field, as the RAG-1-knockout mice also showed significantly more center entries than control mice across all time blocks [F(1,10) = 9.9; P = 0.01]. We then assessed center entries in the open field for all of the animals as a measure of fearfulness independent of overall motor activity during the first testing session (whether in bright or dim lighting). This was done by computing the proportion of center entries divided by the total movements for each of the 5-min time blocks. Repeated-measures ANOVA for all time periods demonstrated that RAG-1-knockout mice made significantly more center entries per total movements under both bright and dim lighting conditions [F(1,22) = 6.03 (P = 0.02) and F(1,22) = 5.5 (P = 0.03), respectively].
FIG. 2.
Comparison of the first 5 min of entries into the center of the open field between RAG-1-knockout (KO) and control mice tested first under bright lighting conditions (mean ± standard error of the mean).
Elevated-plus maze test and acoustic startle and PPI.
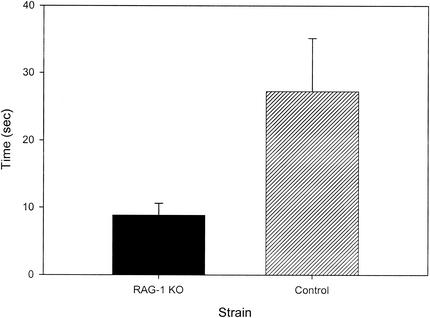
As seen in Fig. 3, the latency to the initiation of movement in the elevated-plus maze was markedly reduced in the RAG-1-knockout mice compared to that in the control mice [F(1,22) = 5.146; P = 0.033]. None of the other variables examined in the elevated-plus maze differed between the groups, including the percentage of time spent in the closed and open areas, the total number of crossings between the various open and closed areas of the maze, and the number of crossings into the open areas of the maze divided by the total number of crossings.
FIG. 3.
Comparison of the latency to initiation of ambulation in the elevated-plus maze between RAG-1-knockout (KO) and control mice (mean ± standard error of the mean).
For both acoustic startle responses and PPI, there were no significant differences between control mice and RAG-1-knockout mice.
Morris water maze.
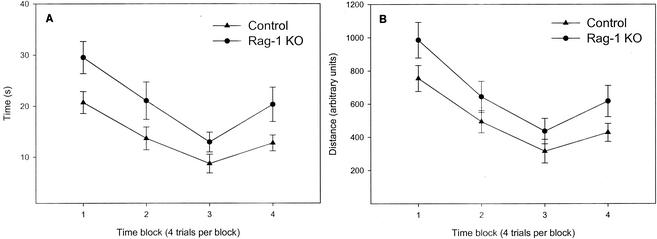
As seen in Fig. 4, in visible-platform testing the RAG-1-knockout mice showed significantly longer escape latencies [F(1,18) = 8.1; P = 0.01] and had longer swim-path distances to reach the platform [F(1,18) = 6.0; P = 0.025]. There were also significant main effects of trial block for both latency [F(3,54) = 13.9; P < 0.001] and distance [F(3,54) = 15.3; P < 0.001], but there were no interactions between trial block and either latency or distance. The swim speed of the RAG-1-knockout mice across the trial blocks in the visible-platform test was significantly reduced [F(1,18); P = 0.011].
FIG. 4.
Comparison of latency and swim-path distance in the visible-platform test of the Morris water maze between RAG-1-knockout (KO) and control mice (mean ± standard error of the mean).
In the submerged-platform test there were no differences between the subjects groups in swim-path distance, latency, or swim speed. In the postacquisition probe trial, there were no differences between the groups in the percentage of time spent in the northwest quadrant (the quadrant from which the platform was removed).
DISCUSSION
The results of this study are the first to demonstrate that neurobehavioral performance is altered in RAG-1-knockout mice. Clear differences in locomotor activity were observed in the RAG-1-knockout mice. The initial level of locomotor activity in the first 5-min time block did not differ between RAG-1-knockout and control mice; however, the RAG-1-knockout mice exhibited an overall increase in locomotor activity relative to controls throughout the remainder of the 1-h test session that was consistent across both lighting conditions. Whereas locomotor activity in the open field in bright light was conducted to assess the response of mice to novel conditions, testing in dim lighting was performed largely to assess basal levels of locomotor activity. From examination of the last 10 min of open-field behavior in dim lighting, for example, when the animals were very well acclimated to the environment (Fig. 1), it is clear that RAG-1-knockout mice have significantly higher levels of basal locomotor activity than control mice. RAG-1-knockout mice also showed a notable loss of habituation (the decline in locomotor activity associated with increasing familiarity with the novel environment) typical of normal mice placed in the open field in bright light, which is known to be aversive or stressful to mice. In fact, among the RAG-1-knockout mice, it is striking that modification of the lighting conditions did not modify their locomotor activity patterns. It is possible that the sustained increase in locomotor activity under bright lighting conditions could represent reduced stress responsiveness in the RAG-1-knockout mice. The propensity for locomotion exhibited by RAG-1-knockout mice was not associated with alterations in prepulse inhibition, indicating that their propensity for hyperlocomotion and altered habituation was not due to a deficit in their ability to filter extraneous sensory stimuli. Reflexive acoustic startle responses were also not different.
