Abstract
We investigated the effect of multiple freeze-thaw cycles on mumps, measles, and rubella virus serum antibody levels with whole-virus immunoglobulin G enzyme-linked immunoassays. Fresh serum samples from nine healthy adult volunteers were divided into six sets of five aliquots each. Samples were taken through a total of 10 freeze-thaw cycles and stored at 4°C until assayed. Each assay measurement was done in replicates of five, and the mean value was reported. After completing 10 freeze-thaw cycles, we found no clinically or statistically significant effect on measured antibody levels and found no discernible detrimental effect on the ability to measure these antibodies by enzyme-linked immunoassays.
Despite the widespread use of banked serum specimens that have undergone multiple freeze-thaw cycles, there is a paucity of data available regarding the effect of numerous freeze-thaw cycles on the measured antibody result. This information is particularly relevant for sensitive assays, such as enzyme-linked immunoassays (EIAs), which measure protein structures (antibodies) prone to denaturation. Because these biologic specimens may be used for multiple investigations over a period of time, concern may exist that repeated freeze-thaw cycles might affect the results of a particular assay by physically damaging the antibody of interest.
The limited data available regarding the effect of multiple freeze-thaw cycles on stored serum samples have focused on serum chemistry determinations (1, 2) or measurement of apolipoproteins (3). Each of these investigations suggested a decline in the absolute value from baseline, but the decline was not deemed clinically significant. Petrakis (5) reports that antibodies are stable when stored at −70°C, but repeated freeze-thaw cycles significantly reduced detectable immunoglobulin G (IgG) and IgM activity. Unfortunately, details regarding the number of freeze-thaw cycles and the conditions of storage and testing of specimens in these studies were not specified. Because of this, we designed an experiment to investigate the effect of multiple freeze-thaw cycles on measles, mumps, and rubella virus (MMR) antibody measurements by utilizing whole-virus EIAs.
MATERIALS AND METHODS
After obtaining informed consent from nine adult volunteers (designated A to I), approximately 30 ml of whole blood was obtained by a standard venipuncture technique. Serum was separated by centrifugation of the sample and divided into six sets of five 0.5-ml aliquots each, for a total of 30 aliquots from each volunteer. One set of aliquots, designated as the baseline, was immediately stored at 4°C for the remainder of the study. The remaining five sets of aliquots were frozen at −80°C. Six hours after the initial freezing, the aliquot sets were removed from the freezer and allowed to stand at room temperature for approximately 2 h until completely thawed; they were then refrozen. After another 6-h freeze and 2-h thaw, one set of aliquots was stored at 4°C for the remainder of the study, and the remaining four sets were refrozen. This 16-h cycle was repeated four additional times, with one aliquot stored in the refrigerator at 4°C and the rest of the aliquots returned to the freezer each time. At this point, aliquot sets 2 through 6 had completed 2, 4, 6, 8, and 10 freeze-thaw cycles respectively, while the first set had been stored at the constant baseline temperature of 4°C.
MMR antibody levels were determined for each set of aliquots, in replicates of five, with whole-virus EIAs (MEASELISA II, MUMPS ELISA II, and RUBESTAT; BioWhittaker, Walkersville, Md.) with the automated WELL PREP 2000 assay system (BioWhittaker). The assay values were reported as the mean of five replicates and plotted for each subject against the number of freeze-thaw cycles. Comparisons were made against baseline MMR antibody level determinations. We tested the null hypothesis that there was no linear decrease in antibody levels by using a random-effects linear regression model of log assay on the number of cycles.
RESULTS
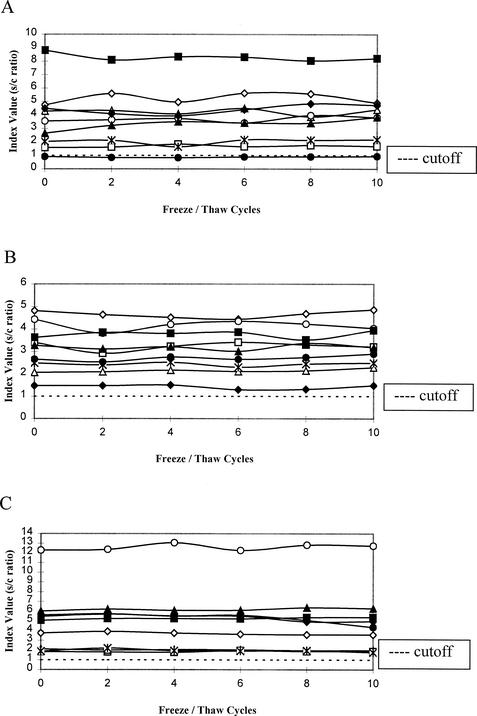
The mean assay values for each specimen (A through I) were plotted in graphs A through C in Fig. 1 for each antibody of interest. These values were plotted against freeze-thaw cycles (baseline and cycles 2, 4, 6, 8, and 10). The cutoff value for seropositivity is also delineated in Fig. 1. (The cutoff value, 1.0, is the lower unit of seropositivity as defined by the manufacturer.) These plots revealed that no changes occurred when antibody levels were analyzed as categorical (i.e., positive, ≥1.0; negative, <0.8; equivocal, ≥1.0) variables in that none dropped below 1.0, except for that for subject F, whose value remained in the equivocal range from the outset. Additionally, no clinical or statistical differences occurred between baseline values and interim or final assay antibody activity levels when antibody levels were analyzed as continuous variables.
FIG. 1.
Antibody levels for MMR. Antibody levels for measles (A), mumps (B), and rubella virus (C) are displayed along the x axis, and the number of freeze-thaw cycles is displayed along the y axis. The horizontal dashed line in each panel indicates the cutoff point, above which results represent seropositivity as established by the kit manufacturer.
