Abstract
Anaplasma marginale is a tick-borne, rickettsial cattle pathogen that is endemic in several areas of the United States. Recent studies (J. de la Fuente, J. C. Garcia-Garcia, E. F. Blouin, J. T. Saliki, and K. M. Kocan, Clin. Diagn. Lab. Immunol. 9:658-668, 2002) demonstrated that infection of cultured tick cells and bovine erythrocytes with one genotype of A. marginale excluded infection with other genotypes, a phenomenon referred to as infection exclusion. The present study was undertaken to confirm the phenomenon of infection exclusion of A. marginale genotypes in a tick vector, Dermacentor variabilis. Only one genotype of A. marginale (Virginia isolate) was detected by PCR in ticks that fed first on a calf infected with a Virginia isolate and second on a calf infected with an Oklahoma isolate. These studies demonstrate that infection exclusion of A. marginale genotypes also occurs in naturally infected ticks, as well as in cattle and cultured tick cells, and results in establishment of only one genotype per tick.
Anaplasmosis, a tick-borne cattle disease caused by the obligate intraerythrocytic bacterium Anaplasma marginale (Rickettsiales: Anaplasmataceae), is endemic in many tropical and subtropical regions, including several areas of the United States. Several geographic isolates of A. marginale, which differ from each other in biology, morphology, and sequence and antigenic characteristics, have been identified in the United States (reviewed in reference 3). Feeding ticks effect biological transmission of A. marginale, while mechanical transmission occurs when infected blood is transferred to susceptible animals by biting flies or by blood-contaminated fomites. Cattle that recover from acute infection remain persistently infected and serve as reservoirs for mechanical transmission and infection of ticks (reviewed in reference 9). A. marginale multiplies only within membrane-bound inclusions in the cytoplasm of host cells. The only known site of A. marginale development in cattle is within erythrocytes (14), while A. marginale develops in several tissues in ticks, including the salivary glands from which A. marginale is transmitted to susceptible cattle (10-12).
Major surface protein 1a (MSP1a) is one of six MSPs that have been described for A. marginale from bovine erythrocytes. MSP1a is encoded by a single gene, msp1α, which is conserved during the multiplication of the parasite in cattle and ticks, therefore resulting in a stable genetic marker of A. marginale geographic isolates (2). The molecular weight of MSP1a is variable among geographic isolates because of a variable number of tandem 28- or 29-amino-acid repeats (1, 3, 4, 7). Recent reports by Palmer et al. (13) and J. de la Fuente, R. A. Van Den Bussche, T. Prado, and K. M. Kocan (submitted for publication) documented genetic heterogeneity in the structure of msp1α sequences that have been recovered from infected animals in endemic areas in Oregon and Oklahoma, respectively. However, only one msp1α genotype was identified in individual cattle that were naturally or experimentally infected and sampled at different stages of infection (2, 13). These findings were recently explained by the demonstration of infection exclusion of other A. marginale genotypes in infected cattle and cultured tick cells (8).
The present study was undertaken to determine whether the phenomenon of infection exclusion of A. marginale also occurs in Dermacentor variabilis, a tick vector of A. marginale in the United States. Two tick-transmissible isolates of A. marginale were used in this study (8), a Virginia isolate obtained originally in 1978 from the USDA Animal Disease Research Laboratory, Beltsville, Md., and an Oklahoma isolate obtained from a naturally infected bovine from Wetumka, Okla., in 1997. Both isolates have been used for tick transmission and cell culture studies in our laboratory (2, 5, 8). Splenectomized calves were inoculated with each isolate, and blood collected at the infection peak was prepared as stabilates and stored in liquid nitrogen until used for infection of the experimental calves.