Since changes in locomotor activity are used to assess fearfulness in rodents exposed to novel conditions, it is often difficult to disentangle the degree to which such changes are motivated by fear. Several measures suggest that the RAG-1-knockout mice were less fearful in response to novel conditions. These include the initial exposure to the open field during the first 5 min, when the fear-related response to novel conditions is most significant. In the open field, mice exhibit natural aversion to bright light and engage in thigmotaxis (explore the outer areas adjacent to the walls more than the center of the open field). Among the mice exposed first to the open field under bright lighting conditions, the RAG-1-knockout mice showed significantly more center entries during the first 5-min time block, although as mentioned above, total movements did not differ between the groups during this interval. Thus, an increased percentage of center entries in the absence of a difference in total movements indicates that the RAG-1-knockout mice were less fearful than the control mice. Likewise, the reduced latency to the initiation of movement by leaving the central platform in the elevated-plus maze and the increased percentage of movements into the center of the open field when controlling for overall movement (in both bright and dim lighting) in RAG-1-knockout mice also suggest that they were less fearful than the control mice. It is noteworthy, however, that in the elevated-plus maze, the percentage of time spent in the open areas and the percentage of entries into open areas versus closed areas, considered to be the primary indices of fearfulness levels in this test, did not differ between the groups. Thus, although RAG-1-knockout mice showed reduced levels of fearfulness for a subset of measures of fear-motivated behavior in both the open-field test and the elevated-plus maze, they do not exhibit a clear-cut profile of behaviors typically associated with reduced fearfulness or emotionality in mice.
Contrary to our working hypothesis, RAG-1-knockout mice did not have spatial learning abnormalities in the Morris water maze. In the visible-platform test, however, the swim-path distance and latency to reach the escape platform were significantly longer in the RAG-1-knockout mice than in the controls. The interpretation of the group differences observed in the visible-platform test is unclear and requires further study. Visible-platform testing was performed first in order to determine if the RAG-1-knockout mice had gross alterations in sensory (e.g., vision), motor, or motivational factors that could have accounted for the postulated differences in spatial learning and memory in submerged-platform testing. One possibility that could explain the minor differences in the performance of the RAG-1-knockout mice in the visible-platform test is that, given their propensity for locomotion and reduced fearfulness, the RAG-1-knockout mice may have been less motivated to reach the platform when it was visible and readily accessible for escape. It is also conceivable that the RAG-1-knockout mice may have a visual impairment that may explain their reduced habituation in bright lighting in the open field as well as their increased latencies in the visible-platform portion of the Morris water maze. We are not aware of any literature of visual deficits associated with RAG-1 gene deletion, and this possibility also appears unlikely, since visual cues are used for spatial learning and memory in submerged-platform testing, in which the groups did not differ. Nonetheless, since RAG-1-knockout mice were tested first for performance when the platform was visible (as is common), it cannot be ruled out that they may have developed a navigational strategy that they subsequently used to effectively locate the submerged platform.
