Abstract
During the 2001 U. S. West Nile virus (WNV) season, 163 specimens were reactive in an in-house WNV-specific immunoglobulin M (IgM) screening enzyme-linked immunosorbent assay (ELISA) and were referred to either the Centers for Disease Control and Prevention or the appropriate state public health laboratory (CDC/SPHL) for additional testing. CDC/SPHL supplied results for 124 specimens that could be further evaluated in-house: 70 specimens were nonreactive in the CDC/SPHL WNV-specific IgM screening assay, and 54 specimens were reactive. These specimens were used to evaluate a modified in-house WNV-specific IgM ELISA that incorporated background subtraction to identify nonspecific reactivity and thus improve assay specificity. Of the 70 CDC/SPHL nonreactive samples, 49 (70%) were nonreactive in the modified ELISA; of the 54 CDC/SPHL reactive samples, 51 (94%) were reactive in the modified ELISA. Confirmatory studies performed by CDC/SPHL indicated that 38 CDC/SPHL screen-reactive specimens represented true WNV infection; all 38 specimens were reactive in the modified in-house WNV-specific IgM ELISA. These findings demonstrate that an in-house ELISA system for WNV-specific IgM effectively identifies patients with WNV infection.
In response to the 1999 emergence of West Nile virus (WNV) as an agent of encephalitis in the United States (3), our facility developed a sensitive in-house enzyme-linked immunosorbent assay (ELISA) to screen for WNV-specific immunoglobulin M (IgM). All samples reactive in the in-house WNV-specific IgM screening ELISA are forwarded to either the Centers for Disease Control and Prevention or the appropriate state public health laboratory (CDC/SPHL), where they are screened for WNV-specific IgM and, if necessary, tested in confirmatory assays for reactivity to specific flaviviruses (5).
The 2001 WNV season offered the opportunity to determine how samples reactive in our in-house WNV-specific IgM screening ELISA fared in WNV-specific IgM assays performed by CDC/SPHL. As shown in this report, many samples that were weakly reactive in our in-house WNV-specific IgM screening ELISA were nonreactive in CDC/SPHL WNV-specific IgM screening assays. We therefore developed a modified version of our WNV-specific IgM ELISA, wherein reactivity in the absence of WNV antigen is subtracted from reactivity in the presence of WNV antigen. This modified ELISA was used to evaluate frozen aliquots of specimens that were reactive in the in-house screening ELISA and forwarded to CDC/SPHL.
Human serum or cerebrospinal fluid (CSF) specimens were submitted to our facility by referring laboratories for WNV-specific IgM testing between 1 June 2001 and 31 January 2002. If possible, an aliquot (0.1-ml minimum) of all samples reactive in the in-house WNV-specific IgM screening ELISA was frozen at −70°C for use in later experiments.
The in-house WNV-specific IgM screening ELISA was similar to IgM capture assays described elsewhere for other infectious organisms (1, 2, 6, 7). Microtiter wells (Polysorb; Nunc, Copenhagen, Denmark; 0.1 ml per well) were coated with rabbit anti-human IgM (Jackson ImmunoResearch, West Grove, Pa.) in carbonate buffer and then blocked with phosphate-buffered saline containing bovine serum albumin (Sigma, St. Louis, Mo.) and sucrose (Sigma). After a 5-min soak in wash buffer (phosphate-buffered saline containing 0.1% Tween 20), wells received 0.1 ml of serum diluted 1:400 or CSF diluted 1:10 in sample buffer (wash buffer containing 0.1% bovine serum albumin). Each assay setup included positive control, negative control, and calibrator sera diluted 1:400 in sample buffer. After 1.5 h at room temperature (RT) and thorough washing, wells received 0.1 ml of WNV antigen (supernatant from Vero cells infected with WNV by a proprietary procedure). Following overnight incubation at 4°C and washing, wells received 0.1 ml of diluted flavivirus monoclonal antibody 6B6-C1 (CDC). After an hour at RT and washing, wells received 0.1 ml of diluted horseradish peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch). After 30 min at RT and washing, wells received 0.1 ml of tetramethylbenzidine (Moss Inc., Pasadena, Md.). Stop buffer (1 N sulfuric acid; Ricca, Arlington, Tex.) was added 10 min later, and absorbance at 450 nm was measured. Index values were determined by dividing the absorbance value for a given control or unknown sample by the absorbance value of the calibrator serum. Index values of ≥0.90 were considered reactive. In validation studies utilizing known positive specimens from the 1999 New York outbreak, the assay demonstrated 100% sensitivity.