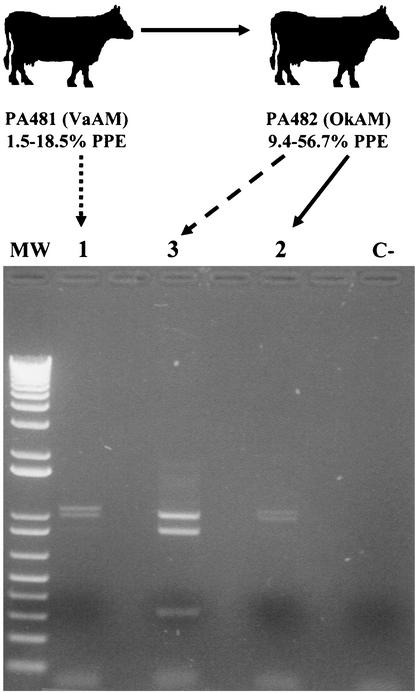
Two mixed-breed calves (4 to 6 months old), determined to be free of infection by an A. marginale-specific enzyme-linked immunosorbent assay (15), were used for the study. The calves were housed in the Anaplasmosis Research Barn and cared for by the Oklahoma State University (OSU) Laboratory Animal Research Unit with the approval of the OSU Institutional Animal Care and Use Committee. The calves were splenectomized to cause higher levels of A. marginale infection, and each calf was then experimentally infected with one of the two A. marginale isolates. The calves were monitored three times a week by examination of stained blood smears and determination of the packed cell volume. Thin smears were made on glass slides from blood collected from the calves by venipuncture in EDTA-treated Vacutainer tubes and stained with Protocol Hema 3 stain (Biochemical Sciences, Inc., Swedesburg, N.J.). The percentage of parasitized erythrocytes (PPE) out of 500 was determined. Once infection was detected in blood smears, the calves were monitored daily. Calf PA482 was inoculated intravenously with 5 ml of the Oklahoma isolate stabilate made from blood collected from calf PA407 at a 16.5% PPE, and calf PA481 was inoculated intravenously with 8 ml of the Virginia isolate stabilate made from blood collected from calf PA433 with a 12.2% PPE. Both calves were infested with approximately 300 D. variabilis male ticks in two orthopedic stockinettes (150 ticks in each) glued to the side of the calf when the parasitemia was 3 to 10%. The ticks were allowed to feed for 7 days, after which they were held in a humidity chamber. The ticks were then allowed to feed for 7 days on a sheep to cause development of A. marginale infection in the ticks' salivary glands. Group 1 ticks fed only on calf PA481, which was infected with the Virginia isolate of A. marginale. Group 3 ticks fed only on calf PA482, which was infected with the Oklahoma isolate of A. marginale. Group 2 ticks were allowed to feed first on calf PA481 and then on calf PA482, before being allowed to feed on the sheep (Fig. 1). After feeding on the sheep, all the ticks were removed; the salivary glands from groups of 30 ticks were dissected and placed in 500 μl of a commercial solution for preserving biological samples for RNA analysis (RNALater; Ambion, Austin, Tex.) to be used for PCR studies. Two groups of tick salivary glands were analyzed, with similar results.
FIG. 1.
Infection exclusion of A. marginale in ticks. Groups of D. variabilis male ticks were allowed to acquisition feed on cows infected with a Virginia isolate of A. marginale (VaAM) (PA481; group 1) and an Oklahoma isolate (OkAM) (PA482; group 3). One group of ticks was allowed to feed first on calf PA481 and subsequently on calf PA482 (group 2). The levels of infection in cattle during tick feeding are indicated as PPE. Only the Virginia isolate was present in the ticks from group 2. DNA was extracted from tick salivary glands after tick transmission feeding on a sheep, and the msp1α genotype was analyzed after PvuII digestion of the PCR products. The samples were analyzed on a 1% ethidium bromide-stained agarose gel. Lane MW, 1-kb Plus DNA ladder (Gibco BRL); lane C-, control sample with no DNA added before PCR. Two groups of tick salivary glands were analyzed, with similar results.
A. marginale from tick salivary glands was characterized by PvuII restriction analysis of the msp1α gene amplified by PCR as previously reported (8). The sequence of the msp1α gene varies among different geographic isolates of A. marginale (1, 3, 4, 7). The msp1α gene of the Virginia isolate has two tandem repeats, while the Oklahoma isolate gene has three tandem repeats (4, 8). The numbers of PvuII sites in the msp1α gene are different for the two isolates: the Oklahoma isolate gene has two sites, at positions 31 and 879 with respect to the translation initiation codon, while the Virginia isolate gene has only one, at position 712 (4, 8). The PvuII digestion products of the msp1α PCR therefore result in distinguishable patterns for the two isolates (8). The PCR product of the Virginia isolate results in two bands, of 1,071 and 997 bp, while the Oklahoma isolate results in three bands, of 997, 848, and 313 bp.