In each of the three analyses, the paired t test comparing log (baseline) to log (10th cycle) found no significant difference: measles, mean change = 0.0731, P = 0.11; mumps, mean change =0.00996, P = 0.69; and rubella virus, mean change = −0.0388, P = 0.27. However, the random-effects linear regression of log (assay) on number of cycles (0 to 10) found significant trends in measles and rubella virus, but not mumps, with estimated slope coefficients and P values as follows: measles, slope = 0.00751, P = 0.012; rubella virus, slope = −0.00461, P = 0.024; and mumps, slope = 0.00138, P = 0.47. The significant P values do not appear to be caused by outliers, either within subjects or between subjects. For measles, the within-subject slopes ranged from −0.00520 to +0.0287, with seven of the nine subjects having positive slopes; for rubella virus, the slopes ranged from −0.0229 to 0.00564, with four of the nine slopes being positive.
DISCUSSION
Frozen serum banks are an important source of scientific and clinical information and are essential to infectious disease and vaccine research. Although not well studied, repeated freeze-thaw cycles, at least theoretically, may damage the entity being measured. Previous studies have yielded inconsistent results. Antibodies have been reported to remain stable in storage at less than −20°C for extended periods of time (4). However, Petrakis (5) revealed that just 1 freeze-thaw cycle significantly reduced IgG levels and IgM activity to 25% below prefreezing levels. Other investigators have demonstrated that IgG antibodies to cytomegalovirus were unstable after more than 5 freeze-thaw cycles, leading to significant variability in antibody testing results (J. A. Helgason, M. F. Jones, A. D. Wold, and T. F. Smith, Abstr. 93rd Gen. Meet. Am. Soc. Microbiol. 1993, abstr. C-3, p. 446, 1993). We assume that this instability in the measured antibody activity level is a result of protein denaturation during the freeze-thaw cycle. Normally, in an aqueous environment, proteins fold spontaneously, so the hydrophobic side chains are encased by polar chains with a surrounding bulk hydration layer. Freezing disrupts this bulk hydration layer, forming a crystal lattice of ice and rendering it inactive. Unfolding allows hydrophobic reactions to occur, thus denaturing the protein.
Examination of the assay values does not suggest any consistent nonlinear trend, nor any threshold effect, such as a number of cycles beyond which the change in antibody levels is more rapid. Direct comparison of baseline and final assay values showed no evidence for change in actual antibody level. However, the random-effects regression showed trends over a number of cycles. Measles antibody levels showed an increasing trend, rubella virus antibody levels showed a decreasing trend, and mumps antibody levels remained constant. Because of the physical effect of freezing, all three assays should have a decreasing or similar trend. We believe that the trends observed are due to instrument drift in the assay and do not reflect changes in the samples, because the interassay coefficient of variation measured after nearly 2,000 measles assays with this kit was 6.63%. The changes observed were small, averaging less than 0.5% change per freeze-thaw cycle. These changes have no apparent clinical significance, and there are no statistically significant differences between baseline and final (i.e., after 10 freeze-thaw cycles) antibody levels. Thus, despite the small number of volunteers, the results are robust enough to demonstrate the lack of effect from the freeze-thaw cycles.
We recognize previous work inconsistently indicated potential deleterious effects, but the literature does not provide adequate methodology. While we found no evidence that freeze-thaw cycles might affect antibody measures, we did not examine antibody function. Furthermore, our enzyme-linked immunosorbent assay tested whole-virus antibody-antigen binding. The freeze-thaw cycle may interfere with different antibody-epitope binding sites. Also, one must consider other factors that might destabilize antibody levels, such as length of storage, aliquot size, dilution, and temperature. We found no effect with MMR. We recommend those working with other antibodies consider similar testing.
In conclusion, our study demonstrates that the IgG antibody activity levels measured for MMR with whole-virus EIAs are stable after 10 freeze-thaw cycles. Although variations in the absolute values were obtained, the values were not clinically significant and did not reflect assay variability. Alternative methods of freezing (i.e., rapid freezing in liquid nitrogen) and thawing were not compared in this protocol, because we hypothesized that any deleterious effect on the proteins resulted from the physical act of freezing and thawing itself. This finding is both important and reassuring to investigators, because it demonstrates the stability of these antibodies through more freeze-thaw cycles than are likely to occur in routine use, thus securing the usefulness of such frozen, banked specimens for future seroepidemiologic research.
Acknowledgments
This work was partially supported by a grant from the NIH (AI33144).
We thank Kim S. Zabel for invaluable editorial assistance.
REFERENCES
- 1.Blincoe, C., and D. W. Marble. 1985. Storage stability of some bovine plasma enzymes. Am. J. Vet. Res. 46:1242-1244. [PubMed] [Google Scholar]
- 2.DiMagno, E. P., D. Corle, J. F. O'Brien, I. J. Masnyk, and R. Aamodt. 1989. Effect of long-term freezer storage, thawing, and refreezing on selected constituents of serum. Mayo Clin. Proc. 64:1226-1234. [DOI] [PubMed] [Google Scholar]
- 3.Palombo, J. D., B. R. Bistrian, and G. L. Blackburn. 1987. Radioimmunoassay of apolipoprotein A-1 in serum and EDTA plasma, and effects of freeze-thaw cycles. Clin. Chem. 33:308. [PubMed] [Google Scholar]
- 4.Paul, J. R., and C. White. (ed.). 1973. Serologic epidemiology. Academic Press, New York, N.Y.
- 5.Petrakis, N. L. 1985. Biologic banking in cohort studies, with special reference to blood. NCI Monogr. 67:193-198. [PubMed] [Google Scholar]