Abstract
Echinococcosis is a cosmopolitan zoonosis caused by adult or larval stages of cestodes belonging to the genus Echinococcus (family Taeniidae). The two major species of medical and public health importance are Echinococcus granulosus and E. multilocularis, which cause cystic echinococcosis (CE) and alveolar echinococcosis (AE), respectively. Both CE and AE are both serious diseases, the latter especially so, with a high fatality rate and poor prognosis if managed inappropriately. This review discusses new concepts and approaches in the immunology and diagnosis of CE, but comparative reference has also been made to AE infection and to earlier pivotal studies of both diseases. The review considers immunity to infection in the intermediate and definitive hosts, innate resistance, evasion of the immune system, and vaccination of intermediate and definitive hosts, and it particularly emphasizes procedures for diagnosis of CE and AE, including the value of immunodiagnostic approaches. There is also discussion of the new advances in recombinant and related DNA technologies, especially application of PCR, that are providing powerful tools in the fields of vaccinology and molecular diagnosis of echinococcosis.
INTRODUCTION
Echinococcosis is a cosmopolitan zoonosis caused by adult or larval stages of cestodes belonging to the genus Echinococcus (family Taeniidae). Larval infection (hydatid disease, hydatidosis) is characterized by long-term growth of metacestode (hydatid) cysts in the intermediate host. The two major species of medical and public health importance are Echinococcus granulosus and E. multilocularis, which cause cystic echinococcosis (CE) and alveolar echinococcosis (AE), respectively. This review emphasizes recent advances in the immunology and diagnosis of CE, but comparative reference is also made to AE infection and to earlier important findings concerning both diseases. The biology, life cycle characteristics, and etiology of Echinococcus have been described comprehensively in a recent review (245), and so only a brief overview is presented here.
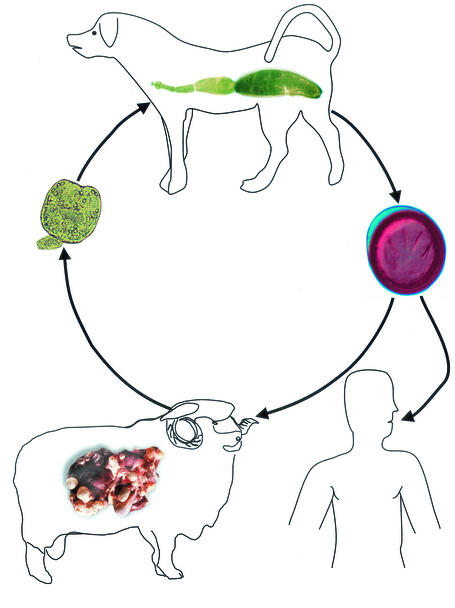
Hydatid cysts of E. granulosus develop in internal organs (mainly the liver and lungs) of humans and intermediate hosts (herbivores such as sheep, horses, cattle, pigs, goats and camels) as unilocular fluid-filled bladders (Fig. 1 to 3). These consist of two parasite-derived layers, an inner nucleated germinal layer and an outer acellular laminated layer surrounded by a host-produced fibrous capsule. Brood capsules and protoscoleces (PSC) bud off from the germinal membrane. Definitive hosts are carnivores such as dogs, wolves, and foxes (Fig. 1). Sexual maturity of adult E. granulosus occurs in the host small intestine within 4 to 5 weeks after the host ingests offal containing viable PSC. Gravid proglottids or released eggs are shed in the feces, and following their ingestion by a human or ungulate host, an oncosphere larva is released that penetrates the intestinal epithelium and enters the lamina propria. The larva is then transported passively through the blood or lymph to the target organs, where it develops into a hydatid cyst. Since the life cycle relies on carnivores eating infected herbivores, humans are usually a dead end for the parasite. Adult worm infections by E. multilocularis occur mainly in red and arctic foxes, although dogs and cats can also act as definitive hosts. Small mammals (usually microtine and arvicolid rodents) act as intermediate hosts. The metacestode of E. multilocularis (Fig. 2) is a tumor-like multivesicular, infiltrating structure consisting of numerous small vesicles embedded in stroma of connective tissue; the larval mass usually contains a semisolid matrix rather than fluid (245). CE and AE are both serious diseases, the latter especially so, with a high fatality rate and poor prognosis in the absence of careful clinical management.
FIG. 1.
Life cycle of E. granulosus. Echinococcus spp. require two mammalian hosts for completion of their life cycle. Segments containing eggs (gravid proglotitids) or free eggs are passed in the feces of the definitive host, a carnivore. The eggs are ingested by an intermediate host, in which the metacestode stage and protoscoleces develop. The cycle is completed if the metacestode and protoscoleces are eaten by a suitable carnivore.
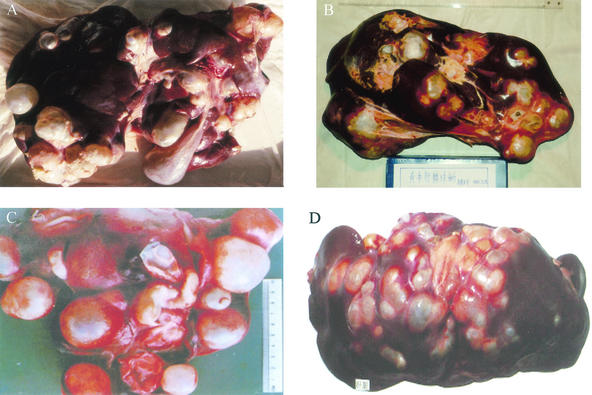
FIG. 3.
Cystic hydatid disease in the livers of various intermediate hosts. (A) sheep; (B) cow; (C) camel; (D) yak.
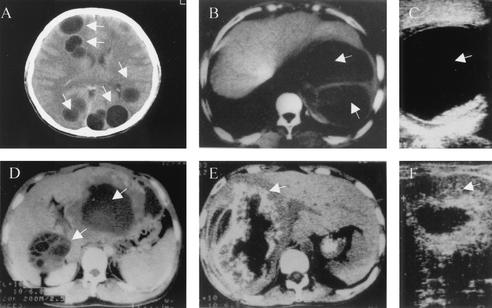
FIG. 2.
Clinical images of CE and AE. (A) CT scan of a brain with CE. (B) CT scan of a liver with CE. (C) Sonogram of a liver with CE. (D) CT scan of a liver with both AE and CE (note the presence of daughter cysts). (E) CT scan of a liver with AE. (F) Sonogram image of a liver with AE. Cysts (CE) or cyst masses (AE) are arrowed.
IMMUNITY IN THE INTERMEDIATE HOST
The immunology of hydatid disease has been divided conceptually into preencystment and postencystment phases (210), which are differentiated by the formation of the laminated layer around the hydatid cyst. This occurs between 2 and 4 weeks postinfection in the animal intermediate or human host following ingestion of the egg and release of the oncosphere.
Innate Resistance and Early Immunity
Primary infection.
Very little is known about the factors affecting innate susceptibility to infection with E. granulosus following ingestion of the infective egg stage and establishment of the primary cyst. Host age, sex, and physiological state may influence the innate susceptibility or resistance to infection (210). Furthermore, experimental infections of mice with eggs or oncospheres of E. granulosus showed that susceptibility varies with different strains of mice (56). It is noteworthy that although cattle are naturally susceptible to infection with E. granulosus, the resultant cysts are invariably infertile and do not produce brood capsules or PSC (246). In contrast, sheep cysts are generally fully fertile, with brood capsules asexually budding from the germinal layer and PSC developing from the inner wall of the brood capsules. It has been suggested that this difference may be due to parasite strain variation, but the same situation applies in cattle and sheep from the same area of endemic infection (41, 146, 273). This implies that cattle may have some natural immunity that inhibits the development and growth of PSC. In contrast, sheep appear to be highly susceptible to infection. Experimental infection of sheep with eggs showed that a high percentage (32 to 48%) of oncospheres survived and developed, suggesting that naive sheep may have only a limited resistance to primary infection (266).
After infection, the earliest detectable immunoglobin G (IgG) response to hydatid cyst fluid (HCF) antigens occurs after 2 to 11 weeks in mice and sheep, respectively (247, 266), and after 4 weeks in vervet monkeys (227). Early infections may be associated with a significant cellular inflammatory response (172, 210) that may cause pathologic changes (12, 80) since there is an increased leukocytosis, mainly of eosinophils, lymphocytes, and macrophages (195). With oncospheres, necrosis of surrounding cells is followed by infiltration of neutrophils and macrophages 3 to 5 days after infection in sheep (195). Experiments in vitro have shown also that neutrophils, in association with antibody, can bring about the killing of E. granulosus oncospheres (226), suggesting a possible role for antibody-dependent cell-mediated cytotoxicity reactions. At the early stages of disease, there is a marked activation of cell-mediated immunity to the parasite (81).
Secondary infection.
In experimentally induced secondary infections in mice, intraperitoneally injected PSC are surrounded by a considerable cellular infiltration within 3 days, initially involving activated macrophages and subsequently including neutrophils, eosinophils, and lymphocytes (208, 221, 222). Interleukin-10 (IL-10), IL-4, and IL-5 secreted in vitro by splenocytes can be detected as early as week 1 postinfection (55). High levels of tumor necrosis factor alpha (TNF), gamma interferon (IFN), IL-6, and specific IgG1 were detectable in serum, and IgG3 was measurable in the peritoneal cavity using protoscolex somatic antigens (55, 111). These data suggest that polarized Th2 reactions are evoked at the very beginning of the immune response to secondary infection. E. granulosus PSC contain immunogenic T-independent antigens (29). Primary antibody responses to protoscolex somatic antigens and the production of IgM and IgG3 in early infection appear to be stimulated mainly by a T-independent mechanism (29).
Similar to E. granulosus, differences in susceptibility to E. multilocularis have been shown in both primary and secondary infection of different mouse strains (26, 53, 74, 75, 106, 184). Susceptibility and resistance are based on the activation of different CD4+ T-cell immune responses (26, 53, 198). Experiments with mice infected with eggs showed that IFN-gamma-, IL-2-, and IL-4-expressing cells in the parasitic lesions were not detectable at the early phase of the infection but were present at the end (26). Similarly, low levels of cytokines in the sera were measurable at the beginning of the infection and high levels were detectable subsequently (26). IL-10 was the most prominent cytokine measurable throughout the course of the infection. Correspondingly, only small amounts of IgM, IgG1, IgG2a, and IgG3 could be detected early on, and higher levels were detectable later (190). A strong, specific intestinal immune response was found in the early stage (190). Both subsets of CD4+ T cells (Th1 and Th2) are involved in primary murine alveolar echinococcosis (26, 74).
In secondary AE in mice, very low levels of Th2 cytokines and IgG1, IgG2a, IgG3, and IgM are produced at the early infection stage, but these levels subsequently increase significantly (73). The degree of antibody response by the murine host does not correlate with susceptibility to E. multilocularis (198).
Established Cysts
Compared with events occurring during early infection, the immune response to established cysts has received much more attention. In humans there is frequent occurrence of elevated antibody levels, particularly of the IgG, IgM, and IgE isotypes (46, 52, 64, 113, 114, 197, 206, 243). In seropositive individuals, there tends to be a predominance of IgG1 and IgG4 antibody recognition of antigen 5 (Ag5) and antigen B (AgB) (see below), respectively (2, 52, 129, 233, 243, 258). This is of some value with respect to immunodiagnosis and is discussed below.
Also involved in the establishment phase is cellular infiltration, which includes eosinophils, neutrophils, macrophages, and fibrocytes (8, 9, 17, 18, 208, 219, 222, 240). However, this generally does not result in a severe inflammatory response and aged cysts tend to become surrounded by a fibrous layer that separates the laminated layer from host tissue. Eosinophilia and the production of high levels of IgE are the common consequences of infection by helminths (30, 37). It has been suggested that the eosinophil has evolved especially as a defense against the tissue stages of parasites that are too large to be phagocytosed (112) and that the IgE-dependent mast cell reaction has evolved primarily to localize eosinophils near the parasite and then enhance their antiparasitic functions (30). Eosinophils are less phagocytic than neutrophils, but, like neutrophils, they can kill larval stages of parasites (204) such as Echinococcus (179) by both dependent and independent mechanisms; their activities are also enhanced by cytokines (36).
Like other helminth infections (12, 80, 159, 180, 193), echinococcosis induces two very distinct Th1 and Th2 cytokine secretion patterns. Th1 cells produce IL-2, IFN-γ, and lymphotoxin, whereas Th2 cells express IL-4, IL-5, IL-6, IL-10, and an induced gene (p600) of unknown function. In tissue culture, the Th1- and Th2-cell patterns are well defined and stable. They are generally cross-inhibitory. IFN-γ inhibits Th2-cell proliferation, whereas IL-10 inhibits the synthesis of Th1 cytokines. In hydatid infections, both cell population profiles remain highly expressing, at least in cysts that survive the immune response. Elevated levels of IL-4, IL-5, IL-6, and IFN-γ are produced in vitro by peripheral blood mononuclear cells (PBMC) isolated from infected human subjects and stimulated by HCF antigens (213-219). Elevated cytokine levels were also measurable in the sera from hydatid disease patients with lung and liver involvement (249). The coexpression of IL-10 and IFN-γ at high levels in human hydatidosis suggests that the immune response to E. granulosus infection is possibly regulated by both Th1 (or Th0) and Th2 profiles. It is not understood why hydatid infection can induce high levels of both Th1 and Th2 cytokines since they usually down-regulate each other (193). It may be due to the very complex mixture of antigens in HCF (177), which probably contain distinct epitopes for each T-cell subset. However, the situation with human subjects is difficult to explain since the involvement of Th0 cells in a late chronic infection is rare (1).
In human subjects undergoing chemotherapy treatment, a Th1 cytokine profile, rather than a Th2 profile, typically dominates (215). It has been suggested that this could be one of the killing mechanisms that set in during the later stages of infection (225). Significantly, increased production of IL-4 and IL-10 in hydatid disease patients corresponds to high levels of IgE and IgG4 (215). Therefore, both IL-4 and IFN-γ regulate the IgE and IgG4 responses (153, 154, 180). AE patients experiencing a relapse of the disease have a tendency to increased production of IL-5 but lower IFN-γ production accompanied by significantly higher levels of IgE and IgG4 compared to patients with a primary infection (94).
In addition to IL-4 and IL-10 production, two other Th2 cytokines, IL-5 and IL-6, are produced in large quantities by hydatid disease patients. IL-5 was shown to be specifically induced by parasite antigens in 90% of patients while control subjects were negative (219). Other studies have shown that IL-5 is associated with the regulation of specific IgE and IgG4 expression (219). In general, IL-5 also regulates the eosinophilic response (45, 125). However, some patients infected with E. granulosus (11%) (218) and most patients infected with E. multilocularis have limited eosinophilia (219, 244).
Sera from patients with active cysts have a range (2 to 500 U/ml) of concentrations of IL-6. The major role of this cytokine is to induce differentiation of B cells into plasma cells, thus contributing to the development of antigen-specific humoral responses (254).
CE patients with relapsing disease have high levels of IgE and IgG4, increased levels of IL-5, IL4, and IL-10, and low levels of IFN-γ produced in vitro by PBMC compared to patients with a primary infection (215, 219). Patients with a primary infection have higher levels of IL-2, IFN-γ, and IL-5. The high level of IL-5 is in agreement with the high levels of IgG4 and IgE observed (219). IFN-γ and IL-6 activities were undetectable in sera from two liver hydatidosis patients who relapsed (250). There is a significant correlation between IgE and IgG4 production in sera from patients with hydatid disease and a trend toward increased IL-4 and IL-10 levels in patients who are high producers of IgE and IgG4 (214).
When specifically stimulated with HCF antigen, PBMC from hydatid disease patients produced higher levels of IL-2 did than those from uninfected donors (125). The apparent bias toward a Th2 response appeared to be related to clinical status and was suggestive of a putative role of Th2-like responses in susceptibility to reinfection by E. granulosus (125). Clearly, these results merit further study.
Primary and secondary infections elicit similar responses, which include elevated levels of TNF-α, IL-1α, IFN-γ, IL-6, and IL-10 (111) and detectable levels of specific IgG1 and IgG3 isotypes (55). The levels of IgM and IgG2a are slightly increased following infection and remain elevated throughout the first 18 weeks of infection. During the 129- to 209-day period following the onset of infection, there is an increase in the level of secreted IL-10 and a slow decrease in the levels of IL-6 and IFN-γ. IgM, IgG, IgG1, and IgG2a levels plateau during this period, whereas IgG3 and TNF-α levels peak on day 190 postinoculation. These data suggest that induction of Th2 antibody-mediated immunity with a parallel expansion of Th1-mediated inflammatory responses is an important mechanism of host defense against the metacestode (111). Local inflammatory reactions to PSC at the site of injection are intense, involving neutrophils, eosinophils, macrophages, and mast cells (221).
Significantly higher levels of IL-10 (257) and IL-5 (222, 244) have been found in AE patients than in controls. In contrast, IL-4 was measurable in only a minority of patients and controls. IL-12 levels were comparable between AE patients and controls and showed a similar distribution pattern to IL-10 with regard to disease progression. These studies suggest that a Th2-dominated immune response occurs in AE in vivo. AE infection results in a strong Mac-1+ cell infiltration of the peritoneal cavity and spleen (53). Peritoneal cells from mice infected with AE at the 1-month stage were rich in macrophages and expressed significantly higher levels of transcripts for the inflammatory cytokine IL-1β and for TNF-α and inducible nitric oxide synthase (257).