The role of RAG-1 in the central nervous system remains to be determined. Although it has been proposed that the same type of V(D)J recombination that is associated with immunological memory is occurring in the brain, RAG-2 gene expression, which also plays an integral role in these events, is not expressed in the brain (4). This, coupled with our finding that spatial learning and memory are not altered in the RAG-1-knockout mice, suggests that the RAG-1 gene is involved in processes unrelated to V(D)J recombination in the brain (e.g., other forms of somatic recombination or genomic stabilization associated with neuronal longevity) (8, 13, 21). Interestingly, dopamine transporter-knockdown mice show patterns of behavior that are similar to those of RAG-1-knockout mice (22). The hippocampal formation and the related limbic regions where the RAG-1 gene is expressed (4) modulate various aspects of neurobehavioral performance via inputs and interaction with brain areas known to mediate dopamine neurotransmission and locomotor activity (e.g., the nucleus accumbens) and fear-motivated behaviors (e.g., the amygdala). It is also possible that physiological changes associated with the severe immunodeficiency of RAG-1-knockout mice could, at least in part, play a mechanistic role in their neurobehavioral functioning. Cytokines such as IL-2 have been shown to act on the limbic neurocircuitry involved in locomotor function (2, 10, 19, 28, 29); and others including IL-1, IL-6, and tumor necrosis factor alpha may activate the hypothalamic-pituitary-adrenal (HPA) axis and the release of neuroactive hormones like corticosterone which can modify emotional behavior (6). In fact, immunological stimuli that activate the HPA axis in normal mice fail to do so in RAG-1-knockout mice (9). To our knowledge, comprehensive assessments of various domains of behavior in SCID mice have not been performed; however, in the Morris water maze they did not exhibit the alterations in the visible-platform test evidenced by RAG-1-knockout mice (20), although changes in motor activity have been found in athymic nude mice (26). Since immunological factors have been shown to influence neurodevelopment and behavior (1, 2, 9, 18, 20, 29), it is possible that the absence of functional T and B lymphocytes in RAG-1-knockout mice rather than the loss of the gene in the brain could account for the behavioral alterations observed in these studies. Future studies may address this question by using adoptive transfer. Likewise, in future studies it will also be important to compare wild-type and RAG-1-knockout littermates (bred from heterozygote × heterozygote crosses) to control for as many of the maternal, developmental, and genetic factors (e.g., contaminant genes associated with the knockout methodology) as possible that may contribute to the observed behavioral phenotype of RAG-1-knockout mice.
In summary, loss of the RAG-1 gene is associated with changes in locomotor activity, habituation in the open field, and fearfulness. Whether these changes are due to the loss of RAG-1 gene expression in the brain, the result of the absence of the RAG-1 gene in the immune system, or some combination of both effects remains to be determined in future research. Study of the neurobiological actions of RAG-1 in the brain may provide new insights into important processes involved in normal brain function and disease.
Acknowledgments
This work was supported by RO1 grants NS42216 and NS38179 (to J.M.P.).
We are grateful to Mark H. Lewis for insights regarding the interpretation of the data.
REFERENCES
- 1.Ader, R., D. L. Felton, and N. Cohen. 2001. Psychoneuroimmunolgy. Academic Press, Inc., San Diego, Calif.
- 2.Anisman, H., and Z. Merali. 1999. Anhedonic and anxiogenic effects of cytokine exposure. Adv. Exp. Med. Biol. 461:199-233. [DOI] [PubMed] [Google Scholar]
- 3.Bassing, C. H., W. Swat, and F. W. Alt. 2002. The mechanism and regulation of chromosomal V(D)J recombination. Cell 109(Suppl.):S45-S55. [DOI] [PubMed] [Google Scholar]
- 4.Chun, J. J., D. G. Schatz, M. A. Oettinger, R. Jaenisch, and D. Baltimore. 1991. The recombination activating gene-1 (RAG-1) transcript is present in the murine central nervous system. Cell 64:189-200. [DOI] [PubMed] [Google Scholar]
- 5.Denenberg, V. H. 1969. Open-field behavior in the rat: what does it mean? Ann. N. Y. Acad. Sci. 159:852-859. [DOI] [PubMed] [Google Scholar]
- 6.Dunn, A. J. 2000. Cytokine activation of the HPA axis. Ann. N. Y. Acad. Sci. 917:608-617. [DOI] [PubMed] [Google Scholar]