This assay was validated in our research, and we have demonstrated that both the Virginia and Oklahoma isolates could be identified simultaneously in infected samples (8). The sensitivity of the assay for infected tick salivary glands was investigated by using for the msp1α PCR DNA that had been extracted from samples in which the A. marginale infection levels were determined by a quantitative msp4 PCR (5, 6). We established that the assay for msp1α was able to detect approximately five pathogens (as copies of msp4) per salivary gland. The infection levels in two groups of salivary glands dissected from ticks that fed first on calf PA481 and then on calf PA482 were equivalent to 700 and 1,500 msp4 copies per salivary gland. Therefore, the detection limit of the msp1α assay (5 msp4 copies per salivary gland) represented less than 1% of the total A. marginale DNA. The product of the msp1α PCR was also sequenced directly by double-stranded dye termination cycle sequencing (Core Sequencing Facility, Department of Biochemistry and Molecular Biology, Noble Research Center, OSU).
Only Virginia and Oklahoma isolates were identified in the ticks that fed on calves PA481 and PA482, which were infected with these isolates, respectively (Fig. 1). In the ticks that fed first on calf PA481 and then on calf PA482, only the Virginia isolate was detected within the detection limit of the assay (Fig. 1). Furthermore, the level of infection in calf PA482, which was infected with the Oklahoma isolate, was higher than the infection levels in calf PA481 (Fig. 1). Nevertheless, as in previous experiments with bovine erythrocytes and cultured tick cells (8), only the isolate first infecting the ticks (Virginia isolate after feeding on PA481) was able to establish infection, excluding the infection with the Oklahoma isolate when the ticks fed on the Oklahoma isolate-infected calf PA482. These experiments confirmed that ticks become infected with only one A. marginale genotype because of the occurrence of infection exclusion. Male ticks, which become persistently infected with A. marginale and can feed on multiple cattle, may have the opportunity to be exposed to more than one A. marginale genotype. However, the results of this study suggest that the males will become infected with only one genotype. The demonstration of infection exclusion of A. marginale in ticks further confirms that different A. marginale genotypes survive in nature by individual transmission events and explains the existence of several A. marginale genotypes in infected cattle in endemic regions (13; de la Fuente et al., submitted). Genotypes introduced into a herd in an endemic area via cattle shipment would most likely be maintained and become endemic if they were transmitted to susceptible cattle. Both persistently infected cattle and ticks could therefore serve as reservoirs of the introduced genotype. These results have important implications for the epidemiology and control of anaplasmosis.
Acknowledgments
This research was supported by project no. 1669 of the Oklahoma Agricultural Experiment Station; the Endowed Chair for Food Animal Research (K. M. Kocan, College of Veterinary Medicine, OSU); National Institutes of Health Centers for Biomedical Research Excellence through a subcontract to J. de la Fuente from the Oklahoma Medical Research Foundation; and the Oklahoma Center for the Advancement of Science and Technology, Applied Research Grant AR00(1)-001.
Dollie Clawson (Department of Veterinary Pathobiology, OSU) is acknowledged for technical assistance. Sue Ann Hudiburg and Janet J. Rogers (Core Sequencing Facility, Department of Biochemistry and Molecular Biology, Noble Research Center, OSU) are acknowledged for oligonucleotide synthesis and DNA sequencing, respectively.