Inhibition of Cyst Growth
It is generally accepted that Echinoccocus is unaffected by the immune response during the developing stage. However, natural infections in sheep indicate that some cysts can be killed during the latter stages of development (275), with the relatively frequent occurrence of dead, calcified metacestodes or necrotic cysts. These are due to the primary cyst having degenerated, leaving the cavity full of host leukocytes and protoscolex-derived daughter cysts (224). There is no direct evidence that the death of such cysts is due to an immunological phenomenon, but it is a likely possibility. If a progression in cyst degeneration does take place, then the immune response may play a role in the death of the parasite. This may signify increased immunological stimulation with cyst progression. Unfortunately, there are no detailed studies of immunological events associated with the degeneration of different types of cyst, and it is therefore unknown which mechanisms may be involved. This is clearly an area for future study. One aspect that is likely to be important is the influence of CD4+ Th lymphocytes on the control of such immunological mechanisms. In addition, IFN-γ and nitric oxide production may play a role (249).
Complement through C5-mediated effectors contributes to host defenses by both restricting the establishment of infection and controlling the growth of established cysts. This contribution may be associated with the ability of C5a to promote eosinophil infiltration (65, 266). Lysis in both immune and normal serum is antibody dependent and complement mediated (116). Protoscoleces of E. multilocularis and E. granulosus are lysed by fresh serum of many different species of mammals (121, 152). The presence of Echinococcus cysts appears to deplete host complement (121, 150, 151). The rapid development of E. multilocularis infection is associated with depletion of serum complement; the use of cobra venom factor to deplete complement results in faster growth of E. multilocularis cyst masses.
In hydatid infections, IL-6 seems to be produced nonspecifically (254) whereas IL-5 production appears antigen specific. The effect of IL-5 on human B cells is controversial (45), but a significant correlation between IL-5 production and IgE and IgG4 expression has been found in hydatid disease patients (219). When CE cysts grow, IgG1 and IgG4 levels are elevated, whereas the concentrations of specific IgG1 and IgG4 decline in cases characterized by cyst infiltration or calcification. This indicates that the IgG4 antibody response is also associated with cystic development and growth and with disease progression whereas the IgG1, IgG2 and IgG3 responses occur predominantly when cysts became infiltrated or are destroyed by the host (52).
In experimental infection, fewer than 10% of PSC survive to form cysts (194, 272). The majority of parasite killing occurs within the first 2 weeks postinfection. Activated macrophages are involved in the killing of Echinococcus PSC (8, 9, 24, 142). Studies in vitro indicate that macrophage-dependent killing of PSC can be increased by IFN-γ (148) and decreased by some cytokines such as IL-10 or IL-4 (142). Therefore, it seems likely that during a secondary infection, an initial Th0 or Th1 response effective in killing parasites becomes polarized to a Th2-type response and that this response seems less effective. This is supported by studies with patients undergoing albendazole chemotherapy who responded better to treatment when they possessed a more dominant Th1-type cytokine profile than when they had a more dominant Th2-type profile (125).
Evading the Immune System
The life span of hydatid cysts of E. granulosus can be as long as 53 years in humans (241) and 16 years in horses (228). The ability of the parasite to survive for such a long time in hosts with the potential to resist infection implies that the parasite possesses strategies for subverting or avoiding protective immune responses. Theoretically, there are two types of mechanisms to subvert the host immune response: passive escape, in which the parasite avoids the damaging effects of an immune response, and immunomodulation, which is an active interaction with the immune system to reduce the impact of a response to the parasite (211). Some of the proposed mechanisms of immune evasion used by the Echinococcus organisms (256) are summarized in Table 1.
TABLE 1.
Proposed mechanisms of avoidance from host-protective responses by the metacestode stage of Echinococcusa
| Mechanism | Reference(s) |
|---|---|
| Sequestration and antigenic disguise | 33, 54, 67, 68, 170, 174 |
| Molecular mimicry | 68, 83 |
| Antigen or DNA polymorphism | 35, 39, 43, 98, 230, 235, 268-270 |
| Production of proteases | 66, 79, 175, 248, 260 |
| Protease inhibitor | 235 |
| Interference with complement activity | 44, 79 |
| Alteration of macrophage and leukocyte funtion | 16, 198, 205, 223 |
| Alteration of lymphoid organ architecture | 3, 4, 6, 7, 9, 220-222 |
| Depletion of T lymphocytes | 34, 155-157, 198, 255 |
| Induction of suppressor cells | 67, 155-157 |
| Alteration of antibody responses | 5, 28 |
| Alteration of lymphocyte proliferative responses | 26, 28, 74, 157, 198 |
| Inhibition of effector cell chemotaxis | 198, 212 |
Modified from reference 256.
A notable feature of the metacestode of E. granulosus is the formation of two capsules. One is the cyst-derived acellular laminated capsule. The other is the host fibrous capsule, which typically surrounds fully developed viable cysts of E. granulosus and is formed probably by filtration of eosinophils (240) and of fibroblasts and mesothelial cells (208). These structures protect the parasite both physically and from immune attack.
The initial response of the host abates with time and is minimal 6 months after infection (81). IFN-γ-, IL-2-, and IL-4-expressing cells could not be detected in lesions of the early phase of infection (26), possibly indicating host immunosuppression. Furthermore, about 30% of CE patients have undetectable antibody levels in their sera (40, 48, 216), a feature which also appears to occur in ovine infections (145, 169, 265). The mechanisms behind this are unclear. Circulating parasite antigen could be mopping up specific antibody, since both circulating antigen and immune complexes are detectable in some seronegative individuals (46). The possibility of antigen-induced specific immunological tolerance has also been raised, suggesting that antibody production during the course of the infection may be regulated, perhaps through periodic release of antigen from cysts and/or general down-regulation of B cells through Th-cell activity.
When using susceptible C57BL/6 mice, spleen cells supplemented with peritioneal cells from E. multilocularis-infected mice induced a complete suppression of splenic proliferation at the early and late stages of infection, and this suppression was reversed to a large extent by the addition of NG-monomethyl-l-arginine and partially by anti-IFN-γ antobodies (53). Spleen cells from late-stage-infected mice express only background levels of IL-10 but greatly increased levels of inducible nitric oxide synthase. The immunosuppression observed in chronic AE is not primarily dependent on IL-10 but rather on nitric oxide production by macrophages from infected animals (53, 74).
Proliferative responses and IL-2 production induced by concanavalin A (ConA) in spleen cells from BALB/c mice are significantly depressed at an early stage after infection (157). With E. multilocularis PSC, addition of plastic-adherent cells from normal syngeneic mice to the nonadherent spleen cells from infected mice did not restore the depressed ConA responsiveness. On the other hand, exogenous IL-2 completely reconstituted the proliferative responses to ConA. Flow cytometry analysis revealed that the number of CD4− CD8+ cells with a low density of CD8 antigen (CD8dull cells) increased in spleens from infected mice 2 weeks after inoculation. Addition of the spleen cell subpopulation containing the CD8dull cells, but not that depleted of the CD8dull cells, to normal spleen cells resulted in marked suppression of the ConA responses. These findings suggest that the CD8dull cells detected in the spleens of mice inoculated with E. multilocularis PSC may play a key role in the suppressive regulation of immune responses (157).
Little is known about antigenic drift or shift in Echinococcus infections compared with some other parasites (50, 104, 105, 108-110). The production of proteases has been reported for a range of helminths and is considered important for the conversion of host tissues into nutrients (128), for host invasion (126), and for migration through host tissues (127). It is unknown whether proteases from Echinococcus function in the cleavage of IgG, as has been recorded in other helminths (19, 20, 158, 202, 203). Nevertheless, secretions from the penetration glands of hatched and activated oncospheres of E. granulosus cause lysis of host tissues; these secretions may protect the parasite against the host immune response while the laminated layer develops (126).
IMMUNITY IN THE DEFINITIVE HOST
Immune reactions of canid definitive hosts to Echinococcus infections have been comprehensively reviewed (113, 114). There is now quite an extensive literature, but until the 1980s, little research had been performed on immune responses to Echinococcus and other taeniid cestodes in their definitive hosts. This may be because the adult worms, being parasites of the gut lumen, were thought unlikely to evoke systemic immune responses and also because of the lack of knowledge of host-protective immune responses to reinfection with taeniid cestodes at the time (163). Mucosal immunity in animals is now recognized as an important phenomenon. In sheep, clearance of the parasitic nematodes Trichostrongylus colubriforms and Haemonchus contortus is associated with the sensitization of mucosal mast cells (MMC), measured by the release of sheep mast cell protease and the number of globular leukocytes, which are possibly degranulated by MMC (242). These cell types are more numerous in the regions of the gastrointestinal tract where these parasites reside (31).
An increase in the level of immune mediators, sheep mast cell protease larval migration inhibition components, and peptidyl leukotriene is correlated with the clearing of T. colubriforms infection (144) and a reduction in fecal egg counts (69). The secretion of leukotrienes and larval migration inhibition from MMC is thought to be a major mechanism of parasite removal (70). IgA and IgE are important in mucosal immunity since they bind directly to antigens and also attract effector cells that bind the constant region of the antibody. Eosinophils and MMC bind immunoglobulin constant regions via Fc receptors, becoming activated to degranulate when bound to the opsonized parasite. This method of antibody-dependent cell-mediated cytotoxicity is well established as an important mechanism by which the host can damage a multicellular parasite (211).
As reviewed by Heath (113, 114), the scolex of adult Echinococcus worms is normally in close contact with the canine intestinal submucosa, but mucosal immune responses, leading to the production of neutralizing IgA antibodies to deal with the secretions of the strobila, have no effect on the scolex. The scolex is in intimate contact with the systemic circulation, even in the Peyer's patches, and it appears to maintain its privileged integrity by suppression of cytotoxic and effector cell activity in the region of the scolex.
Experiments with immunosuppressed golden hamsters subsequently infected with E. multilocularis showed that worms developed faster than in normal animals (147). In addition, dogs that were immunosuppressed and then challenged with PSC of E. granulosus were shown to harbor more worms than did nontreated dogs, suggesting that the definitive host may have some innate resistance to infection by adult worms (D. Heath, personal communication).
Cells from Peyer's patches of dogs infected with E. granulosus produce specific immunoglobulin in vitro (61). Infection depresses the ability of unstimulated cells to proliferate in response to HCF protein but enhances the response to ConA (10). Dogs with enhanced reactivity to ConA and another mitogen, phytohemagglutinin, have significantly fewer worms and a lower number of mature worms than do dogs with less reactivity. After infection, the concentrations of IgG and IgA increased in serum and IgA levels increased in feces. Dogs with high-titer anti-HCF antigen serum antibodies were better protected than were dogs with low titers in serum (10).
VACCINES AND VACCINOLOGY
As indicated above, the life cycles of E. granulosus and E. multilocularis include two hosts: an intermediate host and a definitive host. Effective CE control programs show that prevention of transmission to either host can reduce or even eliminate the infection in human and livestock populations (see below). Therefore, if either or both hosts can be vaccinated, the effect will be to improve and more rapidly expedite control (116). The sylvatic nature of the life cycle of E. multilocularis makes a vaccination approach to control unlikely.
Vaccination of the Intermediate Host
Vaccination of the intermediate host is a burgeoning area that has moved forward considerably in recent years following the development of a recombinant vaccine against Taenia ovis infection in sheep (164). A similar approach has been applied successfully to develop a recombinant vaccine against E. granulosus (164-167). Earlier, a range of different antigens including cyst fluid (51, 62, 63, 118, 191), cyst membranes (191), and PSC (63, 124, 253) had been used as prototype vaccines against E. granulosus. However, oncospheres or oncospheral antigens induce much higher levels of protection in sheep (91, 117, 119, 189) and mice (56, 272) against challenge. One fraction (25 kDa) separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) from a crude preparation of oncospheral antigens was also shown to stimulate a similar level of resistance (117). Lightowlers et al. (167) used antibody prepared against this fraction to screen a cDNA library prepared from oncospheres; selected cDNA clones subsequently expressed in Escherichia coli as a fusion protein with glutathione S-transferase (GST) were tested for protective efficacy by vaccinating sheep and then challenging them with E. granulosus eggs (167). One 16.5-kDa recombinant protein (termed EG95)—50 μg of the GST fusion protein formulated in oil adjuvant or made up with Saponin, Quil A or ISA70 adjuvant—elicited significant protection (mean, 96 to 98%) against the development of hydatid cysts (164, 167).
The immunity (mediated by complement-fixing antibodies) generated by two injections of the vaccine given 1 month apart persists for at least 12 months (115). Thereafter, annual vaccination of domestic livestock is recommended (115, 167). The shelf life of the formulated vaccine is at least 12 months (115). One liter of Escherichia coli culture can yield more than 10,000 vaccine doses, which means that the vaccine could be manufactured cheaply if produced on a large scale. Other noteworthy features of the EG95 vaccine are that immunity can be transferred passively to neonates with antibody from vaccinated dams (115) and that the protection induced is associated with conformational epitopes (261-264). Furthermore, the vaccine conferred a high degree of protection against challenge with different geographical isolates of E. granulosus (166), indicating that it could have wide applicability as a new tool for use in hydatid disease control campaigns. The vaccine therefore provides a valuable new tool to aid in the control of transmission of this important human pathogen and also has the potential to prevent hydatid disease directly through vaccination of humans. Recent research indicates that the EG95-encoding gene belongs to a gene family of six or more genes (43). A closely related protein (designated EM95) that can induce significant levels of protection against challenge infection with E. multilocularis eggs in mice has also been identified in E. multilocularis (90).
Vaccination of the Definitive Host
Compared with the major advances in vaccinating sheep against E. granulosus, attempts to vaccinate canid definitive hosts have yet to achieve a similar level of success. Nevertheless, a series of experiments to induce immunity in dogs through vaccination have been carried out, with some encouraging results. Irradiated E. granulosus PSC used in vaccine trials showed that the preparations could induce inhibition of the growth of worms in the canine intestine (14, 181, 182). Protoscoleces or antigens from PSC can stimulate dogs to reduce the numbers or suppress the growth of worms (92, 251, 252, 274). Protection was also obtained with other antigen sources, such as cyst fluid (14), cyst membranes (93, 251), adult worm extracts (92, 251), and worm secretions (122, 123). There is some evidence for the development of acquired immunity to E. multilocularis in foxes, although detailed knowledge is unavailable (71).
DIAGNOSIS
Early diagnosis of CE and AE can result in significant improvements in the quality of the management and treatment of both diseases. In most cases, the early stages of infection are asymptomatic, so that methods that are cheap and relatively easy to use are required for large-scale screening of populations at high risk. Immunodiagnosis provides such an approach and can also, confirm clinical findings.
The immunodiagnosis of echinococcosis has been comprehensively reviewed in a series of early articles by Schantz and Gottstein (232), Rickard and Lightowlers (209), Lightowlers and Gottstein (165) and Gottstein (100). Here, more recent progress in the development and application of specific diagnosis of Echinococcus infection in humans, animal intermediate hosts and definitive hosts is assessed. Readers should also refer to excellent recent comprehensive reviews of the field (192). Over the past decade, diagnosis of CE and AE has improved due to the use of new or more optimal methods for purification of Echinococcus antigens from somatic materials, by the application of molecular tools for parasite identification and the synthesis of recombinant diagnostic antigens and immunogenic peptides. These approaches have not only improved the sensitivity and specificity of tests for diagnosis of AE and CE but also allowed more reliable characterization of the biological status of parasite materials (reviewed in references 237 and 245).
Immunodiagnosis of Cystic Echinococcosis in Humans
The definitive diagnosis for most human cases of CE is by physical imaging methods, such as radiology, ultrasonography, computed tomography (CT scanning) and magnetic resonance imaging (165), although such procedures are often not readily available in isolated communities. Immunodiagnosis can also play an important complementary role. It is useful not only for primary diagnosis but also for follow-up of patients after surgical or pharmacological treatment. Detection of circulating E. granulosus antigens in sera is less sensitive than antibody detection, which remains the method of choice.
Hydatid serological testing has a very long history, and almost all serological tests that have been developed have been used in the diagnosis of human cases. There are considerable differences among the various tests in both specificity and sensitivity. As the sensitivity of a test increases, so generally does the demand for improved antigens so that sufficient specificity can be achieved to take advantage of the greater sensitivity. An optimum test should be specific with high sensitivity. Insensitive and nonspecific tests, including the Cassoni intradermal test, the complement fixation test, the indirect haemagglutination test, and the latex agglutination test, have been replaced by the enzyme-linked immunosorbent assay (ELISA), the indirect immunofluorescence antibody test, immunoelectrophoresis (IEP), and immunoblotting (IB) in routine laboratory application (165).
Chordi and Kagan (42) were the first to analyze antibody responses in human hydatid infection by IEP with sera from patients and HCF of sheep origin as antigen. Further extensive studies have also focused on HCF antigens that are still considered an invaluable source of antigenic material for immunodiagnosis (Table 2). This research evaluated the immunoreactivity of these antigens with sera from patients with hydatid infection and resulted in the development of new techniques for the preparation of purified antigens (160, 183, 186, 196, 200, 201, 259). Antigen prepared from human HCF was found to be unsuitable for diagnosis because it contains host proteins such as IgG (27). Sheep HCF obtained from fertile cysts has been used routinely to prepare and standardize antigen. Bovine HCF can be used as an alternative antigen source; indeed, it can improve diagnostic sensitivity (130, 206). HCF of camel origin has also been used as antigen in an ELISA format to measure total E. granulosus-specific IgG antibodies and IgG subclasses (206). The diagnostic value of measuring IgG1 (97.7%), as assessed by a rating index (J) for combined sensitivity and specificity, was superior to the use of total IgG (65.1%) and IgG2 to IgG4 (77.8, 57.9, and 39.6%, respectively) (206). These findings have set the stage for field evaluation of the IgG1 assay in areas where human CE is endemic.