For the in-house modified WNV-specific IgM ELISA, the screening assay was modified to include a second, duplicate well of all control, calibrator, and unknown samples. Following sample incubation and washing as described above, WNV antigen was added to the first well, whereas uninfected Vero cell supernatant was added to the second well. The assay was then finished as described for the screening assay. For each specimen (including controls and calibrator), the absorbance value of the well receiving uninfected supernatant was subtracted from the absorbance value of the well receiving WNV antigen. The index value was then calculated with corrected absorbance values; index values of ≥1.0 were considered reactive. This modified assay was also 100% sensitive when evaluated with known positive specimens from the 1999 New York outbreak.
Samples reactive in the in-house WNV-specific IgM screening ELISA were forwarded to either the CDC (Fort Collins, Colo.) or the appropriate SPHL and tested in the CDC screening ELISA for WNV-specific IgM (4, 5). Samples reactive in the CDC screening ELISA were further tested in confirmatory plaque reduction neutralization or hemagglutination inhibition assays in order to determine the flavivirus responsible for infection (4).
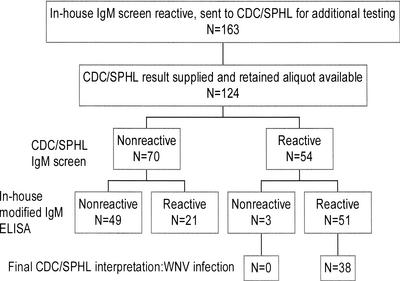
During the 2001 U.S. WNV season, 163 specimens (154 of serum and 9 of CSF) were reactive in the in-house WNV-specific IgM screening ELISA and were forwarded to CDC/SPHL for evaluation. CDC/SPHL supplied results for 138 specimens, and a frozen aliquot was available for 124 of these specimens (118 of serum and 6 of CSF). A summary of the findings for these 124 specimens is presented in Fig. 1; 70 (56%) were nonreactive in the CDC/SPHL WNV-specific IgM screening assay, and 54 (44%) were reactive. These 124 specimens were then tested in the in-house modified WNV-specific IgM ELISA, designed to detect nonspecific reactivity; 49 of 70 (70%) CDC/SPHL nonreactive samples were nonreactive in the in-house modified assay, compared to only 3 of 54 (6%) CDC/SPHL reactive samples (P < 0.001, chi-square test). All 38 specimens interpreted as representing WNV infection by CDC/SPHL were among the specimens that were reactive in the in-house modified WNV-specific IgM ELISA. Thus, had the in-house modified WNV-specific IgM ELISA been in place for the 2001 WNV season, 52 of these 124 specimens (42%) would have been excluded from evaluation by CDC/SPHL, without missing any of the patients deemed to have WNV infection.
FIG. 1.
Summary of tests performed and results obtained for specimens reactive in the in-house WNV-specific IgM screening ELISA during the 2001 WNV season.
The relationship between index values in the in-house screening ELISA and the in-house modified ELISA results is shown in Table 1. Most samples that were reactive in the in-house screening ELISA but nonreactive in the modified in-house ELISA showed index values of ≤2.00 in the screening ELISA (35/49 = 71%). We did not investigate the nature of the nonspecific reactivity identified by the modified ELISA. However, one possible explanation is the presence of human IgM recognizing mouse IgG, which nonspecifically captures the flavivirus monoclonal antibody. Also unclear is the reason(s) why some samples reactive in the in-house modified ELISA were nonreactive in the CDC/SPHL screening assay. This discordance may reflect subtle differences in WNV antigen preparations or other reagents or differences in the criteria for defining reactive versus nonreactive samples. In the present scientific and political climate surrounding WNV infection, however, submission of a small number of samples deemed by CDC/SPHL to be false positives in our in-house ELISA system is preferable to missing true positives.
TABLE 1.
Relationship between in-house screening WNV-specific IgM ELISA index values and in-house modified WNV-specific IgM ELISA index values for samples with known results in the CDC/SPHL screening WNV-specific IgM ELISA
| Group | In-house screening IgM ELISA
|
No. with in-house modified IgM ELISA index:
|
||||
|---|---|---|---|---|---|---|
| Index | n | <1.00 | 1.00-2.00 | 2.01-3.00 | >3.00 | |
| Nonreactive in CDC/SPHL screening assay | 0.90-1.10 | 6 | 6 | |||
| 1.11-2.00 | 38 | 29 | 7 | 2 | ||
| 2.01-3.00 | 15 | 9 | 3 | 3 | ||
| >3.00 | 11 | 5 | 2 | 1 | 3 | |
| Total for nonreactive samples | 70 | 49 | 12 | 6 | 3 | |
| Reactive in CDC/SPHL screening assay | 0.90-1.10 | 1 | 1 | |||
| 1.11-2.00 | 11 | 2 | 6 | 3 | ||
| 2.01-3.00 | 7 | 2 | 3 | 2 | ||
| >3.00 | 35 | 3 | 32 | |||
| Total for reactive samples | 54 | 3 | 8 | 9 | 34 | |
In the CDC/SPHL reactive group (Table 1), there was a clear trend toward strong reactivity (index of >2.0) in the in-house screening ELISA and strong reactivity (index of >2.0) in the in-house modified ELISA; 40 of 54 (74%) specimens fit these criteria. As mentioned earlier, 3 of 54 samples were nonreactive in the in-house modified ELISA but reactive in the CDC/SPHL screening assay; the reason for this discordance remains unclear.