REFERENCES
- 1.Allred, D. R., T. C. McGuire, G. H. Palmer, S. R. Leib, T. M. Harkins, T. F. McElwain, and A. F. Barbet. 1990. Molecular basis for surface antigen size polymorphisms and conservation of a neutralization-sensitive epitope in Anaplasma marginale. Proc. Natl. Acad. Sci. USA 87:3220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowie, M. V., J. de la Fuente, K. M. Kocan, E. F. Blouin, and A. F. Barbet. 2002. Conservation of major surface protein 1 genes of the ehrlichial pathogen Anaplasma marginale during cyclic transmission between ticks and cattle. Gene 282:95-102. [DOI] [PubMed] [Google Scholar]
- 3.de la Fuente, J., J. C. Garcia-Garcia, E. F. Blouin, S. D. Rodríguez, M. A. García, and K. M. Kocan. 2001. Evolution and function of tandem repeats in the major surface protein 1a of the ehrlichial pathogen Anaplasma marginale. Anim. Health Res. Rev. 2:163-173. [PubMed] [Google Scholar]
- 4.de la Fuente, J., R. A. Van Den Bussche, and K. M. Kocan. 2001. Molecular phylogeny and biogeography of North American isolates of Anaplasma marginale (Rickettsiaceae: Ehrlichieae). Vet. Parasitol. 97:65-76. [DOI] [PubMed] [Google Scholar]
- 5.de la Fuente, J., J. C. Garcia-Garcia, E. F. Blouin, and K. M. Kocan. 2001. Major surface protein 1a effects tick infection and transmission of the ehrlichial pathogen Anaplasma marginale. Int. J. Parasitol. 31:1705-1714. [DOI] [PubMed] [Google Scholar]
- 6.de la Fuente, J., K. M. Kocan, J. C. Garcia-Garcia, E. F. Blouin, P. L. Claypool, and J. T. Saliki. 2002. Vaccination of cattle with Anaplasma marginale derived from tick cell culture and bovine erythrocytes followed by challenge-exposure by infected ticks. Vet. Microbiol. 89:239-251. [DOI] [PubMed] [Google Scholar]
- 7.de la Fuente, J., R. A. Van Den Bussche, J. C. Garcia-Garcia, S. D. Rodriquez, M. A. Garcia, A. A. Guglielmone, A. J. Mangold, L. M. Friche Passos, M. F. Barbosa Ribeiro, E. F. Blouin, and K. M. Kocan. 2002. Phylogeography of New World isolates of Anaplasma marginale (Rickettsiaceae: Anaplasmataceae) based on major surface protein sequences. Vet. Microbiol. 88:275-285. [DOI] [PubMed] [Google Scholar]
- 8.de la Fuente, J., J. C. Garcia-Garcia, E. F. Blouin, J. T. Saliki, and K. M. Kocan. 2002. Infection of tick cells and bovine erythrocytes with one genotype of the intracellular ehrlichia Anaplasma marginale excludes infection with other genotypes. Clin. Diagn. Lab. Immunol. 9:658-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewing, S. A. 1981. Transmission of Anaplasma marginale by arthropods, p. 395-423. In Proceedings of the 7th National Anaplasmosis Conference. Mississippi State University, Mississippi State, Miss.
- 10.Kocan, K. M. 1986. Development of Anaplasma marginale: coordinated development of a rickettsial organism and its tick host, p. 472-505. In J. R. Sauer and J. A. Hair (ed.), Morphology, physiology and behavioral ecology of ticks. Ellis Horwood, Ltd., Chichester, United Kingdom.
- 11.Kocan, K. M., D. Stiller, W. L. Goff, P. L. Claypool, W. Edwards, S. A. Ewing, T. C. McGuire, J. A. Hair, and S. J. Barron. 1992. Development of Anaplasma marginale in male Dermacentor andersoni transferred from parasitemic to susceptible cattle. Am. J. Vet. Res. 53:499-507. [PubMed] [Google Scholar]
- 12.Kocan, K. M., W. L. Goff, D. Stiller, P. L. Claypool, W. Edwards, S. A. Ewing, J. A. Hair, and S. J. Barron. 1992. Persistence of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in male Dermacentor andersoni (Acari: Ixodidae) transferred successively from infected to susceptible calves. J. Med. Entomol. 29:657-668. [DOI] [PubMed] [Google Scholar]
- 13.Palmer, G. H., F. R. Rurangirwa, and T. F. McElwain. 2001. Strain composition of the ehrlichia Anaplasma marginale within persistently infected cattle, a mammalian reservoir for tick transmission. J. Clin. Microbiol. 39:631-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ristic, M., and A. M. Watrach. 1963. Anaplasmosis. VI. Studies and a hypothesis concerning the cycle of development of the causative agent. Am. J. Vet. Res. 24:267-276. [PubMed]
- 15.Saliki, J. T., E. F. Blouin, S. J. Rodgers, and K. M. Kocan. 1998. Use of tick cell culture-derived Anaplasma marginale antigen in a competitive ELISA for serodiagnosis of anaplasmosis. Ann. N. Y. Acad. Sci. 849:273-281. [DOI] [PubMed] [Google Scholar]