TABLE 2.
Features of assays for immunodiagnosis of CE based on HCF antigensa
| No. of subjects tested | Antigen source | Assay method | Sensitivity (%) | Specificity (%) | Ig isotype | Reference | ||
|---|---|---|---|---|---|---|---|---|
| CE patients | Healthy subjects | Patients with other diseases | ||||||
| 204 | 90 | 53 | HFF | IHA | 54 | 100 | Ig | 188 |
| 119 | 37 | 54 | HBLF | LA | 86 | 87.9 | Ig | 23 |
| 204 | 90 | 53 | HFF | IEP | 31 | 100 | Ig | 188 |
| 70 | 30 | 73 | ppHCF | ELISA | 89 | 40.8 | IgG | 143 |
| 204 | 90 | 53 | HFF | ELISA | 72 | 97 | IgG | 188 |
| 90 | 28 | 88 | FBHCF | ELISA | 84 | 60 | IgG | 22 |
| 111 (Li) | 0 | 0 | sWHF | ELISA | 89 | ND | IgG | 21 |
| 122 (Lu) | 0 | 0 | sWHF | ELISA | 78 | ND | IgG | 21 |
| 35 | 200 | 339 | HCF | ELISA | 91 | 82.3 | IgG | 199 |
| 52p | 200 | 339 | HCF | ELISA | 96 | 82.3 | IgG | 199 |
| 119 | 37 | 54 | FBHCF | ELISA | 83 | 86.8 | IgG | 23 |
| 204 | 90 | 53 | HFF | IB | 80 | 96 | IgE | 188 |
Abbreviations: HFF, hydatid fluid fraction, rich in Ag5 and AgB; HBLF, heparin-binding lipoprotein fraction; ppHCF, partially purified HCF; FBHCF, fertile bovine hydatid cyst fluid; Li, liver; Lu, lung; sWHF, sheep whole-hydatid-cyst fluid; IHA, indirect hemagglutination assay; LA, latex agglutination assay; ND, not done.
The lipoproteins antigen B (AgB) and antigen 5 (Ag5) (185), the major components of HCF, have received the most attention with regard to diagnosis. Along with HCF, they are the most widely used antigens in current assays for immunodiagnosis of CE. Both antigens have been well characterized by immunoblotting and/or by immunoprecipitation of radiolabeled antigen and SDS-PAGE (13, 168, 234, 236).
Antigen B.
AgB is a polymeric lipoprotein with a molecular mass of 120 kDa (185). It can be measured in patient blood as circulating antigen (149, 171), and it has been suggested that it plays an important role in the biology of the parasite and its relationship with the host (212, 235). AgB is a highly immunogenic molecule (42, 186), a characteristic that underpins its value in serodiagnosis (Table 3). It appears ladder-like under reduced condition on SDS-PAGE, with three bands with molecular sizes of approximately 8 or 12, 16, and 24 kDa (42, 162, 168, 186, 236), suggesting that it comprises polymers of 8-kDa subunits (95, 168). The smallest subunit has proved the most useful target in diagnostic studies (188, 229).
TABLE 3.
Features of assays for immunodiagnosis of CE using native AgBa
| No. of subjects tested | Antigen | Assay method | Sensitivity (%) | Specificity (%) | Ig isotype | Reference | ||
|---|---|---|---|---|---|---|---|---|
| CE patients | Healthy subjects | Patients with other diseases | ||||||
| 204 | 90 | 53 | Gel-EF | ELISA | 74 | 100 | IgG | 188 |
| 90 | 28 | 86 | pp | ELISA | 77 | 85 | IgG | 97 |
| 191 | 50 | 133 | pp | ELISA | 79 | 98 | IgG | 233 |
| 81 | 98 | pp | ELISA | 89 | 86 | IgG | 258 | |
| 31 | 29 | 87 | AEC | ELISA | 77 | 82 | IgG | 229 |
| 90 | 28 | 88 | MAb-AP | ELISA | 77 | 86 | IgG | 22 |
| 81 | 98 | pp | ELISA | 58 | 92 | IgG1 | 258 | |
| 191 | 50 | 133 | pp | ELISA | 57 | 100 | IgG1 | 233 |
| 81 | 98 | pp | ELISA | 53 | 94 | IgG2 | 258 | |
| 81 | 98 | pp | ELISA | 46 | 95 | IgG3 | 258 | |
| 210 | 47 | 79 | pp | ELISA | 63 | 81 | IgG4 | 178 |
| 191 | 50 | 133 | pp | ELISA | 38 | 99 | IgG4 | 233 |
| 81 | 98 | pp | ELISA | 73 | 91 | IgG4 | 258 | |
| 210 | 47 | 79 | pp | ELISA | 63 | 81 | IgG4 | 178 |
| 69 | 82 | 63 | 18 kDa | IB | 10 | 77 | IgG | 143 |
| 35 | 200 | 339 | 8 kDa | IB | 71 | 97 | IgG | 199 |
| 52p | 200 | 339 | 8 kDa | IB | 60 | 97 | IgG | 199 |
| 173 | 29 | 66 (AE) | 8/29/34 kDa | IB | 85 | 65 | IgG | 132 |
| 35 | 200 | 339 | 8/29/34 kDa | IB | 91 | 94 | IgG | 199 |
| 52p | 200 | 339 | 8/29/34 kDa | IB | 81 | 94 | IgG | 199 |
| 204 | 90 | 53 | Gel-EF | IB | 66 | 100 | IgE | 188 |
| 158 | 29 | 152 | pp | IB | 92 | 69 | IgG | 132 |
| 173 | 29 | 115 | pp | IB | 92 | 100 | IgG | 132 |
| 210 | 47 | 79 | pp | dELISA | 93 | 65 | IgG | 178 |
Abbreviations: Gel-EF, eluted fractions from SDS-PAGE; pp, partial purification; AEC, anion-exchange chromatography; MAb-AP, affinity purification by monoclonal antibody; p, posttreatment; dELISA, dot ELISA.
To date, several AgB cDNAs have been cloned, expressed as recombinant proteins, and used for diagnosis; in addition, a number of AgB peptides have been synthesized and used in ELISA for diagnostic purposes (Table 4). Peptide antigens have been considered for use to enhance specificity, and efforts have been made to define discrete epitopes of AgB and other molecules that could be mimicked by synthetic peptides. Shepherd et al. (235) cloned a C-terminal fragment from a PSC cDNA library and expressed this as a 12-kDa protein (235); the complete sequence, termed EgAgB8/1, was cloned subsequently (82). The sequence is highly conserved (82), a fact that underscores its utility for application in immunodiagnosis. Subsequently, another fragment named EgAgB8/2 was also proposed to be an 8-kDa subunit, with 38% similarity to the EgAgB8/1 clone (77).
TABLE 4.
Features of assays for immunodiagnosis of CE based on using recombinant AgB and AgB peptidesa
| No. of subjects tested | Antigen | Assay method | Sensitivity (%) | Specificity (%) | Ig isotype | Reference | ||
|---|---|---|---|---|---|---|---|---|
| CE patients | Healthy subjects | Patients with other diseases | ||||||
| 210 | 47 | 79 | rAgB.MBP | ELISA | 65 | 91 | IgG4 | 178 |
| 64 | 39 | 105 | EG55-GST | ELISA | 89 | 72 | IgG | 120 |
| 31 | 29 | 87 | rAgB8/1 | ELISA | 55 | 80 | IgG | 229 |
| 31 | 29 | 87 | rAgB8/2 | ELISA | 84 | 98 | IgG | 229 |
| 119 | 44 | 123 | rEgPS-3-GST | ELISA | 74 | 87 | IgG | 161 |
| 90 | 28 | 86 | P176 | ELISA | 80 | 93 | IgG | 97 |
| 90 | 28 | 86 | P175 | ELISA | 49 | 94 | IgG | 97 |
| 90 | 28 | 86 | P177 | ELISA | 38 | 92 | IgG | 97 |
| 90 | 28 | 86 | P65 | ELISA | 44 | 96 | IgG | 97 |
| 90 | 28 | 86 | Gu4 | ELISA | 18 | 98 | IgG | 97 |
| 31 | 29 | 87 | GU4 | ELISA | 26 | IgG | 229 | |
| 90 | 28 | 88 | p65# | ELISA | 34-48 | 80-97 | IgG | 22 |
| 90 | 28 | 88 | pGU4# | ELISA | 12-18 | 96-100 | IgG | 22 |
| 210 | 47 | 79 | rAgB.MBP | dELISA | 74 | 88 | IgG | 178 |
| 25 | 9 | 8 | p65 | dELISA | 64 | 100 | IgG | 161 |
| 204 | 90 | 53 | rAgB-GST | IB | 72 | 100 | IgG | 188 |
Abbreviations: dELISA, dot ELISA; rEgPS, recombinant PSC protein; rAgB, recombinant AgB; MBP, maltose binding protein; #, coated with different buffer.
Another recombinant clone, EG55, also corresponding to the smallest subunit of AgB, was expressed as a GST fusion molecule and tested in a sandwich ELISA for its ability to detect specific serum antibodies in CE patients (Table 4). The antigen cross-reacted mainly with sera from AE parients (39.2% subjects reacted) (120). A 12-kDa EgPS-3 recombinant antigen, also corresponding to the smallest subunit of AgB, was similarly tested in ELISA as a GST fusion protein for diagnosis of human CE; 74% of patients recognized the protein, but there was cross-reactivity with sera from AE- and Schistosoma japonicum-infected patients (161). The EgPS-3 recombinant antigen was explored further for diagnostic value with three synthetic peptides prepared based on its predicted amino acid sequence; p65, a 27-mer peptide corresponding to residues 12 to 39 of AgB8/1, showed increased specificity but slightly reduced sensitivity in ELISA (161) (Table 4).
In further efforts to standardize CE diagnosis, Barbieri et al. (22) compared the diagnostic value of p65 and GU4, a 34-mer synthetic peptide corresponding to the C-terminal end of the AgB8/2 subunit (Tables 3 and 4) with that of p89-122, a synthetic peptide derived from Ag5 (Table 5). The p65 peptide provided three- to fourfold higher sensitivity but 30% lower specificity than the other two peptides. A further study showed that a highly antigenic region of AgB resides in the N-terminal extension of the AgB8/1 subunit; an ELISA based on the use of a single peptide designated p176, a 38-mer peptide from the N -terminus of the AgB8/1 subunit, exhibited a diagnostic performance that was superior to that obtained by the use of native AgB (97). The results of this and other studies investigating the sensitivity and specificity of recombinant AgB and AgB peptides in CE diagnosis are summarized in Table 4.
TABLE 5.
Features of assays for immunodiagnosis of CE based on using native, recombinant and synthetic peptides of Ag5a
| No. of subjects tested | Antigen | Assay method | Sensitivity (%) | Specificity (%) | Ig isotype | Reference | ||
|---|---|---|---|---|---|---|---|---|
| CE patients | Healthy subjects | Patients with other disease | ||||||
| 35 | 200 | 289 | Arc5 | IEP | 63 | 97.2 | Ig | 199 |
| 52p | 200 | 289 | Arc5 | IEP | 58 | 97.2 | Ig | 199 |
| 90 | 28 | 88 | MAb-AP | ELISA | 50 | 92 | IgG | 22 |
| 39 | 29 | 51 | MAb-AP | ELISA | 54 | 89 | IgG | 96 |
| 90 | 28 | 88 | P89-122# | ELISA | 14-21 | 77-100 | IgG | 22 |
| 39 | 29 | 51 | rP-29 | ELISA | 61 | 80 | IgG | 96 |
| 39 | 29 | 51 | P89-122# | ELISA | 44 | 100 | IgG | 96 |
| 111 (Li) | ND | ND | pAg 5 | ELISA | 89 | ND | IgG | 21 |
| 122 (Lu) | ND | ND | pAg 5 | ELISA | 78 | ND | IgG | 21 |
Abbreviations: Li, liver; Lu, lung; MAb-AP, affinity purified using monoclonal antibody; #, coated with different buffer; pAg5, purified Ag5; ND, not determined; p, posttreatment.
Antigen 5.
Ag5 is a very-high-molecular-mass (approximately 400-kDa) lipoprotein complex composed of 57- and 67-kDa components that under reducing conditions dissociate into 38- and 22- to 24-kDa subunits (168). Historically, one of the most widely used immunodiagnostic procedures for CE was the demonstration of serum antibodies precipitating antigen 5 (arc 5) by immunoelectrophoresis or similar techniques. Early work suggested absolute diagnostic specificity for detecting E. granulosus infection, but subsequent studies showed that antigen 5 is cross-reactive with human antibodies to other taeniid cestodes, most notably E. multilocularis and Taemia solium, and, indeed, other helminths (236).
Using pooled hydatid disease-specific sera highly reactive with Ag5, a partial cDNA sequence termed Eg6 was isolated (76). The recombinant protein fragment encoded by the sequence was recognized by a monoclonal antibody specific for Ag5 (38). In addition, antibodies eluted from this recombinant protein recognized the 38-kDa subunit of Ag5. Another clone, designated Eg14, was selected and shown to code for an amino acid sequence partially homologous to Eg6 identified with the same monoclonal antibody. Using Eg6 sequence primers, a novel sequence coding for a 29-kDa antigen (termed P-29) was amplified from PSC of E. granulosus. The sequence has 100% identity to the amino acid sequence encoded by Eg6. Additional work has shown that P-29 and Ag5 are immunologically related but are nevertheless different proteins, raising questions about the current state of knowledge of Ag5 (96). Results of studies investigating the sensitivity and specificity of native and recombinant Ag5 and Ag5 peptides in CE diagnosis are summarized in Table 5.
Limitations of Current Tests
Although AgB and Ag5 have proved to be diagnostically valuable, there are difficulties related to their lack of sensitivity and specificity and problems with the standardization of their use (21). Cross-reactivity with antigens from other parasites, notably other taeniid cestodes (165, 171, 188, 199, 229), is a major problem. In addition, results from one- and two-dimensional electrophoresis and microsequencing have suggested that AgB and Ag5 are composed of a family of proteins in cyst fluid, which may complicate their use in diagnosis (271). Furthermore, the older traditional methods still used to purify AgB and Ag5 may limit the purity of the antigens. Echinococcus antigenic components have been isolated and purified through the application of a variety of methods, such as anion-exchange chromatography (13, 95), affinity purification with protein A or monoclonal antibodies (95, 101, 271), isoelectric focusing (132), and affinity chromatography (23, 271).
One recent study highlights the need to standardize techniques and antigenic preparations and to improve the performance of immunodiagnosis by characterizing new antigens and detecting distinct immunoglobulin classes. The diagnostic sensitivity and specificity of IEP, ELISA, and IB in detecting IgG antibodies to native and recombinant AgB and a hydatid fluid fraction in patient sera were compared (188). Sera tested were from patients who had CE grouped according to their type of cysts, from patients with other parasitic diseases, lung or liver carcinomas, or serous cysts, and from healthy controls. Hydatid fluid fraction-IB gave the highest sensitivity (80%) followed by ELISA (72%) and IEP (31%). The diagnostic sensitivity decreased significantly as cysts matured (from type I-II to type VII, classified by ultrasonography). Recombinant and native AgB-IB yielded similar sensitivities (74%), but a large number of clinically or surgically confirmed CE patients (20%) were negative. In these patient sera, the use of IB to assess the usefulness of another recombinant E. granulosus molecule (elongation factor 1β/δ [EF-1β/δ]) in detecting IgE antibodies yielded 33% positivity (188).
The results of this and other studies suggest that hydatid serological testing may be improved by combining several defined antigens (including synthetic peptides) and by designing new E. granulosus-specific peptides that react with otherwise false-negative sera.
Diagnosis for Monitoring Treatment of Cystic Echinococcosis in Humans
Patients with CE need to be carefully monitored after surgery or drug treatment to ensure that they remain free from infection and disease. Antibody detection is a valuable method of monitoring a patient after treatment. In patients from whom cysts have been removed successfully, the IgG4 subclass becomes negative soon after surgery (107). In contrast, patients with relapsing disease maintain high IgG4 titers in ELISA (107, 215, 219), which suggests that the IgG4 subclass is a good marker for hydatidosis follow-up. Specific IgE and IgM ELISAs are also useful in this respect (215, 219, 267).
PBMC isolated from CE patients can be driven in vitro by HCF antigens (125, 216) to produce large amounts of cytokines. IL-4 detection may be useful in the follow up of patients with CE. Furthermore, this can be combined with reverse transcriptase PCR to determine the mRNA expression of cytokines in PBMC to complement the biological assays in the follow-up (213). Detection of circulating antigens is also relevant as a method of post-surgical follow-up of patients and for monitoring the growth dynamics and/or the activity of cysts (78, 165, 207).
EF-1β/δ, a parasite protein present both in PSC and hydatid fluid, is a sensitive marker of infection (176). The higher percentage of humoral immune responses to EF-1β/δ observed in CE patients with calcified cysts than in patients with active cysts suggests that the protein is released into the hydatid fluid after the degeneration of PSC and indicates its possible use in immunosurveillance of CE. Furthermore, EF-1β/δ may play a key role in the allergic disorders (urticaria, itching, and anaphylactic shock) that often complicate the course of CE (176, 187).