- 7.Hanisch, U. K., S. A. Lyons, M. Prinz, C. Nolte, J. R. Weber, H. Kettenmann, and F. Kirchhoff. 1997. Mouse brain microglia express interleukin-15 and its multimeric receptor complex functionally coupled to Janus kinase activity. J. Biol. Chem. 272:28853-28860. [DOI] [PubMed] [Google Scholar]
- 8.Kawaichi, M., C. Oka, R. Reeves, M. Kinoshita, and T. Honjo. 1991. Recombination of exogenous interleukin 2 receptor gene flanked by immunoglobulin recombination signal sequences in a pre-B cell line and transgenic mice. J. Biol. Chem. 266:18387-18394. [PubMed] [Google Scholar]
- 9.Kawashima, N., and A. W. Kusnecov. 2002. Effects of staphylococcal enterotoxin A on pituitary-adrenal activation and neophobic behavior in the C57BL/6 mouse. J. Neuroimmunol. 123:41-49. [DOI] [PubMed] [Google Scholar]
- 10.Lacosta, S., Z. Merali, and H. Anisman. 1999. Influence of acute and repeated interleukin-2 administration on spatial learning, locomotor activity, exploratory behaviors, and anxiety. Behav. Neurosci. 113:1030-1041. [DOI] [PubMed] [Google Scholar]
- 11.Lee, G. S., M. B. Neiditch, R. R. Sinden, and D. B. Roth. 2002. Targeted transposition by the V(D)J recombinase. Mol. Cell. Biol. 22:2068-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lister, R. G. 1987. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 92:180-185. [DOI] [PubMed] [Google Scholar]
- 13.Matsuoka, M., F. Nagawa, K. Okazaki, L. Kingsbury, K. Yoshida, U. Muller, D. T. Larue, J. A. Winer, and H. Sakano. 1991. Detection of somatic DNA recombination in the transgenic mouse brain. Science 254:81-86. [DOI] [PubMed] [Google Scholar]
- 14.McNamara, R. K., and R. W. Skelton. 1993. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res. Brain Res. Rev. 18:33-49. [DOI] [PubMed] [Google Scholar]
- 15.Mombaerts, P., J. Iacomini, R. S. Johnson, K. Herrup, S. Tonegawa, and V. E. Papaioannou. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68:869-877. [DOI] [PubMed] [Google Scholar]
- 16.Morris, R. 1984. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11:47-60. [DOI] [PubMed] [Google Scholar]
- 16a.National Institutes of Health. 2001. Guide for the care and use of laboratory animals. National Institutes of Health, Bethesda, Md.
- 17.Petitto, J. M., and Z. Huang. 2001. Cloning the full-length IL-2/15 receptor-beta cDNA sequence from mouse brain: evidence of enrichment in hippocampal formation neurons. Regul. Pept. 98:77-87. [DOI] [PubMed] [Google Scholar]
- 18.Petitto, J. M., Z. Huang, D. A. Hartemink, and R. Beck. 2002. IL-2/15 receptor-beta gene deletion alters neurobehavioral performance. Brain Res. 929:218-225. [DOI] [PubMed] [Google Scholar]
- 19.Petitto, J. M., D. B. McCarthy, C. M. Rinker, Z. Huang, and T. Getty. 1997. Modulation of behavioral and neurochemical measures of forebrain dopamine function in mice by species-specific interleukin-2. J. Neuroimmunol. 73:183-190. [DOI] [PubMed] [Google Scholar]
- 20.Petitto, J. M., R. K. McNamara, P. L. Gendreau, Z. Huang, and A. J. Jackson. 1999. Impaired learning and memory and altered hippocampal neurodevelopment resulting from interleukin-2 gene deletion. J. Neurosci. Res. 56:441-446. [DOI] [PubMed] [Google Scholar]
- 21.Schatz, D. G., and J. J. Chun. 1992. V(D)J recombination and the transgenic brain blues. New Biol. 4:188-196. [PubMed] [Google Scholar]
- 22.Spielewoy, C., C. Roubert, M. Hamon, M. Nosten-Bertrand, C. Betancur, and B. Giros. 2000. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav. Pharmacol. 11:279-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swerdlow, N. R., D. L. Braff, and M. A. Geyer. 2000. Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav. Pharmacol. 11:185-204. [DOI] [PubMed] [Google Scholar]
- 24.Tonegawa, S. 1983. Somatic generation of antibody diversity. Nature 302:575-581. [DOI] [PubMed] [Google Scholar]
- 25.Trullas, R., and P. Skolnick. 1993. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology 111:323-331. [DOI] [PubMed] [Google Scholar]
- 26.Vidal, J. 1996. Differences of nu/+ and nu/nu mice in some behaviors reflecting temperament traits. Physiol. Behav. 59:341-348. [DOI] [PubMed] [Google Scholar]
- 27.Whimbey, A. E., and V. H. Denenberg. 1967. Two independent behavioral dimensions in open-field performance. J. Comp. Physiol. Psychol. 63:500-504. [DOI] [PubMed] [Google Scholar]
- 28.Zalcman, S., L. Murray, D. G. Dyck, A. H. Greenberg, and D. M. Nance. 1998. Interleukin-2 and -6 induce behavioral-activating effects in mice. Brain Res. 811:111-121. [DOI] [PubMed] [Google Scholar]
- 29.Zalcman, S. S. 2001. Interleukin-2 potentiates novelty- and GBR 12909-induced exploratory activity. Brain Res. 899:1-9. [DOI] [PubMed] [Google Scholar]