Table 2 presents the relationship between index values in the in-house modified WNV-specific IgM ELISA and final interpretation for the 54 CDC/SPHL reactive specimens. The three specimens that were reactive in the CDC/SPHL screening assay, but nonreactive in the in-house modified ELISA, were nonreactive in confirmatory assays for flavivirus infections; thus, the in-house modified ELISA result correctly reflected the clinical status (lack of WNV infection) of these patients. Of the 51 specimens reactive in both the CDC/SPHL screening assay and the in-house modified ELISA, plaque reduction neutralization or hemagglutination inhibition tests indicated that 4 represented dengue virus infection, 1 represented St. Louis encephalitis (SLE) virus infection, and 38 represented WNV infection. No additional reactivity could be identified for 8 of the 51 specimens that were reactive in both the CDC/SPHL screening assay and the in-house modified ELISA. As noted earlier, all 38 specimens representing WNV infection were reactive in the in-house modified WNV-specific IgM ELISA, with 36 of 38 (95%) exhibiting a modified ELISA index value of >2.00. The patients with dengue virus infection or SLE virus infections resided in geographic areas where such infections are not unexpected.
TABLE 2.
Relationship between in-house modified WNV-specific IgM ELISA index values and CDC/SPHL interpretations for 54 samples reactive in the CDC/SPHL screening WNV-specific IgM ELISA
| In-house modified WNV-specific IgM ELISA | No. of samples with final interpretation based on additional CDC/SPHL testing
|
||||
|---|---|---|---|---|---|
| Index | n | No additional reactivity identified | Dengue virus infection | SLE virus infection | WNV infection |
| <1.00 | 3 | 3 | |||
| 1.00-2.00 | 8 | 3 | 2 | 1 | 2 |
| 2.01-3.00 | 9 | 2 | 2 | 5 | |
| >3.00 | 34 | 3 | 31 | ||
| Total | 54 | 11 | 4 | 1 | 38 |
From a cost perspective, performing the in-house WNV-specific IgM screening ELISA, with reflex of reactive specimens to the in-house modified ELISA, is superior to using only the modified ELISA to test all specimens. The 163 samples showing reactivity in the in-house WNV-specific IgM screening ELISA represented <5% of all specimens tested during the indicated time period. Thus, for >95% of specimens, generation of accurate results did not require the additional reagents and labor needed to perform the modified ELISA. This reflexive testing system, wherein sera reactive in the in-house modified WNV-specific IgM ELISA are forwarded to CDC/SPHL, is now in place for the 2002 WNV season.
Acknowledgments
We thank Robert Lanciotti of CDC, Fort Collins, Colo., for valuable discussions and assistance with CDC/SPHL results. Susan Vogeli and Jane Filamor provided expert technical assistance.
REFERENCES
- 1.Anderson, L. J. 1990. Human parvoviruses. J. Infect. Dis. 161:603-608. [DOI] [PubMed] [Google Scholar]
- 2.Helfand, R. F., S. Kebede, H. E. Gary, Jr., H. Beyene, and W. J. Bellini. 1999. Timing of and development of measles-specific immunoglobulin M and G after primary measles vaccination. Clin. Diagn. Lab. Immunol. 6:178-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marfin, A. A., and D. J. Gubler. 2001. West Nile encephalitis: an emerging disease in the United States. Clin. Infect. Dis. 33:1713-1719. [DOI] [PubMed] [Google Scholar]
- 4.Martin, D. A., B. J. Biggerstaff, B. Allen, A. J. Johnson, R. S. Lanciotti, and J. T. Roehrig. 2002. Use of immunoglobulin M cross-reactions in differential diagnosis of human flaviviral infections in the United States. Clin. Diagn. Lab. Immunol. 9:544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin, D. A., D. A. Muth, T. Brown, A. J. Johnson, N. Karabatsos, and J. T. Roehrig. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J. Clin. Microbiol. 38:1823-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monath, T. P., R. R. Nystrom, R. E. Bailey, C. H. Calisher, and D. J. Muth. 1984. Immunoglobulin M antibody capture enzyme-linked immunosorbent assay for diagnosis of St. Louis encephalitis. J. Clin. Microbiol. 20:784-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tardei, G., S. Ruta, V. Chitu, C. Rossi, T. F. Tsai, and C. Cernescu. 2000. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile Virus infection. J. Clin. Microbiol. 38:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]