Brief Comments on the Diagnosis of Alveolar Echinococcosis in Humans
The diagnosis of AE is based on similar findings and criteria to those for the diagnosis of CE. These include case history, clinical findings, morphological lesions identified by imaging techniques, PCR, or immunofluorescence/immunohistochemistry, and immunodiagnosis. The area has been comprehensively reviewed (192). Like CE, serodiagnosis of AE provides a complementary role to other procedures in early detection of the infection. The methods are similar to those used for CE, but serological tests for antibody detection are generally more reliable. AE is a very serious disease with a high fatality rate, so that early detection is vital for successful management and treatment (15).
Em2, a species-specific native antigen isolated from the metacestode of E. multilocularis (101), has been used for immunodiagnosis of human AE with encouraging results (99). The sensitivities of Em2 in ELISA varied depending on the geographical origin of the patient; they ranged between 77 and 92% (103). The Em2plus ELISA, a combination of Em2 with a recombinant protein designated II/3-10, increased the sensitivity to 97%. The Em2plus assay exhibits cross-reaction with CE (in 25.8% of cases), which is higher than for the individual Em2 (5.6%) and 11/3-10 (6.5%), but limited cross-reactivity with other diseases. The Em2plus ELISA has been commercialized for clinical diagnosis of AE (103) and for population screening (32). More recently, an 18-kDa antigen (Em18) from PSC of AE was reported as being a highly species-specific (96.8%) and sensitive (97%) antigen with potential not only for differentiation of AE from either CE or other helminth infections but also for differentiation of active from inactive AE (131-136, 143). Both Em2plus ELISA and Em18 in an IB format have been used for long-term follow-up monitoring of AE patients following pharmacological treatment (173). Furthermore, Em13 and Em10, recombinant proteins expressed from cloned cDNAs from PSC of E. multilocularis, are also valuable in serodiagnosis of AE (Table 6). A purified alkaline phosphatase from E. multilocularis metacestodes has been shown to have exceptional diagnostic characteristics, with 100% specificity without any decrease in sensitivity (100%), and has significant potential for use in routine diagnosis and follow-up of AE patients (231).
TABLE 6.
Features of assays for immunodiagnosis of AE using different antigensa
| No. of subjects tested | Antigen | Assay method | Sensitivity (%) | Specificity (%) | Ig isotype | Reference | ||
|---|---|---|---|---|---|---|---|---|
| AE patients | Healthy subjects | Patients with other disease | ||||||
| 37 | 37 | 95 | pAP | ELISA | 100 | 100 | IgG | 207 |
| 44 | 30 | 99 | Em18 | ELISA | 91 | 89.1 | IgG | 143 |
| 140 | 500 | 348 | Em2 | ELISA | 89.3 | 98.0 | IgG | 103 |
| 74 | 39 | 95 | rEm10 | ELISs | 93.2 | 96.5 | IgG | 120 |
| 140 | 500 | 348 | II/3-10 | ELISA | 86.4 | 96.8 | IgG | 103 |
| 140 | 500 | 348 | Em2plus | ELISA | 97.1 | 90.2 | IgG | 103 |
| 28 | 72 | rEm13 | ELISA | 82.1 | 100 | Ig | 84 | |
| 66 | 29 | 259 | Em18 | IB | 97 | 96.9 | IgG | 132 |
| 33 | 82 | 99 | Em18 | IB | 91 | 92.3 | IgG | 143 |
See the text for details.
Immunodiagnosis of Cystic Echinococcosis in Animals
In comparison with investigations in humans, relatively little research has been directed toward the development of immunodiagnostic techniques for E. granulosus infection in domesticated animals such as sheep and cattle. Currently, the diagnosis of CE in intermediate hosts is based mainly on necropsy procedures. Accurate serological diagnosis of CE infection in livestock is difficult due to serological cross-reactions with several other species of taeniid cestodes including Taenia hydatigena and Taenia ovis (165, 266). Furthermore, natural intermediate host animals produce very poor antibody responses to infection compared with the relatively high levels of specific antibody seen in human infection (165). In sheep, the principal intermediate host of E. granulosus in most regions of endemic infection worldwide, antibodies to various antigens including Ag5 are detectable in the sera of some but not all infected sheep (“nonresponders”) (139). As with human CE, detection of circulating antigen does not appear to be useful for diagnostic purposes (71).
ELISA techniques using a variety of antigens have been applied to the immunodiagnosis of animal CE (138, 141, 266). In experimentally infected sheep, antibodies to hydatid antigens can be detected as early as 4 to 6 weeks postinfection (266) and persist for at least 4 years (138). However, as referred to above, serological cross-reactions between E. granulosus and other cestodes limit the specific diagnosis of hydatid infection by ELISA with crude parasite antigens (138, 266). Affinity purification of crude antigens with antibodies from animals immunized with homologous antigen (141) or affinity depletion of cross-reactive antigens with monoclonal antibody (138) only partly reduces the cross-reactivity. Components of ovine HCF can bind to sheep immunoglobulin nonspecifically and contribute to false-positive reactions, even with sera from cestode-free animals (138). After affinity depletion of crude antigen with both monoclonal antibody and sheep immunoglobulin from animals not infected with hydatid disease, background reactions were greatly reduced. Using this affinity-depleted antigen, it was possible to differentiate serologically between a flock of sheep with hydatid infection and uninfected sheep from the same locality; however, specific diagnosis of infection in individual sheep from another locality was low, and variation in antibody responses to different parasite strains was suggested as a possible cause of these differences (138).
Polysaccharide antigens from either the secretions produced during in vitro cultivation of E. granulosus PSC or from mouse hydatid cyst membranes by phenol extraction have been used to test sera from sheep (140). Although the antibody responses were significantly higher than those of sheep infected with T. hydatigena or T. ovis, very high cross-reacting antibody responses in the sera from T. hydatigena-infected animals were detected with the antigenic secretions from PSC. Neither antigen was sufficiently sensitive or specific for routine serodiagnostic use (140).
DNA techniques are now available that allow the identification of Echinococcus species and of E. granulosus strains by using metacestode material from intermediate hosts (245).
Diagnosis of Echinococcosis in Definitive Hosts
Two major diagnostic methods have been extensively used in dogs: purgation with arecoline compounds and necropsy of the small intestine. Necropsy is the method of choice for foxes and other final hosts. Two main immunodiagnostic approaches have been developed for diagnosis of E. granulosus and E. multilocularis infection in definitive hosts: assays for specific serum antibody and detection of parasite products (coproantigens) in feces.
Specific serum antibodies were shown to be detectable in the blood of dogs after experimental infection with taeniid cestodes, including E. granulosus, using metacestode antigen preparations in ELISA (138-141). Subsequently, others confirmed the appearance of specific antibodies, following experimental infection with E. granulosus in dogs, detected using antigens derived from the oncosphere (25, 238, 239). These latter results suggested that some of the eggs released into the small intestine, following apolysis of proglottids, may hatch and penetrate the intestinal wall, resulting in immunological stimulation of the host. Detection of circulating anti-Em2 antibodies by ELISA may be useful for primary screening of fox populations, but antibody prevalence does not correlate with the actual prevalence of the E. multilocularis intestinal infection (58, 102). Overall, the available ELISA-based methods have poor sensitivity, the specificity is unclear, and there is no correlation with worm burden (85-89); therefore, their usefulness, other than in population-based studies of canine hosts, is questionable.
The other major approach to diagnosis of Echinococcus infection in the definitive host is through detection of adult worm products in feces by using the sandwich ELISA. Craig et al. (47, 49) used a monoclonal antibody specific for an antigen on the surface of E. granulosus oncospheres to distinguish E. granulosus eggs in perianal swabs or samples from environmental sites. This indirect immunofluorescence test is relatively cumbersome, and its sensitivity can be affected by the periodic absence of eggs in fecal samples (47). Nevertheless, the approach has been developed further and used successfully by a number of groups to detect coproantigens of Echinococcus spp. in canine host feces by antibody capture ELSA (11, 59); at least two commercial ELISA kits are now available. Although the various tests that have been developed show some cross-reactivity with other cestode infections (72), they exhibit a high probability of correlation with current infection (57, 59, 137). Further, these tests are capable of detecting patent and prepatent infections with a high degree of sensitivity and specificity, making them reliable tools for epidemiologic investigations (11, 57-60, 72, 184). Some details of investigations showing the sensitivity and specificity of the coproantigen ELISA are shown in Table 7.
TABLE 7.
Dynamics of the coproantigen ELISA for diagnosis of CE and AE in canine hosts
| Host/no. tested | Species | No. of parasites detectable | Sensitivity (%) | Specificity (%) | Reference |
|---|---|---|---|---|---|
| Dogs/94 | E. granulosus | 3-10,000 | 61.5 | 91 | 72 |
| E. granulosus | >20 | 87.5 | 72 | ||
| E. granulosus | >100 | 100 | 72 | ||
| Dogs/242 | E. granulosus | Variable | 63 | 97 | 60 |
| E. granulosus | <100 | 29 | 60 | ||
| E. granulosus | >100 | 92 | 60 | ||
| Dogs/15 | E. granulosus | Variable | 56 | 98a | 59 |
| E. granulosus | >200 | 87 | 59 | ||
| E. granulosus | <200 | 10 | 59 | ||
| Dogs/660 | E. multilocularis | 99.5 | 57 | ||
| Dogs/661 | E. multilocularis | Variable | 80 | 99.5 | 58 |
| Foxes/55 | E. multilocularis | 4-60,000 | 83.6 | 57 | |
| E. multilocularis | >20 | 93.3 | 57 | ||
| Foxes/20 | E. multilocularis | 95 | 58 | ||
| Foxes/35 | E. multilocularis | Variable | 80 | 58 | |
| E. multilocularis | >55 | 93 | 58 | ||
| Foxes/59 | E. multilocularis | Variable | 79.7 | 98a | 59 |
| E. multilocularis | >1,000 | 100 | 59 | ||
| E. multilocularis | <1,000 | 73.9 | 59 | ||
| Cats/263 | E. multilocularis | 98 | 57 |
From 155 carnivores including dogs, foxes, and cats.
There is also interest in detecting parasite DNA (copro-DNA) in fecal samples. No test is available for E. granulosus, but a PCR-based assay has been developed for detecting DNA of E. multilocularis in fecal samples of foxes after isolation of the parasite eggs by a sieving procedure (58); in a total of 55 infected foxes, the specificity was 100% and the sensitivity was 94%. For field application, the coproantigen ELISA has the potential for replacing necropsy examinations, and the copro-PCR is a valuable method for confirmation of positive coproantigen results and for diagnosis in individual animals (58).
Acknowledgments
Our studies have received financial support from various sources, particularly the National Health and Medical Research Council of Australia, the Australian Research Council, the University of Queensland, and the Wellcome Trust.
We much appreciate the typing and formatting skills of Ian Dillon and his help in producing Figure 1. We also acknowledge Wen Hao, Xinjiang Medical University, Xinjiang, People's Republic of China, for providing the clinical pictures.
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Aceti, A., A. Pennica, A. Teggi, L. M. Fondacaro, M. Caferro, O. Leri, G. Tacchi, D. Celestino, G. Quaranta, F. De Rosa, and A. Sebastani. 1993. IgG subclasses in human hydatid disease: prominence of the IgG4 response. Int. Arch. Allergy Immunol. 102:347-351. [DOI] [PubMed] [Google Scholar]
- 3.Ali-Khan, Z. 1978. Cellular changes in the lymphoreticular tissues of C57L/J mice infected with Echinococcus multilocularis cysts. Immunology 34:831-839. [PMC free article] [PubMed] [Google Scholar]
- 4.Ali-Khan, Z. 1978. Echinococcus multilocularis: cell-mediated immune response in early and chronic alveolar murine hydatidosis. Exp. Parasitol. 46:157-165. [DOI] [PubMed] [Google Scholar]
- 5.Ali-Khan, Z. 1974. Host-parasite relationship in echinococcosis. I. Parasite biomass and antibody response in three strains of inbred mice against graded doses of Echinococcus multilocularis cysts. J. Parasitol. 60:231-235. [PubMed] [Google Scholar]
- 6.Ali-Khan, Z. 1974. Host-parasite relationship in echinococcosis. II. Cyst weight, hematologic alterations, and gross changes in the spleen and lymph nodes of C57L mice against graded doses of Echinococcus multilocularis cysts. J. Parasitol. 60:236-242. [PubMed] [Google Scholar]
- 7.Ali-Khan, Z. 1978. Pathological changes in the lymphoreticular tissues of Swiss mice infected with Echinococcus granulosus cysts. Z. Parasitenkd. 58:47-54. [DOI] [PubMed] [Google Scholar]
- 8.Ali-Khan, Z., and R. Siboo. 1980. Pathogenesis and host response in subcutaneous alveolar hydatidosis. I. Histogenesis of alveolar cyst and a qualitative analysis of the inflammatory infiltrates. Z. Parasitenkd. 62:241-254. [DOI] [PubMed] [Google Scholar]
- 9.Ali-Khan, Z., and R. Siboo. 1980. Pathogenesis and host response in subcutaneous alveolar hydatidosis. II. Intense plasmacellular infiltration in the paracortex of draining lymph nodes. Z. Parasitenkd. 62:255-265. [DOI] [PubMed] [Google Scholar]
- 10.Al-Khalidi, N. W., and O. O. Barriga. 1986. Cell-mediated immunity in the prepatent primary infection of dogs with Echinococcus granulosus. Vet. Immunol. Immunopathol. 11:73-82. [DOI] [PubMed] [Google Scholar]
- 11.Allan, J. C., P. S. Craig, J. Garcia Noval, F. Mencos, D. Liu, Y. Wang, H. Wen, P. Zhou, R. Stringer, M. Rogan, and E. Zeyhle. 1992. Coproantigen detection for immunodiagnosis of echinococcosis and taeniasis in dogs and humans. Parasitology 104:347-356. [DOI] [PubMed] [Google Scholar]
- 12.Allen, J. E., and R. M. Maizels. 1996. Immunology of human helminth infection. Int. Arch. Allergy Immunol. 109:3-10. [DOI] [PubMed] [Google Scholar]
- 13.al-Yaman, F. M., and J. Knobloch. 1989. Isolation and partial characterization of species-specific and cross-reactive antigens of Echinococcus granulosus cyst fluid. Mol. Biochem. Parasitol. 37:101-107. [DOI] [PubMed] [Google Scholar]
- 14.Aminzhanov, M. 1980. Immunoprophylaxis of hydatidosis in animals. Tr. Uzbek. Nauchno-Issled. Inst. Vet. 30:15-18. [Google Scholar]
- 15.Ammann, R., K. Tschudi, M. von Ziegler, F. Meister, J. Cotting, J. Eckert, F. Witassek, and A. Freiburghaus. 1988. The long-term course of 60 patients with alveolar echinococcosis in continuous therapy with mebendazole (1976-85). Klin. Wochenschr. 66:1060-1073. (In German.) [DOI] [PubMed] [Google Scholar]
- 16.Annen, J. M., P. Kohler, and J. Eckert. 1981. Cytotoxicity of Echinococcus granulosus cyst fluid in vitro. Z. Parasitenkd. 65:79-88. [DOI] [PubMed] [Google Scholar]
- 17.Archer, G. T., J. E. Robson, and A. R. Thompson. 1977. Eosinophilia and mast cell hyperplasia induced by parasite phospholipid. Pathology 9:137-153. [DOI] [PubMed] [Google Scholar]
- 18.Arias Irigoyen, J., and C. J. Senent Sanchez. 1995. Toxocariasis: a cause of hyper IgE and eosinophilia. J. Investig. Allergol. Clin. Immunol. 5:232-234. [PubMed] [Google Scholar]
- 19.Auriault, C., M. A. Ouaissi, G. Torpier, H. Eisen, and A. Capron. 1981. Proteolytic cleavage of IgG bound to the Fc receptor of Schistosoma mansoni schistosomula. Parasite Immunol. 3:33-44. [DOI] [PubMed] [Google Scholar]
- 20.Auriault, C., J. Pestel, M. Joseph, J. P. Dessaint, and A. Capron. 1981. Interaction between macrophages and Schistosoma mansoni schistosomula: role of IgG peptides and aggregates on the modulation of beta-glucuronidase release and the cytotoxicity against schistosomula. Cell. Immunol. 62:15-27. [DOI] [PubMed] [Google Scholar]
- 21.Babba, H., A. Messedi, S. Masmoudi, M. Zribi, R. Grillot, P. Ambriose-Thomas, I. Beyrouti, and Y. Sahnoun. 1994. Diagnosis of human hydatidosis: comparison between imagery and six serologic techniques. Am. J. Trop. Med. Hyg. 50:64-68. [DOI] [PubMed] [Google Scholar]
- 22.Barbieri, M., V. Fernandez, G. Gonzalez, V. M. Luaces, and A. Nieto. 1998. Diagnostic evaluation of a synthetic peptide derived from a novel antigen B subunit as related to other available peptides and native antigens used for serology of cystic hydatidosis. Parasite Immunol. 20:51-61. [DOI] [PubMed] [Google Scholar]
- 23.Barbieri, M., S. Sterla, J. Battistoni, and A. Nieto. 1993. High performance latex reagent for hydatid serology using an Echinococcus granulosus. lipoprotein antigen fraction purified from cyst fluid in one step. Int. J. Parasitol. 23:565-572. [DOI] [PubMed] [Google Scholar]
- 24.Baron, R. W., and C. E. Tanner. 1977. Echinococcus multilocularis in the mouse: the in vitro protoscolicidal activity of peritoneal macrophages. Int. J. Parasitol. 7:489-495. [DOI] [PubMed] [Google Scholar]
- 25.Barriga, O. O., and N. W. Al-Khalidi. 1986. Humoral immunity in the prepatent primary infection of dogs with Echinococcus granulosus. Vet. Immunol. Immunopathol. 11:375-389. [DOI] [PubMed] [Google Scholar]
- 26.Bauder, B., H. Auer, F. Schilcher, C. Gabler, T. Romig, B. Bilger, and H. Aspock. 1999. Experimental investigations on the B and T cell immune response in primary alveolar echinococcosis. Parasite Immunol. 21:409-421. [DOI] [PubMed] [Google Scholar]
- 27.Baveja, U. K., S. Basak, and T. K. Thusoo. 1997. Immunodiagnosis of human hydatid disease. J. Commun. Dis. 29:313-319. [PubMed] [Google Scholar]
- 28.Baz, A., A. Hernandez, S. Dematteis, H. Carol, and A. Nieto. 1995. Idiotypic modulation of the antibody response of mice to Echinococcus granulosus antigens. Immunology 84:350-354. [PMC free article] [PubMed] [Google Scholar]
- 29.Baz, A., A. Richieri, A. Puglia, A. Nieto, and S. Dematteis. 1999. Antibody response in CD4-depleted mice after immunization or during early infection with Echinococcus granulosus. Parasite Immunol. 21:141-150. [DOI] [PubMed] [Google Scholar]
- 30.Bell, R. G. 1996. IgE, allergies and helminth parasites: a new perspective on an old conundrum. Immunol. Cell Biol. 74:337-345. [DOI] [PubMed] [Google Scholar]
- 31.Bendixsen, T., D. L. Emery, and W. O. Jones. 1995. The sensitization of mucosal mast cells during infections with Trichostrongylus colubriformis or Haemonchus contortus in sheep. Int. J. Parasitol. 25:741-748. [DOI] [PubMed] [Google Scholar]
- 32.Bresson-Hadni, S., J. J. Laplante, D. Lenys, P. Rohmer, B. Gottstein, P. Jacquier, P. Mercet, J. P. Meyer, J. P. Miguet, and D. A. Vuitton. 1994. Seroepidemiologic screening of Echinococcus multilocularis infection in a European area endemic for alveolar echinococcosis. Am. J. Trop. Med. Hyg. 51:837-846. [DOI] [PubMed] [Google Scholar]
- 33.Bresson-Hadni, S., M. Liance, J. P. Meyer, R. Houin, J. L. Bresson, and D. A. Vuitton. 1990. Cellular immunity in experimental Echinococcus multilocularis infection. II. Sequential and comparative phenotypic study of the periparasitic mononuclear cells in resistant and sensitive mice. Clin. Exp. Immunol. 82:378-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bresson-Hadni, S., B. Monnot-Jacquard, E. Racadot, D. Lenys, J. P. Miguet, and D. A. Vuitton. 1991. Soluble IL-2-receptor and CD8 in the serum and the periparasitic granuloma of patients with alveolar echinococcosis. Eur. Cytokine Netw. 2:339-344. [PubMed] [Google Scholar]
- 35.Bretagne, S., B. Assouline, D. Vidaud, R. Houin, and M. Vidaud. 1996. Echinococcus multilocularis: microsatellite polymorphism in U1 snRNA genes. Exp. Parasitol. 82:324-328. [DOI] [PubMed] [Google Scholar]
- 36.Butterworth, A. E. 1984. Cell-mediated damage to helminths. Adv. Parasitol. 23:143-235. [DOI] [PubMed] [Google Scholar]
- 37.Capron, A., and J. P. Dessaint. 1992. Immunologic aspects of schistosomiasis. Annu. Rev. Med. 43:209-218. [DOI] [PubMed] [Google Scholar]
- 38.Chamekh, M., B. Facon, C. Dissous, A. Haque, and A. Capron. 1990. Use of a monoclonal antibody specific for a protein epitope of Echinococcus granulosus antigen 5 in a competitive antibody radioimmunoassay for diagnosis of hydatid disease. J. Immunol. Methods 134:129-137. [DOI] [PubMed] [Google Scholar]
- 39.Chemale, G., K. L. Haag, H. B. Ferreira, and A. Zaha. 2001. Echinococcus granulosus antigen B is encoded by a gene family. Mol. Biochem. Parasitol. 116:233-237. [DOI] [PubMed] [Google Scholar]
- 40.Chemtai, A. K., G. B. Okelo, and J. Kyobe. 1981. Application of immunoelectrophoresis (IEP5) test in the diagnosis of human hydatid disease in Kenya. East Afr. Med. J. 58:583-586. [PubMed] [Google Scholar]
- 41.Chi, P., W. Zhang, Z. Zhang, M. Hasyet, F. Liu, Z. Ding, F. L. Andersen, H. D. Tolley, and P. M. Schantz. 1990. Cystic echinococcosis in the Xinjiang/Uygur Autonomous Region, People's Republic of China. I. Demographic and epidemiologic data. Trop. Med. Parasitol. 41:157-162. [PubMed] [Google Scholar]
- 42.Chordi, A., and I. G. Kagan. 1965. Identification and characterization of antigenic components of sheep hydatid cyst fluid by immunoelectrophoresis. J. Parasitol. 51:63-71. [PubMed] [Google Scholar]
- 43.Chow, C., C. G. Gauci, A. F. Cowman, and M. W. Lightowlers. 2001. A gene family expressing a host-protective antigen of Echinococcus granulosus. Mol. Biochem. Parasitol. 118:83-88. [DOI] [PubMed] [Google Scholar]
- 44.Cicardi, M., D. Frangi, L. Bergamaschini, M. Gardinali, G. Sacchi, and A. Agostoni. 1985. Acquired C1 inhibitor deficiency with angioedema symptoms in a patient infected with Echinococcus granulosus. Complement 2:133-139. [DOI] [PubMed] [Google Scholar]
- 45.Clutterbuck, E. J., E. M. Hirst, and C. J. Sanderson. 1989. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood 73:1504-1512. [PubMed] [Google Scholar]
- 46.Craig, P. S. 1986. Detection of specific circulating antigen, immune complexes and antibodies in human hydatidosis from Turkana (Kenya) and Great Britain, by enzyme-immunoassay. Parasite Immunol. 8:171-188. [DOI] [PubMed] [Google Scholar]
- 47.Craig, P. S., C. N. Macpherson, D. L. Watson-Jones, and G. S. Nelson. 1988. Immunodetection of Echinococcus eggs from naturally infected dogs and from environmental contamination sites in settlements in Turkana, Kenya. Trans. R. Soc. Trop. Med. Hyg. 82:268-274. [DOI] [PubMed] [Google Scholar]
- 48.Craig, P. S., M. T. Rogan, and J. C. Allan. 1996. Detection, screening and community epidemiology of taeniid cestode zoonoses: cystic echinococcosis, alveolar echinococcosis and neurocysticercosis. Adv. Parasitol. 38:169-250. [DOI] [PubMed] [Google Scholar]
- 49.Craig, P. S., E. Zeyhle, and T. Romig. 1986. Hydatid disease: research and control in Turkana. II. The role of immunological techniques for the diagnosis of hydatid disease. Trans. R. Soc. Trop. Med. Hyg. 80:183-192. [DOI] [PubMed] [Google Scholar]
- 50.Cross, G. A. 1996. Antigenic variation in trypanosomes: secrets surface slowly. Bioessays 18:283-291. [DOI] [PubMed] [Google Scholar]
- 51.Dada, B. J., and E. D. Belino. 1981. Immunization of sheep against cystic hydatidosis with homologous and heterologous metacestode antigens. Int. J. Zoonoses 8:20-25. [PubMed] [Google Scholar]
- 52.Daeki, A. O., P. S. Craig, and M. K. Shambesh. 2000. IgG-subclass antibody responses and the natural history of hepatic cystic echinococcosis in asymptomatic patients. Ann. Trop. Med. Parasitol. 94:319-328. [DOI] [PubMed] [Google Scholar]
- 53.Dai, W. J., and B. Gottstein. 1999. Nitric oxide-mediated immunosuppression following murine Echinococcus multilocularis infection. Immunology 97:107-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D'Amelio, R., F. De Rosa, O. Pontesilli, R. Dayal, G. Brighouse, A. Teggi, M. Barnet, and P. H. Lambert. 1989. Hydatid disease: analysis of parasite antigens in circulating immune complexes and in preformed hydatid antigen-antibody complexes. Med. Microbiol. Immunol. 178:177-186. [DOI] [PubMed] [Google Scholar]
- 55.Dematteis, S., A. Baz, M. Rottenberg, C. Fernandez, A. Orn, and A. Nieto. 1999. Antibody and Th1/Th2-type responses in BALB/c mice inoculated with live or dead Echinococcus granulosus protoscoleces. Parasite Immunol. 21:19-26. [DOI] [PubMed] [Google Scholar]
- 56.Dempster, R. P., M. V. Berridge, G. B. Harrison, and D. D. Heath. 1991. Echinococcus granulosus: development of an intermediate host mouse model for use in vaccination studies. Int. J. Parasitol. 21:549-554. [DOI] [PubMed] [Google Scholar]
- 57.Deplazes, P., P. Alther, I. Tanner, R. C. Thompson, and J. Eckert. 1999. Echinococcus multilocularis coproantigen detection by enzyme-linked immunosorbent assay in fox, dog, and cat populations. J. Parasitol. 85:115-121. [PubMed] [Google Scholar]
- 58.Deplazes, P., and J. Eckert. 1996. Diagnosis of the Echinococcus multilocularis infection in final hosts. Appl. Parasitol. 37:245-252. [PubMed] [Google Scholar]
- 59.Deplazes, P., B. Gottstein, J. Eckert, D. J. Jenkins, D. Ewald, and S. Jimenez-Palacios. 1992. Detection of Echinococcus coproantigens by enzyme-linked immunosorbent assay in dogs, dingoes and foxes. Parasitol. Res. 78:303-308. [DOI] [PubMed] [Google Scholar]
- 60.Deplazes, P., S. Jimenez-Palacios, B. Gottstein, J. Skaggs, and J. Eckert. 1994. Detection of Echinococcus coproantigens in stray dogs of northern Spain. Appl. Parasitol. 35:297-301. [PubMed] [Google Scholar]
- 61.Deplazes, P., R. C. Thompson, C. C. Constantine, and W. J. Penhale. 1994. Primary infection of dogs with Echinococcus granulosus: systemic and local (Peyer's patches) immune responses. Vet. Immunol. Immunopathol. 40:171-184. [DOI] [PubMed] [Google Scholar]
- 62.De Rosa, F., S. Dottorini, and S. Pauluzzi. 1977. Vaccination against experimental peritoneal hydatid disease of BALB/C mice with hydatid fluids and their fractions. Ann. Sclavo 19:470-477. (In Italian.) [PubMed] [Google Scholar]
- 63.De Rycke, P. H., and E. Pennoit-de Cooman. 1973. Experimental secondary echinococcosis of Echinococcus granulosus. IV. Vaccination of host mice. Z. Parasitenkd. 42:49-59. [DOI] [PubMed] [Google Scholar]
- 64.Dessaint, J. P., D. Bout, P. Wattre, and A. Capron. 1975. Quantitative determination of specific IgE antibodies to Echinococcus granulosus and IgE levels in sera from patients with hydatid disease. Immunology 29:813-823. [PMC free article] [PubMed] [Google Scholar]
- 65.Diaz, A., F. Irigoin, F. Ferreira, and R. B. Sim. 1999. Control of host complement activation by the Echinococcus granulosus hydatid cyst. Immunopharmacology 42:91-98. [DOI] [PubMed] [Google Scholar]
- 66.Diaz, A., A. C. Willis, and R. B. Sim. 2000. Expression of the proteinase specialized in bone resorption, cathepsin K, in granulomatous inflammation. Mol. Med. 6:648-659. [PMC free article] [PubMed] [Google Scholar]
- 67.Dixon, J. B. 1997. Echinococcosis. Comp. Immunol. Microbiol. Infect. Dis. 20:87-94. [DOI] [PubMed] [Google Scholar]
- 68.Dixon, J. B., and P. Jenkins. 1995. Immunology of mammalian metacestode infections. I. Antigens, protective immunity and immunopathology. Helm. Abstr. 64:532-542. [Google Scholar]
- 69.Douch, P. G., R. S. Green, J. F. Huntley, and P. L. Risdon. 1996. Serum mast cell proteinase responses of sheep to challenge with Trichostrongylus colubriformis and the effect of dexamethasone treatment. Int. J. Parasitol. 26:91-95. [DOI] [PubMed] [Google Scholar]
- 70.Douch, P. G., P. E. Morum, and B. Rabel. 1996. Secretion of anti-parasite substances and leukotrienes from ovine gastrointestinal tissues and isolated mucosal mast cells. Int. J. Parasitol. 26:205-211. [DOI] [PubMed] [Google Scholar]
- 71.Eckert, J., P. Deplazes, P. S. Craig, M. Gemmell, B. Gottstein, D. Heath, D. J. Jenkins, M. Kamiya, and M. Lightowlers. 2001. Echinococcosis in animals: clinical aspects, diagnosis and treatment, p. 72-99. In J. Eckert, M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.), WHOI/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France.
- 72.el-Shehabi, F. S., S. A. Kamhawi, P. M. Schantz, P. S. Craig, and S. K. Abdel-Hafez. 2000. Diagnosis of canine echinococcosis: comparison of coproantigen detection with necropsy in stray dogs and red foxes from northern Jordan. Parasite 7:83-90. [DOI] [PubMed] [Google Scholar]
- 73.Emery, I., C. Leclerc, R. Houin, D. A. Vuitton, and M. Liance. 1997. Lack of H-2 gene influence on mouse susceptibility to secondary alveolar echinococcosis. Int. J. Parasitol. 27:1433-1436. [DOI] [PubMed] [Google Scholar]
- 74.Emery, I., M. Liance, E. Deriaud, D. A. Vuitton, R. Houin, and C. Leclerc. 1996. Characterization of T-cell immune responses of Echinococcus multilocularis-infected C57BL/6J mice. Parasite Immunol. 18:463-472. [DOI] [PubMed] [Google Scholar]
- 75.Emery, I., M. Liance, and C. Leclerc. 1997. Secondary Echinococcus multilocularis infection in A/J mice: delayed metacestode development is associated with Th1 cytokine production. Parasite Immunol. 19:493-503. [DOI] [PubMed] [Google Scholar]
- 76.Facon, B., M. Chamekh, C. Dissous, and A. Capron. 1991. Molecular cloning of an Echinococcus granulosus protein expressing an immunogenic epitope of antigen 5. Mol. Biochem. Parasitol. 45:233-239. [DOI] [PubMed] [Google Scholar]
- 77.Fernandez, V., H. B. Ferreira, C. Fernandez, A. Zaha, and A. Nieto. 1996. Molecular characterisation of a novel 8-kDa subunit of Echinococcus granulosus antigen B. Mol. Biochem. Parasitol. 77:247-250. [DOI] [PubMed] [Google Scholar]
- 78.Ferragut, G., I. Ljungstrom, and A. Nieto. 1998. Relevance of circulating antigen detection to follow-up experimental and human cystic hydatid infections. Parasite Immunol. 20:541-549. [DOI] [PubMed] [Google Scholar]
- 79.Ferreira, A. M., R. Wurzner, M. J. Hobart, and P. J. Lachmann. 1995. Study of the in vitro activation of the complement alternative pathway by Echinococcus granulosus hydatid cyst fluid. Parasite Immunol. 17:245-251. [DOI] [PubMed] [Google Scholar]
- 80.Finkelman, F. D., E. J. Pearce, J. F. Urban, Jr., and A. Sher. 1991. Regulation and biological function of helminth-induced cytokine responses. Immunol. Today 12:A62-A66. [DOI] [PubMed] [Google Scholar]
- 81.Fotiadis, C., C. Sergiou, J. Kirou, T. G. Troupis, J. Tselentis, P. Doussaitou, V. G. Gorgoulis, and M. N. Sechas. 1999. Experimental Echinococcus infection in the mouse model: pericystic cellular immunity reaction and effects on the lymphoid organs of immunocompetent and thymectomized mice. In Vivo 13:541-546. [PubMed] [Google Scholar]
- 82.Frosch, P., M. Hartmann, F. Muhlschlegel, and M. Frosch. 1994. Sequence heterogeneity of the echinococcal antigen B. Mol. Biochem. Parasitol. 64:171-175. [DOI] [PubMed] [Google Scholar]
- 83.Frosch, P. M., M. Frosch, T. Pfister, V. Schaad, and D. Bitter-Suermann. 1991. Cloning and characterisation of an immunodominant major surface antigen of Echinococcus multilocularis. Mol. Biochem. Parasitol. 48:121-130. [DOI] [PubMed] [Google Scholar]
- 84.Frosch, P. M., C. Geier, F. J. Kaup, A. Muller, and M. Frosch. 1993. Molecular cloning of an echinococcal microtrichal antigen immunoreactive in Echinococcus multilocularis disease. Mol. Biochem. Parasitol. 58:301-310. [DOI] [PubMed] [Google Scholar]
- 85.Gasser, R. B., D. J. Jenkins, D. D. Heath, and S. B. Lawrence. 1992. Use of Echinococcus granulosus worm antigens for immunodiagnosis of E. granulosus infection in dogs. Vet. Parasitol. 45:89-100. [DOI] [PubMed] [Google Scholar]
- 86.Gasser, R. B., M. W. Lightowlers, D. L. Obendorf, D. J. Jenkins, and M. D. Rickard. 1988. Evaluation of a serological test system for the diagnosis of natural Echinococcus granulosus infection in dogs using E. granulosus protoscolex and oncosphere antigens. Aust. Vet. J. 65:369-373. [DOI] [PubMed] [Google Scholar]
- 87.Gasser, R. B., M. W. Lightowlers, and M. D. Rickard. 1991. Echinococcus granulosus: antigenic proteins in oncospheres and on the surface of protoscoleces identified by serum antibodies from infected dogs. Res. Vet. Sci. 50:340-345. [DOI] [PubMed] [Google Scholar]
- 88.Gasser, R. B., M. W. Lightowlers, and M. D. Rickard. 1990. A recombinant antigen with potential for serodiagnosis of Echinococcus granulosus infection in dogs. Int. J. Parasitol. 20:943-950. [DOI] [PubMed] [Google Scholar]
- 89.Gasser, R. B., M. W. Lightowlers, M. D. Rickard, R. A. Lyford, and H. J. Dawkins. 1990. Serological screening of farm dogs for Echinococcus granulosus infection in an endemic region. Aust. Vet. J. 67:145-147. [DOI] [PubMed] [Google Scholar]
- 90.Gauci, C., M. Merli, V. Muller, C. Chow, K. Yagi, U. Mackenstedt, and M. W. Lightowlers. 2002. Molecular cloning of a vaccine antigen against infection with the larval stage of Echinococcus multilocularis. Infect. Immun. 70:3969-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gemmell, M. A. 1966. Immunological responses of the mammalian host against tapeworm infections. IV. Species specificity of hexacanth embryos in protecting sheep against Echinococcus granulosus. Immunology 11:325-335. [PMC free article] [PubMed] [Google Scholar]
- 92.Gemmell, M. A. 1962. Natural and acquired immunity factors interfering with development during the rapid growth phage of Echinococcus granulosus in dogs. Immunology 5:496-503. [PMC free article] [PubMed] [Google Scholar]
- 93.Gill, H. S. 1969. Vaccination trials against Echinococcus granulosus infection in dogs. J. Commun. Dis. 1:258-261. [Google Scholar]
- 94.Godot, V., S. Harraga, M. Deschaseaux, S. Bresson-Hadni, B. Gottstein, D. Emilie, and D. A. Vuitton, 1997. Increased basal production of interleukin-10 by peripheral blood mononuclear cells in human alveolar echinococcosis. Eur. Cytokine Netw. 8:401-408. [PubMed] [Google Scholar]
- 95.Gonzalez, G., A. Nieto, C. Fernandez, A. Orn, C. Wernstedt, and U. Hellman. 1996. Two different 8 kDa monomers are involved in the oligomeric organization of the native Echinococcus granulosus antigen B. Parasite Immunol. 18:587-596. [DOI] [PubMed] [Google Scholar]
- 96.Gonzalez, G., P. Spinelli, C. Lorenzo, U. Hellman, A. Nieto, A. Willis, and G. Salinas. 2000. Molecular characterization of P-29, a metacestode-specific component of Echinococcus granulosus which is immunologically related to, but distinct from, antigen 5. Mol. Biochem. Parasitol. 105:177-184. [DOI] [PubMed] [Google Scholar]
- 97.Gonzalez-Sapienza, G., C. Lorenzo, and A. Nieto. 2000. Improved immunodiagnosis of cystic hydatid disease by using a synthetic peptide with higher diagnostic value than that of its parent protein, Echinococcus granulosus antigen B. J. Clin. Microbiol. 38:3979-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gottstein, B. 1991. Echinococcus multilocularis: antigenic variance between different parasite isolates. Parasitol. Res. 77:359-361. [DOI] [PubMed] [Google Scholar]
- 99.Gottstein, B. 1992. Echinococcus multilocularis infection: immunology and immunodiagnosis. Adv. Parasitol. 31:321-380. [DOI] [PubMed] [Google Scholar]
- 100.Gottstein, B. 1992. Molecular and immunological diagnosis of echinococcosis. Clin. Microbiol. Rev. 5:248-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gottstein, B. 1985. Purification and characterization of a specific antigen from Echinococcus multilocularis. Parasite Immunol. 7:201-212. [DOI] [PubMed] [Google Scholar]
- 102.Gottstein, B., P. Deplazes, J. Eckert, B. Muller, E. Schott, O. Helle, P. Boujon, K. Wolff, A. Wandeler, U. Schwiete, and H. Moegle. 1991. Serological (Em2-ELISA) and parasitological examinations of fox populations for Echinococcus multilocularis infections. Zentlbl. Veterinaermed. Reihe B 38:161-168. [DOI] [PubMed] [Google Scholar]
- 103.Gottstein, B., P. Jacquier, S. Bresson-Hadni, and J. Eckert. 1993. Improved primary immunodiagnosis of alveolar echinococcosis in humans by an enzyme-linked immunosorbent assay using the Em2plus antigen. J. Clin. Microbiol. 31:373-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grant, W. N. 1994. Genetic variation in parasitic nematodes and its implications. Int. J. Parasitol. 24:821-830. [DOI] [PubMed] [Google Scholar]
- 105.Grant, W. N., and G. E. Whitington. 1994. Extensive DNA polymorphism within and between two strains of Trichostrongylus colubriformis. Int. J. Parasitol. 24:719-725. [DOI] [PubMed] [Google Scholar]
- 106.Guerret, S., D. A. Vuitton, M. Liance, C. Pater, and J. P. Carbillet. 1998. Echinococcus multilocularis: relationship between susceptibility/resistance and liver fibrogenesis in experimental mice. Parasitol. Res. 84:657-667. [DOI] [PubMed] [Google Scholar]
- 107.Guerri, M. L., M. Davila, M. Rodriguez, F. Javier Nieto, and C. Ladron de Guevara. 2000. Utility of IgG subclasses in the diagnosis and follow up of hydatidosis. Enferm. Infecc. Microbiol. Clin. 18:262-2666. (In Spanish.) [PubMed] [Google Scholar]
- 108.Hanna, R. E. 1980. Fasciola hepatica: an immunofluorescent study of antigenic changes in the tegument during development in the rat and the sheep. Exp. Parasitol. 50:155-170. [DOI] [PubMed] [Google Scholar]
- 109.Hanna, R. E. 1980. Fasciola hepatica: autoradiography of protein synthesis, transport, and secretion by the tegument. Exp. Parasitol. 50:297-304. [DOI] [PubMed] [Google Scholar]
- 110.Hanna, R. E. 1980. Fasciola hepatica: glycocalyx replacement in the juvenile as a possible mechanism for protection against host immunity. Exp. Parasitol. 50:103-114. [DOI] [PubMed] [Google Scholar]
- 111.Haralabidis, S., E. Karagouni, S. Frydas, and E. Dotsika. 1995. Immunoglobulin and cytokine profile in murine secondary hydatidosis. Parasite Immunol. 17:625-630. [DOI] [PubMed] [Google Scholar]
- 112.Haynes, A. P., and J. Fletcher. 1990. Neutrophil function tests. Baillieres Clin. Haematol. 3:871-887. [DOI] [PubMed] [Google Scholar]
- 113.Heath, D. D. 1986. Immunobiology of Echinococcus granulosus, p. 164-188. In R. C. A. Thompson (ed.), The biology of Echinococcus and hydatid disease. George Allen and Unwin, London, United Kingdom.
- 114.Heath, D. D. 1995. Immunology of Echinococcus infections, p. 183-232. In R. C. A. Thompson and A. J. Lymbery (ed.), The biology of Echinococcus and hydatid disease. CAB International, Wallingford, United Kingdom.
- 115.Heath, D. D., and B. Holcman. 1997. Vaccination against Echinococcus in perspective. Acta Trop. 67:37-41. [DOI] [PubMed] [Google Scholar]
- 116.Heath, D. D., B. Holcman, and R. J. Shaw. 1994. Echinococcus granulosus: the mechanism of oncosphere lysis by sheep complement and antibody. Int. J. Parasitol. 24:929-935. [DOI] [PubMed] [Google Scholar]
- 117.Heath, D. D., and S. B. Lawrence. 1996. Antigenic polypeptides of Echinococcus granulosus oncospheres and definition of protective molecules. Parasite Immunol. 18:347-357. [DOI] [PubMed] [Google Scholar]
- 118.Heath, D. D., S. B. Lawrence, and W. K. Yong. 1992. Echinococcus granulosus in sheep: transfer from ewe to lamb of “Arc 5' antibodies and oncosphere-killing activity, but not protection. Int. J. Parasitol. 22:1017-1021. [DOI] [PubMed] [Google Scholar]
- 119.Heath, D. D., S. N. Parmeter, P. J. Osborn, and S. B. Lawrence 1981. Resistance to Echinococcus granulosus infection in lambs. J. Parasitol. 67:797-799. [PubMed] [Google Scholar]
- 120.Helbig, M., P. Frosch, P. Kern, and M. Frosch. 1993. Serological differentiation between cystic and alveolar echinococcosis by use of recombinant larval antigens. J. Clin. Microbiol. 31:3211-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Herd, R. P. 1976. The cestocidal effect of complement in normal and immune sera in vitro. Parasitology 72:325-334. [DOI] [PubMed] [Google Scholar]
- 122.Herd, R. P. 1977. Resistance of dogs to Echinococcus granulosus. Int. J. Parasitol. 7:135-138. [DOI] [PubMed] [Google Scholar]
- 123.Herd, R. P., R. J. Chappel, and D. Biddell. 1975. Immunization of dogs against Echinococcus granulosus using worm secretory antigens. Int. J. Parasitol. 5:395-399. [DOI] [PubMed] [Google Scholar]
- 124.Hernandez, A., and A. Nieto. 1994. Induction of protective immunity against murine secondary hydatidosis. Parasite Immunol. 16:537-544. [DOI] [PubMed] [Google Scholar]
- 125.Hernandez-Pomi, A., R. Borras-Salvador, and A. Mir-Gisbert. 1997. Analysis of cytokine and specific antibody profiles in hydatid patients with primary infection and relapse of disease. Parasite Immunol. 19:553-561. [DOI] [PubMed] [Google Scholar]
- 126.Holcman, B., and D. D. Heath. 1997. The early stages of Echinococcus granulosus development. Acta Trop. 64:5-17. [DOI] [PubMed] [Google Scholar]
- 127.Hotez, P. J., J. Haggerty, J. Hawdon, L. Milstone, H. R. Gamble, G. Schad, and F. Richards. 1990. Metalloproteases of infective Ancylostoma hookworm larvae and their possible functions in tissue invasion and ecdysis. Infect. Immun. 58:3883-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hotez, P. J., N. L. Trang, J. H. McKerrow, and A. Cerami. 1985. Isolation and characterization of a proteolytic enzyme from the adult hookworm Ancylostoma caninum. J. Biol. Chem. 260:7343-7348. [PubMed] [Google Scholar]
- 129.Ioppolo, S., S. Notargiacomo, E. Profumo, C. Franchi, E. Ortona, R. Rigano, and A. Siracusano. 1996. Immunological responses to antigen B from Echinococcus granulosus cyst fluid in hydatid patients. Parasite Immunol. 18:571-578. [DOI] [PubMed] [Google Scholar]
- 130.Irabuena, O., A. Nieto, A. M. Ferreira, J. Battistoni, and G. Ferragut. 2000. Characterization and optimization of bovine Echinococcus granulosus cyst fluid to be used in immunodiagnosis of hydatid disease by ELISA. Rev. Inst. Med. Trop. Sao Paulo 42:255-262. [DOI] [PubMed] [Google Scholar]
- 131.Ito, A., L. Ma, M. Itoh, S. Y. Cho, Y. Kong, S. Y. Kang, T. Horii, X. L. Pang, M. Okamoto, T. Yamashita, M. W. Lightowlers, X. G. Wang, and Y. H. Liu. 1997. Immunodiagnosis of alveolar echinococcosis by enzyme-linked immunosorbent assay using a partially purified Em18/16 enriched fraction. Clin. Diagn. Lab. Immunol. 4:57-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ito, A., L. Ma, P. M. Schantz, B. Gottstein, Y. H. Liu, J. J. Chai, S. K. Abdel-Hafez, N. Altintas, D. D. Joshi, M. W. Lightowlers, and Z. S. Pawlowski. 1999. Differential serodiagnosis for cystic and alveolar echinococcosis using fractions of Echinococcus granulosus cyst fluid (antigen B) and E. multilocularis protoscolex (Em18). Am. J. Trop. Med. Hyg. 60:188-192. [DOI] [PubMed] [Google Scholar]
- 133.Ito, A., M. Nakao, H. Kutsumi, M. W. Lightowlers, M. Itoh, and S. Sato. 1993. Serodiagnosis of alveolar hydatid disease by western blotting. Trans. R. Soc. Trop. Med. Hyg. 87:170-172. [DOI] [PubMed] [Google Scholar]
- 134.Ito, A., P. M. Schantz, and J. F. Wilson. 1995. Em18, a new serodiagnostic marker for differentiation of active and inactive cases of alveolar hydatid disease. Am. J. Trop. Med. Hyg. 52:41-44. [DOI] [PubMed] [Google Scholar]
- 135.Ito, A., X. G. Wang, and Y. H. Liu. 1993. Differential serodiagnosis of alveolar and cystic hydatid disease in the People's Republic of China. Am. J. Trop. Med. Hyg. 49:208-213. [DOI] [PubMed] [Google Scholar]
- 136.Ito, A., H. Wen, P. S. Craig, L. Ma, M. Nakao, T. Horii, X. L. Pang, M. Okamoto, M. Itoh, Y. Osawa, X. G. Wang, and Y. H. Liu. 1997. Antibody responses against Em18 and Em16 serodiagnostic markers in alveolar and cystic echinococcosis patients from northwest China. Jpn. J. Med. Sci. Biol. 50:19-26. [DOI] [PubMed] [Google Scholar]
- 137.Jenkins, D. J., A. Fraser, H. Bradshaw, and P. S. Craig. 2000. Detection of Echinococcus granulosus coproantigens in Australian canids with natural or experimental infection. J. Parasitol. 86:140-145. [DOI] [PubMed] [Google Scholar]
- 138.Jenkins, D. J., and M. D. Rickard. 1984. Haematological and serological data from dogs raised worm-free and monospecifically infected with helminths. Aust. Vet. J. 61:309-311. [DOI] [PubMed] [Google Scholar]
- 139.Jenkins, D. J., and M. D. Rickard. 1986. Specific antibody responses in dogs experimentally infected with Echinococcus granulosus. Am. J. Trop. Med. Hyg. 35:345-349. [DOI] [PubMed] [Google Scholar]
- 140.Jenkins, D. J., and M. D. Rickard. 1985. Specific antibody responses to Taenia hydatigena, Taenia pisiformis and Echinococcus granulosus infection in dogs. Aust. Vet. J. 62:72-78. [DOI] [PubMed] [Google Scholar]
- 141.Jenkins, D. J., and M. D. Rickard. 1986. Specificity of scolex and oncosphere antigens for the serological diagnosis of taeniid cestode infections in dogs. Aust. Vet. J. 63:40-42. [DOI] [PubMed] [Google Scholar]
- 142.Jenkins, P., J. B. Dixon, N. K. Rakha, and S. D. Carter. 1990. Regulation of macrophage-mediated larvicidal activity in Echinococcus granulosus and Mesocestoides corti (Cestoda) infection in mice. Parasitology 100:309-315. [DOI] [PubMed] [Google Scholar]
- 143.Jiang, L., H. Wen, and A. Ito. 2001. Immunodiagnostic differentiation of alveolar and cystic echinococcosis using ELISA test with 18-kDa antigen extracted from Echinococcus protoscoleces. Trans. R. Soc. Trop. Med. Hyg. 95:285-288. [DOI] [PubMed] [Google Scholar]
- 144.Jones, W. O., D. L. Emery, S. J. McClure, and B. M. Wagland. 1994. Changes in inflammatory mediators and larval inhibitory activity in intestinal contents and mucus during primary and challenge infections of sheep with Trichostrongylus colubriformis. Int. J. Parasitol. 24:519-525. [DOI] [PubMed] [Google Scholar]
- 145.Judson, D. G., J. B. Dixon, M. J. Clarkson, and J. Pritchard. 1985. Ovine hydatidosis: some immunological characteristics of the seronegative host. Parasitology 91:349-357. [DOI] [PubMed] [Google Scholar]
- 146.Kamhawi, S., N. Hijjawi, A. Abu-Gazaleh, and M. Abbass. 1995. Prevalence of hydatid cysts in livestock from five regions of Jordan. Ann. Trop. Med. Parasitol. 89:621-629. [DOI] [PubMed] [Google Scholar]
- 147.Kamiya, M., and H. Sato. 1990. Survival, strobilation and sexual maturation of Echinococcus multilocularis in the small intestine of golden hamsters. Parasitology 100:125-130. [DOI] [PubMed] [Google Scholar]
- 148.Kanazawa, T., H. Asahi, H. Hata, K. Mochida, N. Kagei, and M. J. Stadecker. 1993. Arginine-dependent generation of reactive nitrogen intermediates is instrumental in the in vitro killing of protoscoleces of Echinococcus multilocularis by activated macrophages. Parasite Immunol. 15:619-623. [DOI] [PubMed] [Google Scholar]
- 149.Kanwar, J. R., R. K. Kanwar, A. S. Grewal, and V. K. Vinayak. 1994. Significance of detection of immune-complexed 8 kDa hydatid-specific antigen for immunodiagnosis of hydatidosis. FEMS Immunol. Med. Microbiol. 9:231-236. [DOI] [PubMed] [Google Scholar]
- 150.Kassis, A. I., and C. E. Tanner. 1977. Echinococcus multilocularis: complement's role in vivo in hydatid disease. Exp. Parasitol. 43:390-395. [DOI] [PubMed] [Google Scholar]
- 151.Kassis, A. I., and C. E. Tanner. 1977. Host serum proteins in Echinococcus multilocularis: complement activation via the classical pathway. Immunology 33:1-9. [PMC free article] [PubMed] [Google Scholar]
- 152.Kassis, A. I., and C. E. Tanner. 1976. The role of complement in hydatid disease: in vitro studies. Int. J. Parasitol. 6:25-35. [DOI] [PubMed] [Google Scholar]
- 153.King, C. L., C. C. Low, and T. B. Nutman. 1993. IgE production in human helminth infection. Reciprocal interrelationship between IL-4 and IFN-gamma. J. Immunol. 150:1873-1880. [PubMed] [Google Scholar]
- 154.King, C. L., and T. B. Nutman. 1993. IgE and IgG subclass regulation by IL-4 and IFN-gamma in human helminth infections. Assessment by B cell precursor frequencies. J. Immunol. 151:458-465. [PubMed] [Google Scholar]
- 155.Kizaki, T., M. Ishige, W. Bingyan, N. K. Day, R. A. Good, and K. Onoe. 1993. Generation of CD8+ suppressor T cells by protoscoleces of Echinococcus multilocularis in vitro. Immunology 79:412-417. [PMC free article] [PubMed] [Google Scholar]
- 156.Kizaki, T., M. Ishige, S. Kobayashi, W. Bingyan, M. Kumagai, N. K. Day, R. A. Good, and K. Onoe. 1993. Suppression of T-cell proliferation by CD8+ T cells induced in the presence of protoscolices of Echinococcus multilocularis in vitro. Infect. Immun. 61:525-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kizaki, T., S. Kobayashi, K. Ogasawara, N. K. Day, R. A. Good, and K. Onoe. 1991. Immune suppression induced by protoscoleces of Echinococcus multilocularis in mice. Evidence for the presence of CD8dull suppressor cells in spleens of mice intraperitoneally infected with E. multilocularis. J. Immunol. 147:1659-1666. [PubMed] [Google Scholar]
- 158.Kong, Y., Y. B. Chung, S. Y. Cho, and S. Y. Kang. 1994. Cleavage of immunoglobulin G by excretory-secretory cathepsin S-like protease of Spirometra mansoni plerocercoid. Parasitology 109:611-621. [DOI] [PubMed] [Google Scholar]
- 159.Lange, A. M., W. Yutanawiboonchai, P. Scott, and D. Abraham. 1994. IL-4- and IL-5-dependent protective immunity to Onchocerca volvulus infective larvae in BALB/cBYJ mice. J. Immunol. 153:205-211. [PubMed] [Google Scholar]
- 160.Lauriola, L., M. Piantelli, R. Pozzuoli, E. Arru, and P. Musiani. 1978. Echinococcus granulosus: preparation of monosepcific antisera against antigens in sheep hydatid fluid. Zentbl. Bakteriol. Mikrobiol. Hyg. Orig. A 240:251-257. [PubMed] [Google Scholar]
- 161.Leggatt, G. R., and D. P. McManus. 1994. Identification and diagnostic value of a major antibody epitope on the 12 kDa antigen from Echinococcus granulosus (hydatid disease) cyst fluid. Parasite Immunol. 16:87-96. [DOI] [PubMed] [Google Scholar]
- 162.Leggatt, G. R., W. Yang, and D. P. McManus. 1992. Serological evaluation of the 12 kDa subunit of antigen B in Echinococcus granulosus cyst fluid by immunoblot analysis. Trans. R. Soc. Trop. Med. Hyg. 86:189-192. [DOI] [PubMed] [Google Scholar]
- 163.Lightowlers, M. W. 1990. Cestode infections in animals: immunological diagnosis and vaccination. Rev. Sci. Technol. 9:463-487. [DOI] [PubMed] [Google Scholar]
- 164.Lightowlers, M. W., A. Flisser, C. G. Gauci, D. D. Heath, O. Jensen, and R. Rolfe. 2000. Vaccination against cysticercosis and hydatid disease. Parasitol. Today 16:191-196. [DOI] [PubMed] [Google Scholar]
- 165.Lightowlers, M. W., and B. Gottstein. 1995. Echinococcosis/hydatidosis: antigens, immunological and molecular diagnosis, p. 355-410. In R. C. A. Thompson and A. J. Lymbery (ed.), The biology of Echinococcus and hydatid disease. CAB International, Wallingford, United Kingdom.
- 166.Lightowlers, M. W., O. Jensen, E. Fernandez, J. A. Iriarte, D. J. Woollard, C. G. Gauci, D. J. Jenkins, and D. D. Heath. 1999. Vaccination trials in Australia and Argentina confirm the effectiveness of the EG95 hydatid vaccine in sheep. Int. J. Parasitol. 29:531-534. [DOI] [PubMed] [Google Scholar]
- 167.Lightowlers, M. W., S. B. Lawrence, C. G. Gauci, J. Young, M. J. Ralston, D. Maas, and D. D. Heath. 1996. Vaccination against hydatidosis using a defined recombinant antigen. Parasite Immunol. 18:457-462. [DOI] [PubMed] [Google Scholar]
- 168.Lightowlers, M. W., D. Y. Liu, A. Haralambous, and M. D. Rickard. 1989. Subunit composition and specificity of the major cyst fluid antigens of Echinococcus granulosus. Mol. Biochem. Parasitol. 37:171-182. [DOI] [PubMed] [Google Scholar]
- 169.Lightowlers, M. W., M. D. Rickard, and R. D. Honey. 1986. Serum antibody response following parenteral immunization with hydatid cyst fluid in sheep infected with Echinococcus granulosus. Am. J. Trop. Med. Hyg. 35:818-823. [DOI] [PubMed] [Google Scholar]
- 170.Lightowlers, M. W., M. D. Rickard, R. D. Honey, D. L. Obendorf, and G. F. Mitchell. 1984. Serological diagnosis of Echinococcus granulosus infection in sheep using cyst fluid antigen processed by antibody affinity chromatography. Aust. Vet. J. 61:101-108. [DOI] [PubMed] [Google Scholar]
- 171.Liu, D., M. D. Rickard, and M. W. Lightowlers. 1993. Assessment of monoclonal antibodies to Echinococcus granulosus antigen 5 and antigen B for detection of human hydatid circulating antigens. Parasitology 106:75-81. [DOI] [PubMed] [Google Scholar]
- 172.Lloyd, S. 1987. Cysticercosis, vol. II. CRC Press, Inc., Boca Raton, Fla.
- 173.Ma, L., A. Ito, Y. H. Liu, X. G. Wang, Y. Q. Yao, D. G. Yu, and Y. T. Chen. 1997. Alveolar echinococcosis: Em2plus-ELISA and Em18-western blots for follow-up after treatment with albendazole. Trans. R. Soc. Trop. Med. Hyg. 91:476-478. [DOI] [PubMed] [Google Scholar]
- 174.Mackenzie, C. D. 1984. Sequestration—beneficial to both host and parasite. Parasitology 88:593-595. [DOI] [PubMed] [Google Scholar]
- 175.Marco, M., and A. Nieto. 1991. Metalloproteinases in the larvae of Echinococcus granulosus. Int. J. Parasitol. 21:743-746. [DOI] [PubMed] [Google Scholar]
- 176.Margutti, P., E. Ortona, S. Vaccari, S. Barca, R. Rigano, A. Teggi, F. Muhlschlegel, M. Frosch, and A. Siracusano. 1999. Cloning and expression of a cDNA encoding an elongation factor 1beta/delta protein from Echinococcus granulosus with immunogenic activity. Parasite Immunol. 21:485-492. [DOI] [PubMed] [Google Scholar]
- 177.McManus, D. P., and C. Bryant. 1995. Biochemistry, physiology and molecular biology of Echinococcus, p. 355-410. In R. C. A Thompson and A. J. Lymbery (ed.), The biology of Echinococcus and hydatid disease. CAB International, Wallingford, United Kingdom.
- 178.McVie, A., K. Ersfeld, M. T. Rogan, and P. S. Craig. 1997. Expression and immunological characterisation of Echinococcus granulosus recombinant antigen B for IgG4 subclass detection in human cystic echinococcosis. Acta Trop. 67:19-35. [DOI] [PubMed] [Google Scholar]
- 179.Meeusen, E. N., and A. Balic. 2000. Do eosinophils have a role in the killing of helminth parasites? Parasitol. Today 16:95-101. [DOI] [PubMed] [Google Scholar]
- 180.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 181.Movsesijan, M., and Z. Mladenovic. 1970. Active immunisation of dogs against Echinococcus granulosus. Vet. Glas. 24:189-193. [Google Scholar]
- 182.Movsesijan, M., A. Sokolic, and Z. Mladenovic. 1968. Studies on the immunological potentiality of irradiated Echinococcus granulosus forms: immunization experiments in dogs. Br. Vet. J. 124:425-432. [DOI] [PubMed] [Google Scholar]
- 183.Musiani, P., M. Piantelli, L. Lauriola, E. Arru, and R. Pozzuoli. 1978. Echinococcus granulosus: specific quantification of the two most immunoreactive antigens in hydatid fluids. J. Clin. Pathol. 31:475-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nonaka, N., M. Iida, K. Yagi, T. Ito, H. K. Ooi, Y. Oku, and M. Kamiya. 1996. Time course of coproantigen excretion in Echinococcus multilocularis infections in foxes and an alternative definitive host, golden hamsters. Int. J. Parasitol. 26:1271-1278. [DOI] [PubMed] [Google Scholar]
- 185.Oriol, C., and R. Oriol. 1975. Physiocochemical properties of a lipoprotein antigen of Echinococcus granulosus. Am. J. Trop. Med. Hyg. 24:96-100. [DOI] [PubMed] [Google Scholar]
- 186.Oriol, R., J. F. Williams, M. V. Perez Esandi, and C. Oriol. 1971. Purification of lipoprotein antigens of Echinococcus granulosus from sheep hydatid fluid. Am. J. Trop. Med. Hyg. 20:569-574. [DOI] [PubMed] [Google Scholar]
- 187.Ortona, E., P. Margutti, S. Vaccari, R. Rigano, E. Profumo, B. Buttari, A. Chersi, A. Teggi, and A. Siracusano. 2001. Elongation factor 1 beta/delta of Echinococcus granulosus and allergic manifestations in human cystic echinococcosis. Clin. Exp. Immunol. 125:110-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ortona, E., R. Rigano, P. Margutti, S. Notargiacomo, S. Ioppolo, S. Vaccari, S. Barca, B. Buttari, E. Profumo, A. Teggi, and A. Siracusano. 2000. Native and recombinant antigens in the immunodiagnosis of human cystic echinococcosis. Parasite Immunol. 22:553-559. [DOI] [PubMed] [Google Scholar]
- 189.Osborn, P. J., and D. D. Heath. 1982. Immunisation of lambs against Echinococcus granulosus using antigens obtained by incubation of oncospheres in vitro. Res. Vet. Sci. 33:132-133. [PubMed] [Google Scholar]
- 190.Pater, C., V. Muller, S. Harraga, M. Liance, V. Godot, J. P. Carbillet, D. Meillet, T. Romig, and D. A. Vuitton. 1998. Intestinal and systemic humoral immunological events in the susceptible Balb/C mouse strain after oral administration of Echinococcus multilocularis eggs. Parasite Immunol. 20:623-629. [DOI] [PubMed] [Google Scholar]
- 191.Pauluzzi, S., and F. De Rosa. 1969. L'idatidosi sperimentale. V. Vaccinoprofilassi contro l'idatidosi sperimentale secondaria da Echinococcus granulosus del topo BALB/c. Ann. Sclavo 11:518-530. [Google Scholar]
- 192.Pawlowski, I. D., J. Eckert, D. A. Vuitton, R. W. Ammann, P. Kern, P. S. Craig, K. F. Dar, F. De Rosa, C. Filice, B. Gottstein, F. Grimm, C. N. L. MacPherson, N. Sato, T. Todorov, J. Uchino, W. von Sinner, and H. Wen. 2001. Echinococcosis in humans: clinical aspects, diagnosis and treatment, p. 20-71. In J. Eckert, M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.), WHOI/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France.
- 193.Pearce, E. J., P. Caspar, J. M. Grzych, F. A. Lewis, and A. Sher. 1991. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J. Exp. Med. 173:159-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pennoit-De Cooman, E., P. H. De Rycke, and E. J. Van Outryve. 1974. Experimental secondary echinococcosis of Echinococcus granulosus. V. Quantitative aspects of the infection rate. Tropenmed. Parasitol. 25:338-344. [PubMed] [Google Scholar]
- 195.Petrova, R. F. 1968. Blood picture in experimental hydatidosis in sheep. Meteriali Seminara-Soveshch. Borbe Gel'mint. Zhivot. Chimk. Alma-Ala p. 115-116. (In Russian.)
- 196.Piantelli, M., R. Pozzuoli, E. Arru, and P. Musiani. 1977. Echinococcus granulosus: identification of subunits of the major antigens. J. Immunol. 119:1382-1386. [PubMed] [Google Scholar]
- 197.Pinon, J. M., J. Poirriez, H. Lepan, R. Geers, R. Penna, and D. Fernandez. 1987. Value of isotypic characterization of antibodies to Echinococcus granulosus by enzyme-linked immuno-filtration assay. Eur. J. Clin. Microbiol. 6:291-295. [DOI] [PubMed] [Google Scholar]
- 198.Playford, M. C., and M. Kamiya. 1992. Immune response to Echinococcus multilocularis infection in the mouse model: a review. Jpn. J. Vet. Res. 40:113-130. [PubMed] [Google Scholar]
- 199.Poretti, D., E. Felleisen, F. Grimm, M. Pfister, F. Teuscher, C. Zuercher, J. Reichen, and B. Gottstein. 1999. Differential immunodiagnosis between cystic hydatid disease and other cross-reactive pathologies. Am. J. Trop. Med. Hyg. 60:193-198. [DOI] [PubMed] [Google Scholar]
- 200.Pozzuoli, R., P. Musiani, E. Arru, C. Patrono, and M. Piantelli. 1974. Echinococcus granulosus: evaluation of purified antigens immunoreactivity. Exp. Parasitol. 35:52-60. [DOI] [PubMed] [Google Scholar]
- 201.Pozzuoli, R., M. Piantelli, C. Perucci, E. Arru, and P. Musiani. 1975. Isolation of the most immunoreactive antigenes of Echinococcus granulosus from sheep hydatid fluid. J. Immunol. 115:1459-1463. [PubMed] [Google Scholar]
- 202.Pratt, D., R. J. Boisvenue, and G. N. Cox. 1992. Isolation of putative cysteine protease genes of Ostertagia ostertagi. Mol. Biochem. Parasitol. 56:39-48. [DOI] [PubMed] [Google Scholar]
- 203.Provenzano, D., and J. F. Alderete. 1995. Analysis of human immunoglobulin-degrading cysteine proteinases of Trichomonas vaginalis. Infect. Immun. 63:3388-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rainbird, M. A., D. Macmillan, and E. N. Meeusen. 1998. Eosinophil-mediated killing of Haemonchus contortus larvae: effect of eosinophil activation and role of antibody, complement and interleukin-5. Parasite Immunol. 20:93-103. [DOI] [PubMed] [Google Scholar]
- 205.Rakha, N. K., J. B. Dixon, S. D. Carter, P. S. Craig, P. Jenkins, and S. Folkard. 1991. Echinococcus multilocularis antigens modify accessory cell function of macrophages. Immunology 74:652-656. [PMC free article] [PubMed] [Google Scholar]
- 206.Ramzy, R. M., H. Helmy, E. A. El Zayyat, M. M. Rifaat, D. M. Abdel Hameed, and M. H. Abdel-Baki. 1999. An enzyme-linked immunosorbent assay for detection of IgG1 antibodies specific to human cystic echinococcosis in Egypt. Trop. Med. Int. Health 4:616-620. [DOI] [PubMed] [Google Scholar]
- 207.Ravinder, P. T., S. C. Parija, and K. S. Rao. 1997. Evaluation of human hydatid disease before and after surgery and chemotherapy by demonstration of hydatid antigens and antibodies in serum. J. Med. Microbiol. 46:859-864. [DOI] [PubMed] [Google Scholar]
- 208.Richards, K. S., C. Arme, and J. F. Bridges. 1983. Echinococcus granulosus equinus: an ultrastructural study of murine tissue response to hydatid cysts. Parasitology 86:407-417. [DOI] [PubMed] [Google Scholar]
- 209.Rickard, M. D., and M. Lightowlers. 1986. Immunodiagnosis of hydatid disease, p. 217-249. In R. C. A. Thompson (ed.), The biology of Echinococcus and hydatid disease. George Allen and Unwin, London, United Kingdom.
- 210.Rickard, M. D., and J. F. Williams. 1982. Hydatidosis/cysticercosis: immune mechanisms and immunization against infection. Adv. Parasitol. 21:229-296. [DOI] [PubMed] [Google Scholar]
- 211.Riffkin, M., H. F. Seow, D. Jackson, L. Brown, and P. Wood. 1996. Defence against the immune barrage: helminth survival strategies. Immunol. Cell Biol. 74:564-574. [DOI] [PubMed] [Google Scholar]
- 212.Rigano, R., E. Profumo, F. Bruschi, G. Carulli, A. Azzara, S. Ioppolo, B. Buttari, E. Ortona, P. Margutti, A. Teggi, and A. Siracusano. 2001. Modulation of human immune response by Echinococcus granulosus antigen B and its possible role in evading host defenses. Infect. Immun. 69:288-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rigano, R., E. Profumo, B. Buttari, A. Teggi, and A. Siracusano. 1999. Cytokine gene expression in peripheral blood mononuclear cells (PBMC) from patients with pharmacologically treated cystic echinococcosis. Clin. Exp. Immunol. 118:95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rigano, R., E. Profumo, G. Di Felice, E. Ortona, A. Teggi, and A. Siracusano. 1995. In vitro production of cytokines by peripheral blood mononuclear cells from hydatid patients. Clin. Exp. Immunol. 99:433-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rigano, R., E. Profumo, S. Ioppolo, S. Notargiacomo, E. Ortona, A. Teggi, and A. Siracusano. 1995. Immunological markers indicating the effectiveness of pharmacological treatment in human hydatid disease. Clin. Exp. Immunol. 102:281-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rigano, R., E. Profumo, S. Ioppolo, S. Notargiacomo, A. Teggi, and A. Siracusano. 1998. Cytokine patterns in seropositive and seronegative patients with Echinococcus granulosus infection. Immunol. Lett. 64:5-8. [DOI] [PubMed] [Google Scholar]
- 217.Rigano, R., E. Profumo, S. Ioppolo, S. Notargiacomo, A. Teggi, and A. Siracusano. 1999. Serum cytokine detection in the clinical follow up of patients with cystic echinococcosis. Clin. Exp. Immunol. 115:503-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rigano, R., E. Profumo, and A. Siracusano. 1997. New perspectives in the immunology of Echinococcus granulosus infection. Parassitologia 39:275-277. [PubMed] [Google Scholar]
- 219.Rigano, R., E. Profumo, A. Teggi, and A. Siracusano. 1996. Production of IL-5 and IL-6 by peripheral blood mononuclear cells (PBMC) from patients with Echinococcus granulosus infection. Clin. Exp. Immunol. 105:456-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Riley, E. M., and J. B. Dixon. 1987. Experimental Echinococcus granulosus infection in mice: immunocytochemical analysis of lymphocyte populations in local lymphoid infections during early infection. Parasitology 94:523-532. [DOI] [PubMed] [Google Scholar]
- 221.Riley, E. M., J. B. Dixon, P. Jenkins, and G. Ross. 1986. Echinococcus granulosus infection in mice: host responses during primary and secondary infection. Parasitology 92:391-403. [DOI] [PubMed] [Google Scholar]
- 222.Riley, E. M., J. B. Dixon, D. F. Kelly, and D. A. Cox. 1985. The immune response to Echinococcus granulosus: sequential histological observations of lymphoreticular and connective tissues during early murine infection. J. Comp. Pathol. 95:93-104. [DOI] [PubMed] [Google Scholar]
- 223.Robinson, R. D., and C. Arme. 1986. Echinococcus granulosus: effect of protoscoleces on murine activation. Helminthologia 23:210. [Google Scholar]
- 224.Rogan, M. T. 1988. Observations on the origin of daughter cysts within hydatid cysts of Echinococcus granulosus. Ann. Trop. Med. Parasitol. 82:405-406. [DOI] [PubMed] [Google Scholar]
- 225.Rogan, M. T., and P. S. Craig. 1997. Immunology of Echinococcus granulosus infections. Acta Trop. 67:7-17. [DOI] [PubMed] [Google Scholar]
- 226.Rogan, M. T., P. S. Craig, E. Zehyle, G. Masinde, H. Wen, and P. Zhou. 1992. In vitro killing of taeniid oncospheres, mediated by human sera from hydatid endemic areas. Acta Trop. 51:291-296. [DOI] [PubMed] [Google Scholar]
- 227.Rogan, M. T., I. Marshall, G. D. Reid, C. N. Macpherson, and P. S. Craig. 1993. The potential of vervet monkeys (Cercopithecus aethiops) and baboons (Papio anubis) as models for the study of the immunology of Echinococcus granulosus infections. Parasitology 106:511-517. [DOI] [PubMed] [Google Scholar]
- 228.Roneus, O., D. Christensson, and N. G. Nilsson. 1982. The longevity of hydatid cysts in horses. Vet. Parasitol. 11:149-154. [DOI] [PubMed] [Google Scholar]
- 229.Rott, M. B., V. Fernandez, S. Farias, J. Ceni, H. B. Ferreira, K. L. Haag, and A. Zaha. 2000. Comparative analysis of two different subunits of antigen B from Echinococcus granulosus: gene sequences, expression in Escherichia coli and serological evaluation. Acta Trop. 75:331-340. [DOI] [PubMed] [Google Scholar]
- 230.Rozenzvit, M. C., L. H. Zhang, L. Kamenetzky, S. G. Canova, E. A. Guarnera, and D. P. McManus. 1999. Genetic variation and epidemiology of Echinococcus granulosus in Argentina. Parasitology 118:523-530. [DOI] [PubMed] [Google Scholar]
- 231.Sarciron, E. M., S. Bresson-Hadni, M. Mercier, P. Lawton, C. Duranton, D. Lenys, A. F. Petavy, and D. A. Vuitton. 1997. Antibodies against Echinococcus multilocularis alkaline phosphatase as markers for the specific diagnosis and the serological monitoring of alveolar echinococcosis. Parasite Immunol. 19:61-68. [DOI] [PubMed] [Google Scholar]
- 232.Schantz, P. M., and B. Gottstein. 1986. Echinococcosis (hydatidosis), vol. 1. Academic Press, Inc., Orlando, Fla.
- 233.Shambesh, M. K., P. S. Craig, H. Wen, M. T. Rogan, and E. Paolillo. 1997. IgG1 and IgG4 serum antibody responses in asymptomatic and clinically expressed cystic echinococcosis patients. Acta Trop. 64:53-63. [DOI] [PubMed] [Google Scholar]
- 234.Shapiro, S. Z., G. M. Bahr, and P. R. Hira. 1992. Analysis of host components in hydatid cyst fluid and immunoblot diagnosis of human Echinococcus granulosus infection. Ann. Trop. Med. Parasitol. 86:503-509. [DOI] [PubMed] [Google Scholar]
- 235.Shepherd, J. C., A. Aitken, and D. P. McManus. 1991. A protein secreted in vivo by Echinococcus granulosus inhibits elastase activity and neutrophil chemotaxis. Mol. Biochem. Parasitol. 44:81-90. [DOI] [PubMed] [Google Scholar]
- 236.Shepherd, J. C., and D. P. McManus. 1987. Specific and cross-reactive antigens of Echinococcus granulosus hydatid cyst fluid. Mol. Biochem. Parasitol. 25:143-154. [DOI] [PubMed] [Google Scholar]
- 237.Siles-Lucas, M. M., and B. B. Gottstein. 2001. Molecular tools for the diagnosis of cystic and alveolar echinococcosis. Trop. Med. Int. Health 6:463-475. [DOI] [PubMed] [Google Scholar]
- 238.Singh, B. P., and D. N. Dhar. 1988. Indirect fluorescent antibody test for the detection of antibodies to Echinococcus granulosus in experimentally infected pups. Vet. Parasitol. 28:185-190. [DOI] [PubMed] [Google Scholar]
- 239.Sixl, W., E. Wisidagama, D. Stunzner, H. Withalm, and B. Sixl-Voigt. 1988. Serological examinations of dogs (Canis familiaris) in Colombo/Sri Lanka. Geogr. Med. Suppl. 1:89-92. [PubMed] [Google Scholar]
- 240.Slais, J., and M. Vanek. 1980. Tissue reaction to spherical and lobular hydatid cysts of Echinococcus granulosus (Batsch, 1786). Folia Parasitol. 27:135-143. [PubMed] [Google Scholar]
- 241.Spruance, S. L. 1974. Latent period of 53 years in a case of hydatid cyst disease. Arch. Intern. Med. 134:741-742. [PubMed] [Google Scholar]
- 242.Stankiewicz, M., W. E. Jonas, P. C. Douch, B. Rabel, S. Bisset, and W. Cabaj. 1993. Globule leukocytes in the lumen of the small intestine and the resistance status of sheep infected with parasitic nematodes. J. Parasitol. 79:940-945. [PubMed] [Google Scholar]
- 243.Sterla, S., H. Sato, and A. Nieto. 1999. Echinococcus granulosus human infection stimulates low avidity anticarbohydrate IgG2 and high avidity antipeptide IgG4 antibodies. Parasite Immunol. 21:27-34. [DOI] [PubMed] [Google Scholar]
- 244.Sturm, D., J. Menzel, B. Gottstein, and P. Kern. 1995. Interleukin-5 is the predominant cytokine produced by peripheral blood mononuclear cells in alveolar echinococcosis. Infect. Immun. 63:1688-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Thompson, C. R. A., and D. P. McManus. 2001. Aetiology: parasites and life-cycles, p. 1-19. In J. Eckert, M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.), WHOI/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France.
- 246.Thompson, R. C., L. M. Kumaratilake, and J. Eckert. 1984. Observations on Echinococcus granulosus of cattle origin in Switzerland. Int. J. Parasitol. 14:283-291. [DOI] [PubMed] [Google Scholar]
- 247.Torres Rodriguez, J. M., and C. Wisnivesky. 1978. Kinetics of the serological response in the experimental primary hydatid disease of mice infected with Echinococcus granulosus embryophores. Ann. Parasitol. Hum. Comp. 53:479-486. [PubMed] [Google Scholar]
- 248.Tort, J., P. J. Brindley, D. Knox, K. H. Wolfe, and J. P. Dalton. 1999. Proteinases and associated genes of parasitic helminths. Adv. Parasitol. 43:161-266. [DOI] [PubMed] [Google Scholar]
- 249.Touil-Boukoffa, C., B. Bauvois, J. Sanceau, B. Hamrioui, and J. Wietzerbin. 1998. Production of nitric oxide (NO) in human hydatidosis: relationship between nitrite production and interferon-gamma levels. Biochimie 80:739-744. [DOI] [PubMed] [Google Scholar]
- 250.Touil-Boukoffa, C., J. Sanceau, B. Tayebi, and J. Wietzerbin. 1997. Relationship among circulating interferon, tumor necrosis factor-alpha, and interleukin-6 and serologic reaction against parasitic antigen in human hydatidosis. J. Interferon Cytokine Res. 17:211-217. [DOI] [PubMed] [Google Scholar]
- 251.Turner, E. L., D. A. Berberian, and E. W. Dennis. 1936. The production of artificial immunity in dogs against Echinococcus granulosus. J. Parasitol. 22:14-28. [Google Scholar]
- 252.Turner, E. L., D. A. Berberian, and E. W. Dennis 1933. Successful artificial immunization of dogs against Taenia echinococcus. Proc. Soc. Exp. Biol. Med. 30:618-619. [Google Scholar]
- 253.Turner, E. L., E. W. Dennis, and D. A. Berberian. 1937. The production of artificial immunity against hydatid disease in sheep. J. Parasitol. 23:43-61. [Google Scholar]
- 254.Van Snick, J. 1990. Interleukin-6: an overview. Annu. Rev. Immunol. 8:253-278. [DOI] [PubMed] [Google Scholar]
- 255.Vuitton, D. A., S. Bresson-Hadni, L. Laroche, D. Kaiserlian, S. Guerret-Stocker, J. L. Bresson, and M. Gillet. 1989. Cellular immune response in Echinococcus multilocularis infection in humans. II. Natural killer cell activity and cell subpopulations in the blood and in the periparasitic granuloma of patients with alveolar echinococcosis. Clin. Exp. Immunol. 78:67-74. [PMC free article] [PubMed] [Google Scholar]
- 256.Wakelin, D. 1997. Immune response to Echinococcus infection: parasite avoidance and host protection. Parassitologia 39:355-358. [PubMed] [Google Scholar]
- 257.Wellinghausen, N., P. Gebert, and P. Kern. 1999. Interleukin (IL)-4, IL-10 and IL-12 profile in serum of patients with alveolar echinococcosis. Acta Trop. 73:165-174. [DOI] [PubMed] [Google Scholar]
- 258.Wen, H., and P. S. Craig. 1994. Immunoglobulin G subclass responses in human cystic and alveolar echinococcosis. Am. J. Trop. Med. Hyg. 51:741-748. [DOI] [PubMed] [Google Scholar]
- 259.Williams, J. F., M. V. Perez Esandi, and R. Oriol. 1971. Evaluation of purified lipoprotein antigens of Echinococcus granulosus in the immunodiagnosis of human infection. Am. J. Trop. Med. Hyg. 20:575-579. [DOI] [PubMed] [Google Scholar]
- 260.Willis, A. C., A. Diaz, A. Nieto, and R. B. Sim. 1997. Echinococcus granulosus antigen 5 may be a serine proteinase. Parasite Immunol. 19:385. [DOI] [PubMed] [Google Scholar]
- 261.Woollard, D. J., C. G. Gauci, D. D. Heath, and M. W. Lightowlers. 1998. Epitope specificities and antibody responses to the EG95 hydatid vaccine. Parasite Immunol. 20:535-540. [DOI] [PubMed] [Google Scholar]
- 262.Woollard, D. J., C. G. Gauci, D. D. Heath, and M. W. Lightowlers. 2000. Protection against hydatid disease induced with the EG95 vaccine is associated with conformational epitopes. Vaccine 19:498-507. [DOI] [PubMed] [Google Scholar]
- 263.Woollard, D. J., C. G. Gauci, and M. W. Lightowlers. 1999. Synthetic peptides induce antibody against a host-protective antigen of Echinococcus granulosus. Vaccine 18:785-794. [DOI] [PubMed] [Google Scholar]
- 264.Woollard, D. J., D. D. Heath, and M. W. Lightowlers. 2000. Assessment of protective immune responses against hydatid disease in sheep by immunization with synthetic peptide antigens. Parasitology 121:145-153. [DOI] [PubMed] [Google Scholar]
- 265.Yong, W. K., and D. D. Heath. 1979. ‘Arc 5' antibodies in sera of sheep infected with Echinococcus granulosus, Taenia hydatigena and Taenia ovis. Parasite Immunol. 1:27-38. [DOI] [PubMed] [Google Scholar]
- 266.Yong, W. K., D. D. Heath, and F. Van Knapen. 1984. Comparison of cestode antigens in an enzyme-linked immunosorbent assay for the diagnosis of Echinococcus granulosus, Taenia hydatigena and Taenia ovis infections in sheep. Res. Vet. Sci. 36:24-31. [PubMed] [Google Scholar]
- 267.Zarzosa, M. P., A. Orduna Domingo, P. Gutierrez, P. Alonso, M. Cuervo, A. Prado, M. A. Bratos, M. Garcia-Yuste, G. Ramos, and A. Rodriguez-Torres. 1999. Evaluation of six serological tests in diagnosis and postoperative control of pulmonary hydatid disease patients. Diagn. Microbiol. Infect. Dis. 35:255-262. [DOI] [PubMed] [Google Scholar]
- 268.Zhang, L., A. Eslami, S. H. Hosseini, and D. P. McManus. 1998. Indication of the presence of two distinct strains of Echinococcus granulosus in Iran by mitochondrial DNA markers. Am. J. Trop. Med. Hyg. 59:171-174. [DOI] [PubMed] [Google Scholar]
- 269.Zhang, L., R. B. Gasser, X. Zhu, and D. P. McManus. 1999. Screening for different genotypes of Echinococcus granulosus within China and Argentina by single-strand conformation polymorphism (SSCP) analysis. Trans. R. Soc. Trop. Med. Hyg. 93:329-334. [DOI] [PubMed] [Google Scholar]
- 270.Zhang, L. H., D. D. Joshi, and D. P. McManus. 2000. Three genotypes of Echinococcus granulosus identified in Nepal using mitochondrial DNA markers. Trans. R. Soc. Trop. Med. Hyg. 94:258-260. [DOI] [PubMed] [Google Scholar]
- 271.Zhang, L. H., and D. P. McManus. 1996. Purification and N-terminal amino acid sequencing of Echinococcus granulosus antigen 5. Parasite Immunol. 18:597-606. [DOI] [PubMed] [Google Scholar]
- 272.Zhang, W., H. You, Z. Zhang, G. Turson, A. Hasyet, and D. P. McManus. 2001. Further studies on an intermediate host murine model showing that a primary Echinococcus granulosus infection is protective against subsequent oncospheral challenge. Parasitol. Int. 50:279-283. [DOI] [PubMed] [Google Scholar]
- 273.Zhang, W. B., Z. Z. Zhang, H. Alili, and P.S. Chi. 1994. An investigation of factors influencing the hyperendemic of echinococcosis in Xinjiang. Chin. J. Zoonoses 10:204-205. (In Chinese.) [Google Scholar]
- 274.Zhang, W. B., Z. Z. Zhang, and P. S. Chi. 1999. Vaccination of dogs against Echinococcus granulosus using soluble antigens of protoscoleces. Chin. J. Vet. Sci. Technol. 29:21-22. (In Chinese.) [Google Scholar]
- 275.Zhang, W. B., and D. Z. Zhao. 1992. A comparison of calcification of hydatid cysts in sheep pastured and post-fed. Chin. J. Vet. Sci. Technol. 22:28-29. (In Chinese.) [Google Scholar]